Abstract
Background
Peutz-Jeghers syndrome (PJS) is an autosomal dominant hereditary disease characterized by mucocutaneous pigmentation and gastrointestinal hamartomatous polyposis. The germline mutations in the serine/threonine kinase 11 (STK11) gene have been shown to be associated with the disease. Individuals with PJS are at increased risk for development of various neoplasms. The aim of the present study was to characterize the genotype and phenotype of Czech patients with PJS.
Methods
We examined genomic DNA of 8 individuals from five Czech families by sequencing analysis of STK11 gene, covering its promotor region, the entire coding region and the splice-site boundaries, and by multiplex ligation-dependent probe amplification (MLPA) assay designed for the identification of large exonic deletions or duplications of STK11 gene.
Results
We found pathogenic mutations in STK11 gene in two families fulfilling the diagnostic criteria of PJS and in one of three sporadic cases not complying with the criteria. The patient with the frameshift mutation in STK11 gene developed aggressive gastric cancer. No other studied proband has developed a carcinoma so far.
Conclusion
Our results showed that a germline mutation of STK11 gene can be found not only in probands fulfilling the PJS diagnostic criteria, but also in some sporadic cases not complying with the criteria. Moreover, we observed a new case of aggressive gastric cancer in a young patient with a frameshift mutation of STK11 gene.
Similar content being viewed by others
Background
Peutz-Jeghers syndrome (PJS; OMIM 175200) is an autosomal dominant disorder characterized by mucocutaneous pigmentation and gastrointestinal hamartomatous polyposis with an increased risk of cancer [1–4]. The cumulative risk of all cancers in PJS patients by the age of 60 years is 60% and is increased approximately by 8-fold as compared to general population [5]. Histopathologically, polyps in PJS are characterized as hamartomas. However, adenomatous changes may occur in polyps and they can become malignant. In addition to an elevated risk of gastrointestinal cancers, it has been described an increased risk of cancer development at other sites, particularly in the breast, ovary, uterus, cervix, pancreas, lung and testis [3, 6–9]. Testicular sex cord and Sertoli cell tumors, leading to sexual precocity and gynecomastia [10–12], sex cord tumors with annular tubules and cervical adenoma malignum [13] have also been reported.
The gene responsible for PJS, denoted STK11, which encodes a serine/threonine kinase and mapps to chromosome 19p13.3, acts as a tumor suppressor [4, 14, 15]. It plays a role in the p53-dependent apoptosis pathway, in the vascular endothelial growth factor signaling pathway and in the polarization of epithelial cells [16–18].
About one-third of patients with PJS are diagnosed before the age of 10 years and up to 60% cases develop their first clinical manifestations until the third decade of life [19]. In most cases, initial symptoms are abdominal pain due to intussusceptions, obstruction and gastrointestinal bleeding with anemia [20, 21]. A working definition of PJS has been suggested by Giardiello [3], where for individuals with a histopathologically confirmed hamartoma, the diagnosis of definite PJS requires two of the following three findings: a family history consistent with the autosomal dominant inheritance, mucocutaneous hyperpigmentation, or small-bowel polyposis. Tomlinson and Houlston [22] have modified the classification criteria for PJS for individuals without a family history of PJS, in whom the diagnosis depends on the presence of two or more histologically verified Peutz-Jeghers-type hamartomatous polyps.
There are some differential syndromes of PJS which could be misdiagnosed. The pigmentation of the perioral region is an external hallmark of PJS. It is not present in other hamartomatous polyposis syndromes which include Cowden syndrome (CS; OMIM 158350), Bannayan-Riley-Ruvalcaba syndrome (BRRS; OMIM 153480) and Juvenile polyposis syndrome (JPS; OMIM 174900). Laugier-Hunziker syndrome (LHS) is another differential diagnosis of PJS characterized by benign melanotic pigmentation of the oral cavity and lips, associated with spotted macular pigmentation of the fingerprints and longitudinal melanonychia. LHS is known to be a benign disease without gastrointestinal polyposis and with no systemic manifestation [23].
We report here a clinicopathological manifestation and mutational analysis of STK11 gene in eight PJS individuals from five unrelated Czech families.
Methods
Patients
Eight patients from five unrelated families were included in the study (table 1). Four probands from two families fulfilled and three sporadic cases did not fulfill criteria to establish the diagnosis of definite PJS [3, 22]. In one individual, we made a presumptive diagnosis of PJS due to a first-degree relative with PJS and the presence of mucocutaneous hyperpigmentation. All eight patients except one (A-2) underwent endoscopic procedures to examine the inspectable part of GIT.
Family A includes mother (case A-1) and her daughter (case A-2).
Case A-1 was a 29-year-old female with negative family history. The diagnosis of PJS was made at her 10 years of age due to hyperpigmentation of the lips, buccal mucosa, and perinasal region. X-ray examination of abdomen did not reveal any polyp. The patient was free from any abdominal symptoms. At her 24 years of age she underwent gastroscopy because of dyspepsia lasting for a few months. A rigid mucosa of the stomach was noted, but it was histolopathologically negative. Sixteen months later and seven months after giving birth, two hamartomatous polyps 4.5 cm and 1.5 cm in diameter and multiple small polyps 1–3 mm in diameter were found in her stomach. Colonoscopy revealed tubulous adenoma, 3 cm in diameter in her caecum. Enteroclysis did not show any pathology of the small intestine. Later on, she has been followed up every six months. During the follow up, a tubulovillous adenoma from sigmoid colon and a hamartomatous polyp from the transverse colon were removed, and at her 27 years of age, a well differentiated mucinous adenocarcinoma in her left inferior lung lobe was surgically removed. One year later, during second gestation, adenocarcinoma of the stomach was found. The patient refused termination of her pregnancy. Therefore, an operation was performed without any previous neoadjuvant therapy. Unfortunately, because of deterioration of her performance state, premature birth was induced at the 30-th week of the gestation. Three months later the patient died of gastric cancer.
Case A-2, a 7-year-old girl, presented with pale brown patches on the lower lip, which have been noted since her 2 years of age. Examination of her GIT was not performed.
The younger daughter, 4 years old, was not included in the study. She was free of any symptoms typical for PJS.
Family B comprises mother (case B-1) and her two sons (cases B-2 and B-3).
Case B-1, a 46-year-old female has presented with perioral and buccal pigmentation, lasting since childhood. She was found to have colonic and small intestinal hamartomatous polyps already at 36 years of age. The polyps from the stomach were histopathologically classified as hyperplastic with diffuse mixed inflammatory infiltration in stroma and focal epithelial metaplasia. Subsequently, repeated colonoscopy and enteroscopy with polypectomy have been performed. Eventually, total colectomy was performed due to excessive polyposis and recurrent GIT problems. Histopatholological examination of the polyps did not reveal any malignancy. Her family history is missing.
Case B-2 represented a 17-year-old boy with perioral brown pigmentation, mostly on the lips. At his 10 years of age, hamartomatous polyps in small the intestine were detected. Afterwards, he has been frequently examined by gastroduodenoscopy and colonoscopy and polypectomies have been performed. One polyp from the antrum of the stomach, 3 mm in diameter, was classified as hyperplastic with stromal inflammation. Three colon polyps, 3 mm in diameter, showed the typical features of hamartomatous lesions, branching strands of muscular tissue and numerous cystic dilatations of the glandular lumens of various sizes.
Case B-3, a 13-year-old boy, manifested with mucocutaneous brown to dark blue pigmentations on the lip, mostly on the lower one. At his 6 years of age, rectal bleeding due to a polyp, 55 × 35 × 20 mm, in the rectum was noted. Histopathological examination of the polyp showed a tubulovillous adenoma with mild dysplasia in the superficial colonic epithelium. Later on, he has undergone frequent gastroduodenoscopies and colonoscopies with polypectomies. Three polyps were classified as hamartomatous and one as a tubulovillous adenoma with low-grade dysplasia, 3 mm in diameter. All four polyps were excised from the sigmoid colon.
The remaining cases (C-1, D-1 and E-1) were sporadic.
Case C-1, a 20-year-old female has presented brown to dark blue pigmentations since her 2 years of age. At her 14 years of age, enteroclysis her small intestine showed one adenomatous polyp. Frequent colonoscopies and enteroclysies with negative results have been performed. At the time of the molecular analysis of her genomic DNA for germline STK11 mutation, another capsule endoscopy examination was performed. A few (less than ten) diminutive polyps in the stomach and one polyp in the ileum were found. The polyp from the ileum showed characteristic histopathological features of adenoma. Polyps from the stomach were not biopsied. Her family history was negative.
Case D-1, a 50-year-old male with perioral and buccal pigmentation was found to have two hyperplastic polyps, one in the sigmoideum and the other one in the colon ascendens. Tubulovillous adenoma with low-grade dysplasia was excised from the ascending colon. The mother of the patient had colon cancer in 72 years of age and his father, a smoker, had lung cancer in 76 years of age. They were without hyperpigmentation.
Case E-1, a 10-year-old boy, was referred because of perioral and buccal pigmentation. Examination of his GIT did not reveal any polyp. His parents and step-siblings are without any PJS symptoms.
Genetic analysis
After receiving a written and signed informed consent, the genomic DNA of the patients was isolated from blood leukocytes using the Genomic DNA Purification Kit (Gentra Systems, Minneapolis, MI, USA) according to manufacturer's guide. The genomic DNA was amplified using intronic primers [24, 25] flanking the nine exons and the promoter region of STK11 gene. PCR reactions were performed in a total volume of 30 μl containing 100 ng of genomic DNA, 50 pmols of each primer, 0.2 mM of each dNTP, 1 × Taq buffer containing 1.5 mM MgCl2 and 1 unit of Taq polymerase (Fermentas, Vilnius, Lithuania). The amplification was performed in 2720 Thermal cycler (Applied Biosystems, Foster City, CA, USA) with the following protocol: an initial denaturation of 1 min at 95°C was followed by 33 cycles of 1 min at 95°C, 1 min at 53°C, 1 min at 72°C, and a final extension step of 7 min at 72°C. PCR products were purified using the SureClean PCR purification kit (Bioline, London, UK). DNA sequencing was performed using the purified PCR product, the BigDye Terminator v3.1 Cycle Sequencing kit and ABI 3130 Genetic Analyzer (both from Applied Biosystem) according to the manufacturer's instructions. Patients with negative results of DNA sequencing analysis were further examined by means of the multiplex ligation-dependent probe amplification (MLPA) method for the identification of large exonic deletions or duplications, using the P101-STK11 MLPA kit (MRC Holland, Amsterdam, The Netherlands) and the following MLPA protocol http://www.mlpa.com/WebForms/WebFormMain.aspx?Tag=wl2zCji\rCGANQgZPuTixtCplCA1mmwJoFo/xHPnTgc%7C. Samples were run and data were analyzed on ABI 3130 Genetic Analyzer in conjunction with Genotyper software (version 4.0; Applied Biosystems). Electropherograms were evaluated by visual examination of peak heights of the STK11 fragments in relation to the adjacent control fragments and in comparison with external control DNA samples.
The study was conducted with approval by central ethical committee of Ministry of Health, Czech Republic, in accordance with the tenets of the Helsinki declaration.
Results and discussion
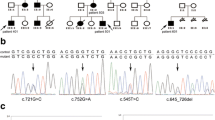
Excised polyps were histopathologically classified as hamartomatous in the cases A-1, B-1, B-2, and B-3, as adenomatous in the case C-1, and as hyperplastic in the case D-1. Case E-1 was free of any polyps (Table 1). All studied individuals had pigmentation of the lips and buccal mucosa, while it was most visible in the children patients. None of the probands had pigmentation of extremities. A positive family history of cancer was only noted in the case D-1. Mutation analysis revealed three different germline mutations. In the family A, a germline mutation (c.350dupT) in exon 2 (Fig. 1) was detected. This mutation is predicted to introduce a frameshift at codon Leu117, 46 novel amino acid residues, and a premature termination codon (p.Leu117PhefsX46). It was found in heterozygosity in both examined patients (A-1 and A-2).
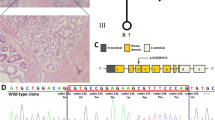
Individuals from the family B (cases B-1, B-2, and B-3) harboured deletion of a part of the promoter region and exon 1 (Fig. 2). Case C-1 was a carrier of deletion of the whole STK11 gene (Fig. 3). In the cases D-1 and E-1, we have not revealed any variation of STK11 gene by using the aforementioned methods.
PJS is a relatively very well characterized disorder with a clear cut phenotype [22]. However, in sporadic cases, the diagnosis of PJS may be uncertain. Although multiple hamartomatous polyps of the GIT are pathognomonic of the PJS, hyperplastic and adenomatous polyps are commonly present [17]. Recently, it has been reported that STK11 deletions are not a rare cause of Peutz-Jeghers syndrome and account for up to 30% of patients with PJS [26, 27]. There was no difference in the clinical phenotype between the patients with point mutations or with large genomic deletions [26]. However, the detailed phenotype of patients with different types of mutations was not reported.
Members of the family B (cases B-1, B-2, and B-3) had almost uniform clinical symptoms with variable age of onset of the first symptoms and detection of polyps. They are carriers of a germline mutation (deletion of a part of the promoter region and exon 1) of STK11. All three affected individuals had mucocutaneous hyperpigmentation predominantly on the lips and on the buccal mucosa, being the most prominent in the youngest patient (B-3) and very pale in the mother (B-1). Sons of the latter patient, i.e. the patients B-2 and B-3, were initially classified as PJS-suspected because of striking hyperpigmentation of the lips. Both of them are under careful surveillance. A few endoscopic examinations of the GIT have been performed so far, with polypectomy of hamartomatous polyps. This confirmed the diagnosis of definite PJS. Polyps were localized in the small intestine and colon. No polyps were detected in the stomach.
Deletion of the promoter region and exon 1 was reported in three independent studies in 12 PJS families overall [26–28]. It could be a recurrent mutation, probably a consequence of an unequal recombination mediated by repetitive Alu (SINE elements) sequences. In accord with UCSC Genome Browser, the region of chr19:1,129,999–1,196,665, where STK11 gene is located, is rich for these repetitive sequences, which can be involved in large chromosome rearrangements. The implication of Alu repetitive elements in unequal genomic recombinations were described for another tumor suppressor gene, MSH2, implicated in Lynch syndrome (HNPCC) [29].
We failed to find any variation of STK11 gene in sporadic cases D-1 and E-1, which would explain their phenotype. On the other hand, they did not fulfill criteria for the diagnosis of definite PJS [3, 22]. We included these cases to the study on the basis of the result from the case C-1. Especially in case E-1 PJS polyps could develop later on. Studies with more individuals not fulfilling PJS diagnostic criteria were reported. None of the patients harboured a germline mutation of STK11 gene [26, 30]. Some studies suggested there could be another locus responsible for PJS phenotype [31, 32]. Other authors stated according to their results that another locus is unlikely and the causative variation could be in regulatory regions such as promoter, enhancers, or splicing sites deep in introns, which are not detectable by conventional methods [26, 27, 33].
The risk of developing various types of GIT cancers (in the esophagus, stomach, small bowel and colon) was determined in several studies [3, 5, 7, 8, 34]. The cumulative risk for stomach cancer was 29% [8]. Amos et al. noted that gastric polyps are very common among individuals with PJS [30]. However, they did not specify the proportion of patients with a detectable PJS germline mutation and the gastric polyps/cancer. There are several case-reports and reviews reporting gastric cancer in PJS patients [3, 20, 35–44]. In our group of probands, the case A-1 had developed gastric cancer at 28 years of age and died one year later. No genotype-phenotype correlations were published in PJS patients with gastric cancer [7, 30, 33]. Konishi et al. reviewed 103 PJS patients with malignancy from literature and found out that the mean age of 8 cases with gastric cancer was 31.2 years as compared to 39.7 years in duodenal carcinoma (9 cases), and 48 years in colorectal carcinoma (13 cases). According to the literature and our results we suppose that gastric cancer has very aggressive course in some individuals with PJS and despite the very frequent endoscopic examinations with relevant treatment the next course is usually poor. Therefore, more attention should be paid to patients with molecularly confirmed PJS, especially those who have polyposis of the stomach. It would be particularly interesting to find out if there is a correlation between the genotype and phenotype in relation to the development of gastric cancer. There have been only two reports dealing with gastric cancer in PJS patients and mutational analysis of STK11 gene so far [24, 43]. Shinmura et al. described two PJS females (sisters) with gastric cancer in whom a STK11 germline mutation (c.890delG) was identified [24]. Takahashi et al. reported a 14-year-old girl with sporadic PJS and early-onset gastric cancer harboring a frameshift (c.757_758insT) STK11 mutation [43]. Similarly, as in our family A, the mutations led to a truncated protein lacking the kinase domain. These results suggest that the truncation mutations leading to loss of STK11 kinase domain could act in a dominant negative fashion and be responsible of tumor development. Schumacher et al. summarized clinical and mutational data from 132 PJS cases (83 without and 49 with cancer) to find correlation between the type/site of mutation and cancer. They proposed two different mechanisms of tumor development. One is based on the loss of STK11 functions due to truncation mutations and subsequent LOH as a second hit. This hypothesis is not in accord with findings of other authors, where a second hit was not a requisite condition for tumor development [24, 43].
Conclusion
In summary, we found germline mutations of STK11 gene in three families. One patient (C-1) with the germline mutation did not fulfill the criteria for establishing the diagnosis of PJS. Therefore, the variability in time of onset of symptoms should be always kept in mind when establishing the diagnosis of PJS and managing this disease.
Abbreviations
- STK11 :
-
serine/threonine kinase 11
- PJS:
-
Peutz-Jeghers syndrome
- MLPA:
-
multiplex ligation-dependent probe amplification
- BRRS:
-
Bannayan-Riley-Ruvalcaba syndrome
- JPS:
-
Juvenile polyposis syndrome
- LHS:
-
Laugier-Hunziker syndrome
- GIT:
-
gastrointestinal tract
- SINE:
-
short interspersed element
- UCSC:
-
University of California Santa Cruz
- MSH2 :
-
mutS homologue 2
- HNPCC:
-
hereditary nonpolyposis colon cancer
- LOH:
-
loss of heterozygosity.
References
Jeghers H, Mc KV, Katz KH: Generalized intestinal polyposis and melanin spots of the oral mucosa, lips and digits; a syndrome of diagnostic significance. N Engl J Med. 1949, 241 (26): 1031-1036.
McGarrity TJ, Kulin HE, Zaino RJ: Peutz-Jeghers syndrome. Am J Gastroenterol. 2000, 95 (3): 596-604. 10.1111/j.1572-0241.2000.01831.x.
Giardiello FM, Welsh SB, Hamilton SR, Offerhaus GJ, Gittelsohn AM, Booker SV, Krush AJ, Yardley JH, Luk GD: Increased risk of cancer in the Peutz-Jeghers syndrome. N Engl J Med. 1987, 316 (24): 1511-1514.
Hemminki A, Tomlinson I, Markie D, Jarvinen H, Sistonen P, Bjorkqvist AM, Knuutila S, Salovaara R, Bodmer W, Shibata D, et al: Localization of a susceptibility locus for Peutz-Jeghers syndrome to 19p using comparative genomic hybridization and targeted linkage analysis. Nat Genet. 1997, 15 (1): 87-90. 10.1038/ng0197-87.
Hearle N, Schumacher V, Menko FH, Olschwang S, Boardman LA, Gille JJ, Keller JJ, Westerman AM, Scott RJ, Lim W, et al: Frequency and spectrum of cancers in the Peutz-Jeghers syndrome. Clin Cancer Res. 2006, 12 (10): 3209-3215. 10.1158/1078-0432.CCR-06-0083.
Gruber SB, Entius MM, Petersen GM, Laken SJ, Longo PA, Boyer R, Levin AM, Mujumdar UJ, Trent JM, Kinzler KW, et al: Pathogenesis of adenocarcinoma in Peutz-Jeghers syndrome. Cancer Res. 1998, 58 (23): 5267-5270.
Lim W, Olschwang S, Keller JJ, Westerman AM, Menko FH, Boardman LA, Scott RJ, Trimbath J, Giardiello FM, Gruber SB, et al: Relative frequency and morphology of cancers in STK11 mutation carriers. Gastroenterology. 2004, 126 (7): 1788-1794. 10.1053/j.gastro.2004.03.014.
Giardiello FM, Brensinger JD, Tersmette AC, Goodman SN, Petersen GM, Booker SV, Cruz-Correa M, Offerhaus JA: Very high risk of cancer in familial Peutz-Jeghers syndrome. Gastroenterology. 2000, 119 (6): 1447-1453. 10.1053/gast.2000.20228.
Spigelman AD, Murday V, Phillips RK: Cancer and the Peutz-Jeghers syndrome. Gut. 1989, 30 (11): 1588-1590. 10.1136/gut.30.11.1588.
Wilson DM, Pitts WC, Hintz RL, Rosenfeld RG: Testicular tumors with Peutz-Jeghers syndrome. Cancer. 1986, 57 (11): 2238-2240. 10.1002/1097-0142(19860601)57:11<2238::AID-CNCR2820571128>3.0.CO;2-C.
Coen P, Kulin H, Ballantine T, Zaino R, Frauenhoffer E, Boal D, Inkster S, Brodie A, Santen R: An aromatase-producing sex-cord tumor resulting in prepubertal gynecomastia. N Engl J Med. 1991, 324 (5): 317-322.
Young S, Gooneratne S, Straus FH, Zeller WP, Bulun SE, Rosenthal IM: Feminizing Sertoli cell tumors in boys with Peutz-Jeghers syndrome. Am J Surg Pathol. 1995, 19 (1): 50-58.
Podczaski E, Kaminski PF, Pees RC, Singapuri K, Sorosky JI: Peutz-Jeghers syndrome with ovarian sex cord tumor with annular tubules and cervical adenoma malignum. Gynecol Oncol. 1991, 42 (1): 74-78. 10.1016/0090-8258(91)90234-V.
Hemminki A, Markie D, Tomlinson I, Avizienyte E, Roth S, Loukola A, Bignell G, Warren W, Aminoff M, Hoglund P, et al: A serine/threonine kinase gene defective in Peutz-Jeghers syndrome. Nature. 1998, 391 (6663): 184-187. 10.1038/34432.
Jenne DE, Reimann H, Nezu J, Friedel W, Loff S, Jeschke R, Muller O, Back W, Zimmer M: Peutz-Jeghers syndrome is caused by mutations in a novel serine threonine kinase. Nat Genet. 1998, 18 (1): 38-43. 10.1038/ng0198-38.
Karuman P, Gozani O, Odze RD, Zhou XC, Zhu H, Shaw R, Brien TP, Bozzuto CD, Ooi D, Cantley LC, et al: The Peutz-Jegher gene product LKB1 is a mediator of p53-dependent cell death. Mol Cell. 2001, 7 (6): 1307-1319. 10.1016/S1097-2765(01)00258-1.
Ylikorkala A, Rossi DJ, Korsisaari N, Luukko K, Alitalo K, Henkemeyer M, Makela TP: Vascular abnormalities and deregulation of VEGF in Lkb1-deficient mice. Science. 2001, 293 (5533): 1323-1326. 10.1126/science.1062074.
Martin SG, St Johnston D: A role for Drosophila LKB1 in anterior-posterior axis formation and epithelial polarity. Nature. 2003, 421 (6921): 379-384. 10.1038/nature01296.
Tovar JA, Eizaguirre I, Albert A, Jimenez J: Peutz-Jeghers syndrome in children: report of two cases and review of the literature. J Pediatr Surg. 1983, 18 (1): 1-6. 10.1016/S0022-3468(83)80262-0.
Utsunomiya J, Gocho H, Miyanaga T, Hamaguchi E, Kashimure A: Peutz-Jeghers syndrome: its natural course and management. Johns Hopkins Med J. 1975, 136 (2): 71-82.
McGarrity TJ, Amos C: Peutz-Jeghers syndrome: clinicopathology and molecular alterations. Cell Mol Life Sci. 2006, 63 (18): 2135-2144. 10.1007/s00018-006-6080-0.
Tomlinson IP, Houlston RS: Peutz-Jeghers syndrome. J Med Genet. 1997, 34 (12): 1007-1011. 10.1136/jmg.34.12.1007.
Mignogna MD, Lo Muzio L, Ruoppo E, Errico M, Amato M, Satriano RA: Oral manifestations of idiopathic lenticular mucocutaneous pigmentation (Laugier-Hunziker syndrome): a clinical, histopathological and ultrastructural review of 12 cases. Oral Dis. 1999, 5 (1): 80-86.
Shinmura K, Goto M, Tao H, Shimizu S, Otsuki Y, Kobayashi H, Ushida S, Suzuki K, Tsuneyoshi T, Sugimura H: A novel STK11 germline mutation in two siblings with Peutz-Jeghers syndrome complicated by primary gastric cancer. Clin Genet. 2005, 67 (1): 81-86. 10.1111/j.1399-0004.2005.00380.x.
Hearle NC, Tomlinson I, Lim W, Murday V, Swarbrick E, Lim G, Phillips R, Lee P, O'Donohue J, Trembath RC, et al: Sequence changes in predicted promoter elements of STK11/LKB1 are unlikely to contribute to Peutz-Jeghers syndrome. BMC Genomics. 2005, 6 (1): 38-10.1186/1471-2164-6-38.
Aretz S, Stienen D, Uhlhaas S, Loff S, Back W, Pagenstecher C, McLeod DR, Graham GE, Mangold E, Santer R, et al: High proportion of large genomic STK11 deletions in Peutz-Jeghers syndrome. Hum Mutat. 2005, 26 (6): 513-519. 10.1002/humu.20253.
Hearle NC, Rudd MF, Lim W, Murday V, Lim AG, Phillips RK, Lee PW, O'Donohue J, Morrison PJ, Norman A, et al: Exonic STK11 deletions are not a rare cause of Peutz-Jeghers syndrome. J Med Genet. 2006, 43 (4): e15-10.1136/jmg.2005.036830.
Volikos E, Robinson J, Aittomaki K, Mecklin JP, Jarvinen H, Westerman AM, de Rooji FW, Vogel T, Moeslein G, Launonen V, et al: LKB1 exonic and whole gene deletions are a common cause of Peutz-Jeghers syndrome. J Med Genet. 2006, 43 (5): e18-10.1136/jmg.2005.039875.
Li L, McVety S, Younan R, Liang P, Du Sart D, Gordon PH, Hutter P, Hogervorst FB, Chong G, Foulkes WD: Distinct patterns of germ-line deletions in MLH1 and MSH2: the implication of Alu repetitive element in the genetic etiology of Lynch syndrome (HNPCC). Hum Mutat. 2006, 27 (4): 388-10.1002/humu.9417.
Amos CI, Keitheri-Cheteri MB, Sabripour M, Wei C, McGarrity TJ, Seldin MF, Nations L, Lynch PM, Fidder HH, Friedman E, et al: Genotype-phenotype correlations in Peutz-Jeghers syndrome. J Med Genet. 2004, 41 (5): 327-333. 10.1136/jmg.2003.010900.
Mehenni H, Blouin JL, Radhakrishna U, Bhardwaj SS, Bhardwaj K, Dixit VB, Richards KF, Bermejo-Fenoll A, Leal AS, Raval RC, et al: Peutz-Jeghers syndrome: confirmation of linkage to chromosome 19p13.3 and identification of a potential second locus, on 19q13.4. Am J Hum Genet. 1997, 61 (6): 1327-1334. 10.1086/301644.
Olschwang S, Markie D, Seal S, Neale K, Phillips R, Cottrell S, Ellis I, Hodgson S, Zauber P, Spigelman A, et al: Peutz-Jeghers disease: most, but not all, families are compatible with linkage to 19p13.3. J Med Genet. 1998, 35 (1): 42-44. 10.1136/jmg.35.1.42.
Chow E, Meldrum CJ, Crooks R, Macrae F, Spigelman AD, Scott RJ: An updated mutation spectrum in an Australian series of PJS patients provides further evidence for only one gene locus. Clin Genet. 2006, 70 (5): 409-414. 10.1111/j.1399-0004.2006.00704.x.
Lim W, Hearle N, Shah B, Murday V, Hodgson SV, Lucassen A, Eccles D, Talbot I, Neale K, Lim AG, et al: Further observations on LKB1/STK11 status and cancer risk in Peutz-Jeghers syndrome. Br J Cancer. 2003, 89 (2): 308-313. 10.1038/sj.bjc.6601030.
Aideyan UO, Kao SC: Gastric adenocarcinoma metastatic to the testes in Peutz-Jeghers syndrome. Pediatr Radiol. 1994, 24 (7): 496-497. 10.1007/BF02015010.
Cochet B, Carrel J, Desbaillets L, Widgren S: Peutz-Jeghers syndrome associated with gastrointestinal carcinoma. Report of two cases in a family. Gut. 1979, 20 (2): 169-175. 10.1136/gut.20.2.169.
Dong K, Li B: Peutz-Jeghers syndrome: case reports and update on diagnosis and treatment. Chin J Dig Dis. 2004, 5 (4): 160-164. 10.1111/j.1443-9573.2004.00179.x.
Foley TR, McGarrity TJ, Abt AB: Peutz-Jeghers syndrome: a clinicopathologic survey of the "Harrisburg family" with a 49-year follow-up. Gastroenterology. 1988, 95 (6): 1535-1540.
Halbert RE: Peutz-Jeghers syndrome with metastasizing gastric adenocarcinoma. Report of a case. Arch Pathol Lab Med. 1982, 106 (10): 517-520.
Hizawa K, Iida M, Matsumoto T, Kohrogi N, Kinoshita H, Yao T, Fujishima M: Cancer in Peutz-Jeghers syndrome. Cancer. 1993, 72 (9): 2777-2781. 10.1002/1097-0142(19931101)72:9<2777::AID-CNCR2820720940>3.0.CO;2-6.
Payson BA, Moumgis B: Metastasizing carcinoma of the stomach in Peutz-Jeghers syndrome. Ann Surg. 1967, 165 (1): 145-151. 10.1097/00000658-196701000-00021.
Taguchi T, Suita S, Taguchi S, Tanaka S: Peutz-Jeghers syndrome in children: high recurrence rate in short-term follow-up. Asian J Surg. 2003, 26 (4): 221-224.
Takahashi M, Sakayori M, Takahashi S, Kato T, Kaji M, Kawahara M, Suzuki T, Kato S, Kato S, Shibata H, et al: A novel germline mutation of the LKB1 gene in a patient with Peutz-Jeghers syndrome with early-onset gastric cancer. J Gastroenterol. 2004, 39 (12): 1210-1214. 10.1007/s00535-004-1474-y.
Horn RC, Payne WA, Fine G: The Peutz-Jeghers syndrome. (Gastrointestinal polyposis with mucocutaneous pigmentation): report of a case terminating with disseminated gastrointestinal cancer. Arch Pathol. 1963, 76: 29-37.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2350/10/69/prepub
Acknowledgements
We thank to all the patients and their families for agreeing to participate in the study. Written consent was obtained from the patients or their relative for publication of study. We are grateful to Iveta Nimsova for the DNA isolation.
Grant support: VZ MZO 00064203
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
PV carried out molecular genetic studies including DNA sequencing, MLPA analysis for all the families, and drafted the manuscript. AP identified and diagnosed the patients. JR performed GIT examinations and provided histopathological information. AK designed the study and revised the manuscript. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Vasovčák, P., Puchmajerová, A., Roubalík, J. et al. Mutations in STK11gene in Czech Peutz-Jeghers patients. BMC Med Genet 10, 69 (2009). https://doi.org/10.1186/1471-2350-10-69
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2350-10-69