Abstract
Soil organic carbon (SOC) is a crucial component that significantly affects the soil fertility, soil remediation, and carbon sequestration. Here, we review the redox-induced transformation of potentially toxic elements (PTEs) through the abiotic impact of SOC. The complex composition of SOC includes humus, pyrogenic carbon (e.g., biochar), dissolved organic matter, and anthropogenic carbon (e.g., compost), with varying concentrations and properties. The primary redox moieties on organic carbon are surface functionalities (e.g., phenol, quinone, and N/S-containing functional groups), environmentally persistent free radicals, and graphitic structures, and their contents are highly variable. Owing to these rich redox moieties, organic carbon can directly affect the reduction and oxidation of PTEs in the soil, such as Cr(VI) reduction and As(III) oxidation. In addition, the interactions between organic carbon and soil redox moieties (i.e., O2, Fe, and Mn minerals) cause the transformation of PTEs. The formation of reactive oxygen species, Fe(II), and Mn(III)/Mn(II) is the main contributor to the redox-induced transformation of PTEs, including Cr(VI) reduction and As(III)/Cr(III)/Tl(I) oxidation. We articulated both the positive and negative effects of organic carbon on the redox-induced transformation of PTEs, which could guide soil remediation efforts. Further scientific studies are necessary to better understand the potential transformations of PTEs by SOC, considering the complicated soil moieties, variable organic carbon composition, and both biotic and abiotic transformations of PTEs in the environment.
Graphical Abstract

Highlights
• Redox reactions of potentially toxic elements (PTEs) with organic carbon are elaborated, which will affect the fate and toxicity of PTEs.
• Organic carbon can directly/indirectly oxidize or reduce PTEs, and interactions with soil moieties also affect redox reactions of PTEs.
• Future studies are needed to better understand the multiple roles of organic carbon and their impact on the fate and transport of PTEs in soil.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
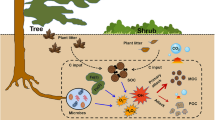
Soil is the largest terrestrial carbon pool, containing approximately 1.5 × 1012 kg organic carbon (Wang et al. 2020e), which stores over twofold higher than the atmospheric and biotic pools (Lehmann and Kleber 2015). Organic carbon and organic matter normally represent a similar series of organic compounds in soil, whereas the concept of organic carbon is typically used to emphasize the carbon element in organic matter compared with organic nitrogen and phosphate in the soil. The concentration of soil organic carbon (SOC) ranges widely (Lamichhane et al. 2019). For instance, the SOC concentration in China could change from 16.01 to 42.62 Mg C ha− 1 due to the different soil properties, and it increased by about 140 kg C ha− 1 year− 1 (Zhao et al. 2018b). In addition to the changeable concentration, SOC has a complicated and variable composition, including plant and animal residues with different degrees of decomposition, substances synthesized through various natural and anthropogenic reactions in soil, and the biomass of living soil organisms and their associated metabolic products. The varying composition and concentration of organic carbon greatly affect the biogeochemical processes in different soils.
Humus (humic acid, fulvic acid, and humin) and some non-humic dissolved organic matter (e.g., organic acids) are the most widely studied organic carbon components to obtain a deep understanding of their crucial roles in different soil reactions (Ondrasek et al. 2019; Paul 2016; Polak et al. 2019; Wang et al. 2020e; Yang et al. 2020). Recently, pyrogenic carbon (e.g., biochar) from biomass burning in fields, which is rich in Amazonian dark earth, has garnered increasing attention owing to its high stability for long-term carbon sequestration (Lehmann 2007; Lehmann et al. 2021). In addition, the porous structure, rich surface functionality, and condensed graphitic carbon of biochar offer outstanding capacity for the regulation of other soil properties, including the immobilization of pollutants (Gong et al. 2022; Wang and Wang 2019; Xu et al. 2021c) and fertility (Baki and Abedi-Koupai 2018; Marcińczyk and Oleszczuk 2022). In addition to naturally generated organic carbon, human activities might result in the introduction of some organic carbon in the soil, such as carbon-based materials for soil application, leading to further variations in soil properties (Marcińczyk and Oleszczuk 2022; Xie et al. 2015). Living organisms, including bacteria, fungi, actinomycetes, soil animals, and archaea, also comprise a non-negligible portion of organic carbon in the soil; however, their content and composition are highly site-specific and time-dependent.
The impact of different organic carbon on fertility, soil remediation, and carbon sequestration is systematically studied (Hoffland et al. 2020; Lehmann and Kleber 2015; Maestrini et al. 2015; Ondrasek et al. 2019; Smreczak and Ukalska-Jaruga 2021; Verbeeck et al. 2020; Woolet and Whitman 2020), and their potential role in the abiotic redox-induced transformation of pollutants in soil has been highlighted recently (Aftabtalab et al. 2022; LaCroix et al. 2021; Xu et al. 2020c, 2022b). The considerable redox capacity of organic carbon could directly affect the redox reaction of potentially toxic elements (PTEs), which are the trace elements with the potential toxicity to humans. The mobility and toxicity of PTEs will be greatly altered during the redox-induced transformation process (Ali et al. 2020), thus affecting their risks to humans. For instance, the oxidation of arsenic (As(III) to As(V)) and thallium (Tl(I) to Tl(III)) by the oxidative moieties of SOC decreases their mobility (Chen et al. 2022; Cuong et al. 2021; Xu et al. 2021b). In addition, the reduction of chromium (Cr(VI) to Cr(III)) by organic carbon reverses the charge of ions from negative to positive, leading to different combination processes in the soil (Xu et al. 2022a, b). The indirect impact of organic carbon on redox-induced transformation is also critical, such as coupling the redox reaction with iron minerals or electron mediation (Qiao et al. 2021). The chelation effect of dissolved organic carbon, especially for that with rich O- or N-moieties, might affect the transformation of PTEs because it can increase the availability of redox-active moieties (e.g., Fe ions) in the soil, thereby enhancing the redox-induced transformation of PTEs. The transformation of PTEs is vital because it not only affects their mobility and stability but also their toxicity. For instance, As(V) and Cr(III) are usually less toxic than their other valence states (As(III) and Cr(VI)).
To the best of our knowledge, no critical review has comprehensively studied the abiotic redox-induced transformation of typical PTEs in soil with a wide range of organic carbon content. Various kinds of non-living organic carbon, including humus, biochar, non-humic dissolved organic matter, and anthropogenic carbon, are included in this review because they represent the majority of SOC (> 80%) and are relatively stable compared to living organic carbon. Notably, although the concept of humus has been refuted recently since the contemporary techniques failed to find convincing evidence and the definition was not precise enough (Lehmann and Kleber 2015; Weng et al. 2021), we still use these concepts to describe the humic substances as a composition of SOC in this review since they are widely used in related papers. This review critically summarizes the following: (1) typical SOC with different redox properties; (2) direct redox-induced transformation of PTEs with SOC; (3) indirect transformation of PTEs with SOC coupled with active soil moieties; and (4) remediation strategies and potential risks based on the PTEs transformation process through SOC. This review can advance our understanding about the fates and risks of PTEs with organic carbon in the soil and guide sustainable remediation of PTEs.
2 Organic carbon in the soil and its redox reactivity
2.1 Humus
Humus (humic substances) is a traditional concept with different definitions: strict operation according to what can be extracted with an alkaline solution, with further subcategories of humic acids (HA), fulvic acids (FA), and unextractable humin; as an existing substance that is not merely an operational construct; or as a combination of the two (Lehmann and Kleber 2015). As stated before, we still use these concepts to describe the humic substances as a composition of SOC since they have been extensively used in published papers, although the concept of humus has been refuted recently (Lehmann and Kleber 2015; Weng et al. 2021). Different small assimilable biopolymers and monomers are generated through the decomposition process of dead plants and animal matter under different strategies, and humus comprises these molecules with supramolecular structures (Fig. 1) (Lehmann and Kleber 2015; Paul 2016). Different theories, such as humification, have been developed for describing the formation of humus, and they all agree that the detailed composition of humus is highly related to the feedstock precursors and formation strategies, leading to the highly variable properties of humus (Lehmann and Kleber 2015).
Existence and transformation of organic carbon in the soil with the following processes: (1) progressive decomposition of biomass residues to CO2 by microbes; (2) humification of different biopolymers and monomers by microbes and minerals; (3) pyrolysis or incomplete combustion of biomass with wildfire; (4) release of a small fraction of organic carbon through physical, chemical, and biological degradation; and (5) deployment of carbon-based materials for different applications, wherein artificial carbon could be partially degraded over a long duration
Humin is an insoluble component even during strong-base extraction because of its highly condensed structure and combination with minerals, and the inherent quinone and sulfur-containing moieties are the redox-active centers (Pham et al. 2021, 2022). HA and FA are soluble in a strong-base solution, but only HA precipitates when the strong-base extract is acidified, owing to its higher molecular weight and less surface functionality of the HA (Tang et al. 2014). Both HA and FA contain various redox-active functional groups, such as phenol, hydroxyl, and quinone, thereby enabling them to participate in the redox reaction directly (Eqs. 1–3 and Fig. 2) (Bai et al. 2020; LaCroix et al. 2021). For instance, the quinone can accept electrons with the formation of hydroquinone, thus causing oxidation (Eq. 1). In addition, the transition among hydroxyl, carbonyl, and carboxyl accompanied by the electron exchange also leads to the redox reaction. The quinone-hydroquinone/phenol moieties (E0 = 0.70 V, Table 1), instead of the aliphatic structure, are the primary electron-donating or withdrawing moieties (He et al. 2019; Klüpfel et al. 2014b; Walpen et al. 2018), offering a reversible redox reactivity (on 822 μmol e− g of HA) (Aeschbacher et al. 2010). In addition, the persistent free radicals (PFRs), hydroxyl, and N/S-containing functionalities might be responsible for up to 50% of the redox reactivity of the humic substances (He et al. 2019; Shi et al. 2021). The higher molecular weight fraction of humus may contain a higher amount of carbon-centered radicals, leading to a higher reducing capacity than that of the lower molecular weight fraction with oxygen-centered radicals (Shi et al. 2021).

In addition to the direct redox reaction with PTEs, humus can serve as the redox buffer and mediator to facilitate electron transfer (Fig. 2) (Bai et al. 2019; Zou et al. 2021). The potential capacity of humus for radical generation has been evaluated (Chen et al. 2018; Wu et al. 2021), which significantly affects the transformation of PTEs. The PFRs or reductive functionality of humus can reduce O2 with the formation of H2O2 and free radicals (Eqs. 4–7) (Kim et al. 2022; Page et al. 2012). Although several studies have also reported that humic substances are radical scavengers (Cheng et al. 2022; Donham et al. 2014), the radical activation process by humus can be facilitated after complexation with minerals (Liao et al. 2019a; Wang et al. 2021b; Yu et al. 2021; Zeng et al. 2020), leading to a higher probability of radical activation in the soil. The redox-active minerals could donate parts of electrons to complete the formation of radicals with HA (Yu et al. 2021), and the homogeneous H2O2 decomposition by HA-chelated metal complexes would facilitate ·OH formation (Liao et al. 2019a; Zeng et al. 2020). Solar irradiation could also enhance the generation of radicals with humus owing to the excited states of humus (Wan et al. 2021a; Wang et al. 2021b; Wu et al. 2021), and this phenomenon might mainly occur in the surface layer of soil.
2.2 Biochar
Biochar is a relatively newer concept than humus, but it has a similarly long history in the soil. Biochar is mainly produced from biomass combustion in the complete or partial absence of oxygen (Fig. 1), leading to the formation of carbon-rich residues with higher stability (Pignatello et al. 2017). Previously, it had some older names with similar concepts, such as black carbon, carbonized biomass, pyrogenic carbon, and charcoal, but the expression of biochar was first defined in 2006 by Lehmann et al. (2006), highlighting its carbon sequestration nature. Although current studies mainly focused on the engineered preparation of biochar for different soil applications, its natural source from the on-site combustion of plant-based biomass is still critical.
The redox properties of biochar are highly related to the production temperature because of the transition in surface functionality (Klüpfel et al. 2014a; Xin et al. 2021; Zhang et al. 2018b, 2019b). Similar to humus, the quinone and phenol moieties are vital for the redox performance of biochar (Fig. 2). Moreover, phenol would transform to quinone with the increase of temperature, leading to the transition of reductive biochar to oxidative biochar (Klüpfel et al. 2014a; Xin et al. 2021). In addition, other oxygen-containing functionalities (e.g., carboxyl, hydroxyl, and carbonyl) may be responsible for the electron transfer capacity of biochar (Xu et al. 2020c; Zhang et al. 2019b; Zhong et al. 2019). In addition to the reversible electron transfer through surface functionality (geo-battery), the conductivity of biochar facilitates a fast electron transfer routine, also denoted as the geo-conductor mechanism (Fig. 2) (Sun et al. 2017, 2018; Yang et al. 2021). The formation of a graphitic-like structure with condensed aromatic rings during high-temperature pyrolysis (> 700 °C) is vital to the fast electron transfer process, and this might be the unique electron transfer routine for biochar as opposed to humus. Moreover, the highly ordered aromatic ring might stabilize the PFRs on the biochar, leading to higher PFRs content than humus (Ruan et al. 2019). Biochar-derived dissolved organic matter also contains considerable activity for the redox reaction (Xu et al. 2021a; Zhang et al. 2019a).
Engineered biochar produced by the pyrolysis of selected waste under designated conditions is increasingly studied as a soil amendment. Compared with the naturally occurring biochar or other pyrogenic carbon in the soil, biochar manufactured purposefully through a wide range of feedstocks and precise temperatures with different modification or activation processes could accomplish high performance for the desired applications (Li et al. 2020; Lü et al. 2022; Wen et al. 2017; Xu et al. 2022a). The engineered biochar can demonstrate a higher redox reactivity with rich surface functionality and a higher surface area than natural biochar for the redox-related remediation of PTEs (Alkurdi et al. 2019; Li et al. 2020; Lü et al. 2022). However, if the biochar is designed for soil conditioning and fertility enhancement, its impact on the redox-induced transformation of PTEs needs further consideration based on the biochar properties such as surface functionality, carbon structure, particle size, and surface area. In addition, the newly-added biochar may have a different redox reactivity than the naturally occurring biochar, because the latter generally undergoes a long-term aging process with increasing O-functionalities and decreasing electron-donating capacities (Wang et al. 2020c; Xu et al. 2021c).
Owing to the rich mineral content in the soil, biochar is known to extensively combine with soil minerals (Yang et al. 2016; Zhao and Zhou 2019), resulting in a variable electron transfer capacity and route (Xu et al. 2021c, 2022b). The formation of an organo-mineral coating on the biochar surface can protect the carbon phase of biochar with a decrease in the redox reactivity (Jing et al. 2022; Yang et al. 2016). In addition, the electrons from biochar might be “captured” by minerals with the formation of reductive species (e.g., Fe(II)), causing the decrease in electron reactivity (Xu et al. 2020b, 2022b). In contrast, some redox-active metal ions might facilitate the electron transfer through valence transformation (Xu et al. 2021c), and cationic ions can overcome the charge repulsion between biochar and anions, enhancing the electron transfer probability (Xu et al. 2021c; Yang et al. 2018).
2.3 Non-humic dissolved organic matter
Non-humic dissolved organic matter refers to a series of free, small organic carbon constituents without supramolecular humus structures (Polak et al. 2019), as the widely used concept of dissolved organic matter (DOM) or dissolved organic carbon (DOC) might have some overlapping characteristics with humus. The main components are organic acids, poly-/mono-saccharides, phenol-aromatics, and lipids (Yang et al. 2020). Although the content of DOC is lower than that of humus and its composition is highly variable, its impact is still critical for the redox processes of PTEs, especially for organic acids. Recent studies have been conducted on the impact of organic acids and saccharides on the redox-induced transformation of PTEs in soil (Deng et al. 2020; Liu et al. 2019; Xu et al. 2019c).
Organic acids are a significant part of the non-humic dissolved organic matter in the soil, especially in the rhizosphere (Adeleke et al. 2017; Hubova et al. 2017). Among the different organic acids, oxalic, citric, malic, acetic, formic, and tartaric acids have been identified to play significant roles in soil because of their relatively higher contents (Adeleke et al. 2017; Xu et al. 2019c). The α-hydroxyl on organic acids has a higher reducing capacity and might participate in the electron-donating process, while the carboxyl group typically has a limited impact on the direct electron donation (Liu et al. 2019; Xu et al. 2019c). The strong chelation effect of hydroxyl and carboxyl groups on organic acids might also affect the redox-induced transformation of PTEs, because of their different redox potentials and solubilities after chelation (Liu et al. 2019; Saad et al. 2017). For instance, organic ligands could lead to the formation of Cr-ligands through chelation, facilitating the Cr(VI) reduction process (Liu et al. 2019). In addition, chelation with redox-active metals (e.g., Fe) contributes to further electron transfer with PTEs (Saad et al. 2017; Xu et al. 2020b). The N−/S-containing functional groups (e.g., −NH2 and −SH) in amino acids also play a non-negligible role in the redox cycle of the elements in soil owing to their strong redox reactivity and chelation effect (Bhattacharyya et al. 2019).
2.4 Anthropogenic carbon sources
In addition to naturally occurring organic carbon, different carbon-based materials have been added to soil for various applications, including carbon sequestration, soil fertility enhancement, and environmental remediation (Mazarji et al. 2021; Pignatello et al. 2017; Rashid et al. 2021). A wide range of carbon-containing materials have been added to the soil, and they might have different reactivities for the redox-induced transformation of PTEs in the environment.
In addition to biochar-based materials, as mentioned before, there has been a significant interest in using natural organic materials for soil amendment, such as biopolymers (starch and lignin) (Pang et al. 2019; Rashid et al. 2021; Yaashikaa et al. 2022). These materials might also have a considerable redox potential, which could be enhanced after modification (He et al. 2019). Composting is a common method for organic modification and depolymerization (Farid et al. 2022), and it degrades the stable carbon structure with the formation of a humus-like structure containing rich quinone and phenol moieties (Duran et al. 2022; He et al. 2019), thus leading to high redox reactivity and performance. The addition of inorganic catalysts, such as minerals, during the composting process can accelerate depolymerization and humic substance formation (Jiang et al. 2021; Wu et al. 2018; Zheng et al. 2021).
Other carbon-based materials, such as activated carbon, graphene, and carbon nanotubes, can also be added to the soil for remediation (Li et al. 2018; Mazarji et al. 2021; Park et al. 2016). The primary redox-reactive moieties on these carbon materials should be similar to pyrogenic carbon (e.g., phenolic and quinone moieties); however, they usually exhibit higher reactivity, a large surface area, rich active sites, small particle size, and well-formed graphitic structure, thereby significantly influencing the transformation of PTEs (Wang et al. 2020a). The aging process also has a critical impact on the redox performance of the anthropogenic carbon materials, and it is a combined process involving oxidation, aggregation, and fragmentation (Ding et al. 2022a; Park et al. 2016), leading to different performances in the redox-induced transformation of PTEs.
3 Direct redox reaction between PTEs and organic carbon
3.1 Reduction
The SOC had a higher reducing potential, leading to the direct reduction of several PTEs. Chromium (Cr) is the most widely studied PTEs because the reduction of Cr(VI) to Cr(III) decreases the toxicity of Cr. The electrical properties of Cr also change from negative (CrO42−/HCrO4−) to positive (Cr(OH)X(3-X)+), leading to a different fate of Cr in the soil. Surface functionalities such as phenolic functionality, PFRs, and α-hydroxyl on organic carbon are the main reducing moieties for Cr(VI) reduction (Xu et al. 2020b, c; Zhao et al. 2018a). The structural defects on the graphitic carbon structure, which widely occur on the pyrogenic carbon and graphene, also provide considerable reducing activity for Cr(VI) reduction (Xu et al. 2020c). The reduction of Cr(VI) by organic carbon is facilitated under the following conditions: (i) an acidic environment (Xu et al. 2019b); (ii) accelerated electron transfer with redox-active metal ions (i.e., iron (Fe) and manganese (Mn)) (Xu et al. 2020b, 2021c); (iii) condensed graphitic structure on pyrogenic carbon (e.g., biochar) for fast electron transfer (Sun et al. 2017; Xu et al. 2020c); (iv) cations for overcoming the charge repulsion (Xu et al. 2021c); and (v) accumulation of Cr(VI) and organic carbon on the solid surface.
In addition to Cr(VI) reduction, SOC can reduce other PTEs with similar critical reducing moieties. The formation of zero-valent mercury (Hg(0)) through Hg(II) reduction by the reduced quinones from organic carbon was found, which was two to six times faster than that from the photochemical reduction of Hg(II) (Zheng et al. 2012). The reduction of copper (Cu(II) to Cu(I) or even Cu(0)) could also be achieved by organic carbon with a high reducing capacity and the reaction was sensitive to the O2 concentration, because the oxic environment causes the re-oxidation of Cu(I) to Cu(II) (Fulda et al. 2013; Xing et al. 2020). In addition, the reductive formation of nano-sized silver particles might occur with the phenol-like groups of organic matter, which contribute to 11–31% of the Ag(0) particles in soil (Huang et al. 2019). The reduction of Tl(III) to Tl(I), Sb(V) to Sb(III) (antimony), As(V) to As(III), and Sn(IV) to Sn(II) (tin) by the reductive surface functionalities of organic carbon has also been observed in soil under reducing conditions with lower Eh, which increases the solubility and toxicity of these elements with significant potential risks (Aftabtalab et al. 2022; Rinklebe et al. 2020; Zhong et al. 2022).
3.2 Oxidation
The oxidizing potential of organic carbon is normally lower than its reducing potential due to the nature of organic carbon. Quinone and semi-quinine-types PFRs are the primary oxidizing moieties on organic carbon, which can directly oxidize the As(III) to As(V) under an alkaline environment (Zhong et al. 2019). A rich surface oxidative functionality with a good aromatic structure usually offers a higher capacity for the oxidation of As(III). Hg(0) oxidation by the surface functionality of organic carbon was also evaluated, and the low-molecular-weight thiol compounds, glutathione, and mercaptoacetic acid could be potential oxidizing moieties (Zheng et al. 2012, 2019). However, the organic carbon might fail to directly oxidize some PTEs because of the higher demand for the oxidizing potential, while the indirect impact of the redox cycle on other elements might be the main reaction route.
4 Redox-induced transformation of PTEs by soil moieties with organic carbon
4.1 Redox-induced transformation of PTEs by the redox cycle of O2 with organic carbon
The redox cycle of organic carbon with O2 usually results in a higher redox capacity and can even be the determining process for the redox-induced transformation of PTEs that can hardly be affected by organic carbon directly. The interaction between O2 and surface functionalities, such as the phenolic functionality, is believed to affect both the reduction and oxidation of PTEs with the formation of reactive oxygen species (ROS).
4.1.1 Formation of ROS with O2
Reactive oxygen species can be considered as a series of oxidants obtained by the incomplete reduction of O2 compared with the complete reduction to H2O (Yu and Kuzyakov 2021). The main ROS in soil include the superoxide anion (O2·-), hydroperoxyl radical (HO2·), hydrogen peroxide (H2O2), and hydroxyl radicals (OH·) (Fig. 3 and Table 1) (Yu and Kuzyakov 2021). The Fenton-like reaction with iron, photocatalytic reaction, and input from rainwater are believed to be the primary input of ROS in soil, while the reduction of O2 by organic carbon might also potentially form ROS (Eqs. 4–7) (Trusiak et al. 2018). Although several studies have found that ROS have a higher possibility of reacting with organic carbon and generating H2O and CO2 (Patel et al. 2021; Yu and Kuzyakov 2021), the incomplete reduction by organic carbon could still contribute to the formation of a considerable amount of ROS, thereby altering the fate of PTEs (Zhong et al. 2019).
Reduced humic acids with rich reductive surface functionalities could support the reduction of O2 with the formation of H2O2 and OH· under dark conditions, and the yield could reach 42–160 mmol per mole of electrons donated by the reduced HA (Page et al. 2012). Similarly, the reduction of O2 with the formation of ROS occurred with pyrogenic carbon in the absence of light (Fang et al. 2015; Zhong et al. 2019, 2020). In addition, DOM has a high potential to activate O2, especially for those with lower molecular weights (Du et al. 2021). The potential reactive moieties on the organic carbon for the formation of ROS could be the oxygen functionalities, nitrogen functionalities, and PFRs (Chen et al. 2017; Huang et al. 2021; Navalon et al. 2017). Furthermore, defects and heteroatom doping are believed to have a higher reactivity for ROS generation (Navalon et al. 2017; Song et al. 2019; Wan et al. 2021b), which mainly occurs on the anthropogenic carbon after modification. The acidic environment could facilitate the electron transfer process with more ROS formation, and the interaction with redox-active ions would further enhance this process (Du et al. 2021); this will be discussed in a subsequent section. Recent studies have also found that the photocatalytic process could drive ROS formation with organic carbon, including humus (Zhang et al. 2020), biochar (Chen et al. 2017; Rangarajan et al. 2022), and DOM (Fu et al. 2016).
4.1.2 Reduction of PTEs with the ROS
The generated ROS, especially O2·- with a high reducing potential (E0 = − 0.33 V, Table 1), could reduce different PTEs based on their high reactivities. During this electron transfer process, the ROS serves as the reducing moiety, which then changes back to O2 or oxidative species. For instance, phenolics and PFRs on humin could trigger the generation of O2·- with O2, and the generated O2·- could reduce Cr(VI) to Cr(III) (Xu et al. 2020a). This electron transfer process contributed to almost 30% of the total Cr(VI) reduction by humin, while the remaining reduction was achieved by direct electron transfer (Xu et al. 2020a). In addition, the ROS generated by biochar might cause the reduction of Cr(VI), although their contribution could be limited compared to the direct reduction by reductive surface functionality and PFRs (Zhu et al. 2020). Several studies have reported that the generated ROS can directly react with PFRs on pyrogenic carbon (Zhao et al. 2018a; Zhu et al. 2020), resulting in the lower contribution of ROS to Cr(VI) reduction by pyrogenic carbon. A lower pH should be the premise for reducing Cr(VI) by ROS, while a higher pH might cause the re-oxidation of Cr(III) by the generated ROS (Liang et al. 2021). In addition to Cr(VI) reduction, other PTEs such as Ag(I) might also be reduced by O2·- generated by the organic carbon (Huang et al. 2019). Notably, the reduction of Cu(II) to Cu(I) by O2·- might have a lower possibility because the oxidation of Cu(I) by O2·- or O2 is dominant under oxic conditions, especially with organic carbon (Xing et al. 2020).
4.1.3 Oxidation of PTEs with the ROS
Compared to the reduction of PTEs in soil, ROS usually has a higher oxidation potential for PTEs. The oxidation of As(III) is a widely studied redox process in the soil; and different ROS generated from organic carbon, including hydrogen peroxide (H2O2) and hydroxyl radical (OH·), are responsible for this process (Xu et al. 2022a; Zhong et al. 2019, 2020). The formation of ROS from organic carbon through electron donation is considered to be the rate-limiting step for the entire electron transfer process; thus, an acidic environment and rich reductive moieties on the organic carbon could facilitate this redox process (Zhong et al. 2019). The Cr(III) oxidation by ROS has been reported, and this process could be enhanced by an alkaline environment and the formation of a Cr(III)-ligand complex (Liang et al. 2021; Luo and Chatterjee 2010; Oze et al. 2016). The oxidation of Cu(I) also occurs with the formation of ROS, such as O2·-, leading to the limited Cu(I) content in the oxic environment (Xing et al. 2020). Although Tl(I) oxidation can be achieved with species such as hydroxyl radicals (OH·) (Li et al. 2019; Xu et al. 2019a), the oxidation of Tl(I) with organic carbon and O2 has seldom been reported owing to the relatively lower concentration of Tl in soil (Belzile and Chen 2017) and the higher oxidation demand (E0 = 0.77 V, Table 1).
4.2 Redox-induced transformation of PTEs with Fe and organic carbon
4.2.1 Transformation of Fe minerals with organic carbon
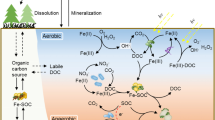
Iron (Fe), the most abundant redox-active metal element in the soil, plays a vital role in the dynamics of toxic elements in the environment (Han et al. 2019; Xu et al. 2022b), and its speciation is greatly affected by organic carbon in the soil (Chen et al. 2021; Frankl et al. 2022). The direct redox reaction is widely found in soil because of electron transfer from the reductive moiety to the ferric mineral, resulting in the formation of Fe(II)-containing minerals, sorbed Fe(II), or Fe2+ ions (Fig. 3) (Cismasu et al. 2016; Kim et al. 2019; Klüpfel et al. 2014b; Li et al. 2021a; Xu et al. 2022b). This process also transforms the Fe mineral (Fig. 3) because the formed Fe(II) can serve as a reactive moiety to accelerate the transformation process with organic carbon (Boland et al. 2014; Chen et al. 2015; Sheng et al. 2020; ThomasArrigo et al. 2018). Relevant studies have suggested that organic carbon, such as dissolved organic ligands, can modify the relative rates of olation and oxolation reactions that assemble the labile Fe(III) into various product minerals. This leads to the formation of different iron minerals, including goethite, magnetite, and lepidocrocite, based on the DOM concentration (Sheng et al. 2020). In addition, the presence of organic carbon inhibits the oxidation of Fe(II) to Fe(III) as well as the crystallization of iron minerals, and more iron minerals might remain in the amorphous phase or low-crystallinity phase (i.e., ferrihydrite) with highly-disordered structure (Chen and Thompson 2021; ThomasArrigo et al. 2019). Co-precipitation of the iron mineral with organic carbon would also occur during the interaction; this causes a highly dynamic phase in the iron mineral, namely, the mixing of solid Fe with the aqueous Fe(II), even without secondary mineral formation (Zhou et al. 2018). The chelation effect of organic ligands causes the dissolution of iron minerals, resulting in a higher amount of soluble iron with potent redox reactivity (Saad et al. 2017; Xu et al. 2020b; Zeng et al. 2020). All these organic carbon-related transformations of iron minerals affect the redox-induced transformation of PTEs in the soil.
4.2.2 Reduction of PTEs by Fe mineral with organic carbon
The Fe(II) formed by organic carbon can serve as a direct reducing agent for PTEs, such as Cr(VI) (Liao et al. 2019b; Xu et al. 2020b). During this process, Fe served as the mediator to transfer the electrons from organic carbon to PTEs; namely, soluble Fe(III) could obtain electrons from biochar with the formation of Fe(II), which quickly reacts with Cr(VI) with the re-formation of Fe(III) (Xu et al. 2020b). In addition to the soluble Fe(II), the Fe(II) combined with clay mineral could serve as the reducing moiety for the Cr(VI) with organic carbon (Liu et al. 2019). However, some studies have reported that Fe(II) complexed with organic carbon might have lower reactivity for Cr(VI) reduction with a higher probability of reacting with O2 (Pan et al. 2017). This electron transfer process may also occur in the solid phase of iron minerals. For this process, organic carbon can combine with the iron mineral and reduce Fe(III) to Fe(II). The electron on Fe(II) can be transferred through the iron mineral by Fe(II)/Fe(III) transformation, which reduces the sorbed Cr(VI) afterward with the re-formation of Fe(III) (Liao et al. 2019b; Xu et al. 2022b). This process should be more vital in soil because of the prevalent combination of iron mineral-organic carbon-PTEs. Moreover, the low-crystallinity iron minerals might have a higher possibility than high-crystallinity iron minerals owing to their lower stability and higher reactivity (Xu et al. 2022b).
Compared to those on Cr(VI) reduction, studies on the reduction of other PTEs are relatively insufficient, which might be related to the low possibility of redox transfer with Fe(II). For instance, the abiotic reduction of As(V) by the Fe(II) with organic carbon can hardly be achieved in soil under normal temperature (Johnston et al. 2018), and it often requires support from organisms (Qiao et al. 2019).
4.2.3 Oxidation of PTEs through Fe mineral and organic carbon
The generated Fe(II) could serve as a reducing agent for the activation of O2 with ROS formation (i.e., Fenton-like reaction, Eqs. 8–10) (Merino et al. 2021; Usman et al. 2022), although the chelation of organic carbon with Fe might buffer this electron transfer process (Daugherty et al. 2017; Jones et al. 2020; Yu et al. 2021). This Fenton-like reaction could also happen with Fe sorbed on the clay mineral with organic carbon (Hong et al. 2019). Some studies have suggested that organic carbon could act as a mediator to expand the area of ROS production with Fe(II)-containing minerals, leading to the higher and faster production of ROS (Yu et al. 2021). Further comparison between the ROS generation efficiency of organic carbon with and without Fe minerals is required to evaluate the potential synergistic impact. The generated ROS can be the determining oxidative species for different PTEs, such as As(III), Cu(I), and Pu(IV) (Gubler and ThomasArrigo 2021; Pan et al. 2021; Xu et al. 2019a, 2022a), with a mechanism as previously mentioned. Transformation and crystallization of iron minerals with organic carbon also alter the direct electron-accepting capacity of iron (Aeppli et al. 2019a, b), and the direct oxidation by Fe(III) (e.g., As(III) oxidation by Fe(III)) could be achieved with the support of rich quinone functionality on organic carbon (An et al. 2022).
4.3 Redox-induced transformation of PTEs by the redox cycle of Mn with organic carbon
4.3.1 Transformation of Mn minerals with organic carbon
Manganese (Mn) minerals are ubiquitous oxidants in the soil systems, and the redox reactions between organic carbon and Mn oxide occur extensively (Fig. 3) (Ma et al. 2020; Zhang et al. 2021). Manganese could be the dominant redox moiety in soil, especially in the surface layer, even when compared with iron minerals (Jones et al. 2020). Redox reactions between organic carbon and birnessite (MnO2) cause the reduction of Mn and the oxidation of organic matter (Ding et al. 2022b; Trainer et al. 2021). The DOM was more aliphatic and had a lower molecular weight after reacting with Mn, and the highly aromatic DOM underwent greater changes (Trainer et al. 2021). This redox process is generally accompanied by the dissolution of Mn oxide and the release of Mn(III)-ligand complex, which subsequently transforms into Mn(II) and oxidized organic compounds (Wang and Stone 2006). In addition to the reduction process, the oxidation of Mn(II) to Mn(III) occurs during the interaction with organic carbon (Wang et al. 2020d; Xu et al. 2021c). Organic carbon in the soil can serve as the oxidizing moiety, activating agent for ROS, and ligands to support Mn(III) formation, and the generated Mn(III) contains a solid oxidizing potential (Gao et al. 2018; Oldham et al. 2017). This Mn(III)-ligand complex has a higher possibility for internal electron transfer to transform back into aqueous Mn(II) and oxidized degradation products, especially in an acidic environment (Li et al. 2021b); this process might also contribute to ROS generation (Jones et al. 2020).
The multiple redox processes of Mn minerals with organic carbon are strongly related to the basic properties of organic carbon in the soil. Different surface functionalities might lead to the contrasting transformations of Mn minerals owing to the different redox reactivity. For instance, −OH and − NH2 functionalities could promote the electron transfer to MnO2, whereas some electron-withdrawing functionalities, such as −NO2 and − COOH groups, might inhibit the electron transfer (Zhao et al. 2020). Environmental conditions, such as the pH and O2 content, may affect the redox-induced transformation of Mn with organic carbon. For example, co-existing O2 may oxidize Mn(II) newly produced through reductive dissolution, especially with support from the mineral surface (Li et al. 2021b; Ma et al. 2020).
4.3.2 Redox-induced transformation of PTEs with Mn and organic carbon
Mn(IV) can effectively oxidize the reduced PTE species (e.g., As(III), Cr(III), Tl(I), and Sb(III)) in soil (Ao et al. 2022; Cruz-Hernández et al. 2019; Fu et al. 2018; Liang et al. 2021; Wick et al. 2020; Zhang et al. 2018a), while the Mn(III) and Mn(II) formed from the reduction of Mn(IV) usually contain lower oxidizing potential (Lafferty et al. 2010; Zhang et al. 2018a). In particular, reducing Mn(IV) together with organic carbon might alleviate the oxidative transformation of some PTEs. In contrast, some studies found that labile Mn(III) could be more active than Mn(IV) with the formation of a Mn(III)-organic ligand complex (Luther et al. 2018; Wang et al. 2020d). This could form ROS through the Mn(II)/Mn(III) cycle for the direct oxidation of PTEs. In addition, the reductive formation of Mn(III) is generally accompanied by the formation of vacancy-rich Mn oxides, which could be effective for the oxidative sorption of PTEs such as Tl(I) (Wick et al. 2019; Xu et al. 2019a). Mn(II) could also serve as an electron-mediating agent to facilitate the electron transfer between PTEs and organic carbon, such as the reduction of Cr(VI) by organic acids and biochar (Sarkar et al. 2013; Xu et al. 2021c). In addition, Fenton-like reaction caused by the activation of O2 by Mn(II) has been reported, which might also affect the fate of PTEs (e.g., Sb(III) oxidation) (Wang et al. 2022; Wu et al. 2022).
5 Implications to the soil PTEs remediation
Based on the above information, we conclude that organic carbon can significantly affect the speciation of PTEs through redox reactions, and this process is highly related to the redox moieties in soil, including O2, Fe, and Mn. Therefore, it is critical to consider these redox processes during the remediation of soil PTEs (Fig. 4).
5.1 Strategies for the utilization of organic carbon for PTEs immobilization
The addition of carbon-based materials has been widely studied to alter the fate of PTEs for lower toxicity and mobility. Introducing biochar into the soil is a common method to improve the carbon content in the soil, and it is believed to have great electron-donating capacities (Xu et al. 2022a, b). This supports the reduction of highly toxic Cr(VI) to less toxic Cr(III), which has a higher potential for immobilization in soil. The addition of catalysts to facilitate the electron transfer between organic carbon and PTEs can be a potential method to effectively utilize the redox reactivity of organic carbon (Fei et al. 2022). For instance, the graphitic structure can act as an electron conductor to facilitate the electron transfer from organic acids to Cr(VI), thus achieving the Cr(VI) reduction (Xu et al. 2019c). Redox-active minerals and surface functionalities can also participate in this electron transfer process through valence transformation (Xu et al. 2021c). Notably, this process might cause the oxidation of organic carbon, posing the potential risks to soil health and carbon emissions.
Although the inherent electron-accepting capacity of organic carbon is relatively low, it can support the ROS formation as a rich electron pool for the oxidation of several PTEs with the addition of oxidizing agent. For instance, oxidants, such as persulfate or O2, could be activated by electrons from organic carbon, leading to the oxidation of pollutants (Wu et al. 2020; Zhou et al. 2021). For example, the oxidation of As(III) to As(V) leads to lower toxicity and mobility, which are critical for As immobilization in soil. In addition, the oxidative transformation of Tl(I) to Tl(III) could result in rapid precipitation and sorption, leading to a higher immobilization potential (Biagioni et al. 2017; Chen et al. 2022). However, organic carbon might also inhibit ROS generation with the oxidant, which needs more consideration during practical applications.
Moreover, organic carbon also acts as an activating agent for mineral-based materials through the redox process. For example, the surface passivation of zero-valent-iron with the formation of Fe(III) is the rate-limiting step for redox-related immobilization; however, a suitable amount of organic carbon could lead to the reductive dissolution of Fe(III) to Fe(II), resulting in a higher removal rate of Cr(VI) (Lv et al. 2013; Xie et al. 2022). In addition, the reduction of Mn-based minerals may be critical for the oxidation-determined immobilization process (Wick et al. 2019). For instance, vacancy-containing birnessite (MnO2) with rich Mn(III) has higher oxidation and immobilization potentials for Tl(I) (Marafatto et al. 2021; Wick et al. 2019); therefore, organic carbon could potentially support the Mn(III) formation to enhance the Tl(I) immobilization. Reductive dissolution by organic carbon might also support the de-passivation of MnO2, leading to a higher oxidizing potential for PTEs (Lafferty et al. 2010). Notably, organic carbon has a dual impact on mineral materials for the immobilization of PTEs based on its concentration and properties (Di Palma et al. 2018; Xie et al. 2022), and this negative impact will be discussed in the following section.
5.2 Potential risks related to SOC during the PTEs immobilization
In addition to the direct redox reaction with PTEs, increasing the organic carbon content has been widely applied in other applications. According to its redox reactivity, organic carbon might cause unwanted transformation or mobilization of PTEs. For instance, adding organic carbon to the soil would increase the mobility of As (Gong et al. 2022), and its reducing potential might cause the reduction of As(V) to As(III), which is highly toxic (Alkurdi et al. 2019; Qin et al. 2020). The reductive formation of Tl(I), Sb(III), and Sn(II) with higher solubility was also reported owing to the broader Eh caused by the addition of biochar, increasing their mobility and potential toxicity (Rinklebe et al. 2019, 2020). In addition, rich organic carbon causes the reductive dissolution of Fe and Mn minerals with the formation of Fe(II) and Mn(III). As mentioned before, the formation of Fe(II) and Mn(III) is highly related to mineral transformation and the generation of ROS, leading to the potential oxidative formation of Cr(VI) with high toxicity.
Organic carbon in the soil requires more consideration when applying metal-based materials (e.g., zero-valent-iron and MnO2) to the soil for PTEs remediation. Owing to the redox-related transformations between organic carbon and Fe/Mn minerals, the organic carbon content significantly affects the effectiveness of the metal-based materials. The interactions between organic carbon and iron-based materials might cause the dissolution of Fe(II) into the soil and thus facilitate the redox-induced transformation of PTEs, which can be a potential risk. For instance, adding zero-valent-iron materials could effectively reduce and immobilize Cr(VI) through reduction and co-precipitation, whereas a high organic carbon concentration might cause the re-oxidation of Cr(III) by Fe (Di Palma et al. 2018, Xie et al. 2022), possibly owing to the formation of Fe(II) and ROS (Liang et al. 2021; Usman et al. 2022). This process could cause the potential remobilization of Cr(VI), and its contribution has been largely ignored compared with the competitive impact of organic carbon (Xie et al. 2022). Similar interactions might also occur with Mn minerals, and several studies have reported the decrease in the oxidation potential with the reductive dissolution of Mn minerals (Lafferty et al. 2010, Zhang et al. 2018a).
6 Future perspectives
In this review, we scrutinized the composition of SOC, primary redox-active moieties on organic carbon, and their impact on the redox-induced transformation of PTEs. The phenolic and quinone functionalities, PFRs, and graphitic structure could be the critical redox-active moieties of organic carbon in the soil, and they could directly affect the redox-induced transformation of Cr, As, Cu, Hg, etc., in the environment. In addition, the interactions between organic carbon and O2, Fe, or Mn minerals lead to the formation of ROS, the reductive formation of Fe(II) and Fe(II)-organic ligands with the catalytic transformation of iron minerals, and the transformation of Mn(IV)/Mn(III)/Mn(II) with Mn-containing minerals. These processes significantly affect the fate of PTEs through either oxidation or reduction reactions. It is critical to consider both the positive and negative impacts of organic carbon on the transformation of PTEs during soil remediation. Utilizing organic carbon as the redox reagent or activator can be a promising method for the transformation and immobilization of PTEs, but the potential risk caused by organic carbon also needs further evaluation. Such fundamental knowledge of PTEs transformation with organic carbon can help us better understand the bio-geochemistry of PTEs and guide the remediation of PTEs in soil.
Several issues regarding organic carbon and its impact on the redox-induced transformation of PTEs need to be addressed in future studies. First, organic carbon contains both reducing and oxidizing moieties, and their capacities are strongly related to the chemical structure and surface properties (Li et al. 2020). Although several studies have revealed the structure-function relationship of the redox reactivity of specific organic carbon, the overall concern of all types of SOC and their synergistic impact on redox-related performance is still required. Moreover, a more detailed consideration of both micro (i.e., molecular and atomic structures) and macro aspects (i.e., different properties related to organic carbon evolution and peculiarity) is critical for evaluating the redox potential of organic carbon and its impact on the redox-induced transformation of PTEs. A theory-driven Pourbaix Diagram (Eh/pH diagram) of PTEs with varying SOC containing different moieties is also suggested in future studies.
Second, the addition of carbon into the soil has been widely conducted as a potential method for fertility enhancement, environmental remediation, and carbon sequestration, leading to the increased organic carbon content in the soil. Notably, this newly added carbon could have different properties compared to the indigenous organic carbon owing to its formation process and aging impact (LaCroix et al. 2021; Wang et al. 2020c). Thus, it is vital to consider the unintended consequence and potential risk of adding carbon to the soil in future studies, including both the direct mobilization of PTEs and the indirect transformation with the soil moieties.
Third, current studies have provided detailed explanation for the potential redox interactions between organic carbon and several primary soil moieties, respectively, including O2, Fe-minerals, and Mn-minerals. An integrated consideration of these soil moieties with organic carbon and their impact on the redox-induced transformation of PTEs under temporally and spatially variable geochemical conditions could be a promising research area in future studies, which is much more relevant to natural soil conditions. In addition, potential contribution of other redox moieties (e.g., sulphur (Wang et al. 2021a, Zhao et al. 2019) and chlorine (Hou et al. 2018; Wang et al. 2020b)) and electron transfer between different PTEs (e.g., As and Cr (Dong et al. 2014)) need further consideration.
Fourth, this review focused on the abiotic transformation processes of organic carbon with soil moieties and PTEs. In field conditions, biotic processes involving organic carbon could also be critical for determining the fate of PTEs, such as the redox-related transformation of As and Tl (An et al. 2022; Wei et al. 2020, 2021). The consideration of both abiotic/biotic processes and their synergistic interplay could be an important research area in future studies.
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- SOC:
-
Soil organic carbon
- PTEs:
-
Potentially toxic elements
- HA:
-
Humic acids
- FA:
-
Fulvic acids
- PFRs:
-
Persistent free radicals
- DOM:
-
Dissolved organic matter
- DOC:
-
Dissolved organic carbon
- ROS:
-
Reactive oxygen species
References
Adeleke R, Nwangburuka C, Oboirien B (2017) Origins, roles and fate of organic acids in soils: a review. S Afr J Bot 108:393–406
Aeppli M, Kaegi R, Kretzschmar R, Voegelin A, Hofstetter TB, Sander M (2019a) Electrochemical analysis of changes in iron oxide reducibility during abiotic ferrihydrite transformation into goethite and magnetite. Environ Sci Technol 53(7):3568–3578
Aeppli M, Vranic S, Kaegi R, Kretzschmar R, Brown AR, Voegelin A, Hofstetter TB, Sander M (2019b) Decreases in iron oxide reducibility during microbial reductive dissolution and transformation of ferrihydrite. Environ Sci Technol 53(15):8736–8746
Aeschbacher M, Sander M, Schwarzenbach RP (2010) Novel electrochemical approach to assess the redox properties of humic substances. Environ Sci Technol 44(1):87–93
Aftabtalab A, Rinklebe J, Shaheen SM, Niazi NK, Moreno-Jiménez E, Schaller J, Knorr K-H (2022) Review on the interactions of arsenic, iron (oxy)(hydr)oxides, and dissolved organic matter in soils, sediments, and groundwater in a ternary system. Chemosphere 286:131790
Ali W, Mao K, Zhang H, Junaid M, Xu N, Rasool A, Feng X, Yang Z (2020) Comprehensive review of the basic chemical behaviours, sources, processes, and endpoints of trace element contamination in paddy soil-rice systems in rice-growing countries. J Hazard Mater 397:122720
Alkurdi SSA, Herath I, Bundschuh J, Al-Juboori RA, Vithanage M, Mohan D (2019) Biochar versus bone char for a sustainable inorganic arsenic mitigation in water: what needs to be done in future research? Environ Int 127:52–69
An W, Wu C, Xue S, Liu Z, Liu M, Li W (2022) Effects of biochar/AQDS on As(III)-adsorbed ferrihydrite reduction and arsenic (As) and iron (Fe) transformation: abiotic and biological conditions. Chemosphere 291:133126
Ao M, Sun S, Deng T, Zhang F, Liu T, Tang Y, Li J, Wang S, Qiu R (2022) Natural source of Cr(VI) in soil: the anoxic oxidation of Cr(III) by Mn oxides. J Hazard Mater 433:128805
Bai Y, Sun T, Angenent LT, Haderlein SB, Kappler A (2020) Electron hopping enables rapid electron transfer between quinone−/hydroquinone-containing organic molecules in microbial iron(iii) mineral reduction. Environ Sci Technol 54(17):10646–10653
Bai Y-N, Wang X-N, Wu J, Lu Y-Z, Fu L, Zhang F, Lau T-C, Zeng RJ (2019) Humic substances as electron acceptors for anaerobic oxidation of methane driven by ANME-2d. Water Res 164:114935
Baki M, Abedi-Koupai J (2018) Preparation and characterization of a superabsorbent slow-release fertilizer with sodium alginate and biochar. J Appl Polym Sci 135(10):45966
Belzile N, Chen Y-W (2017) Thallium in the environment: a critical review focused on natural waters, soils, sediments and airborne particles. Appl Geochem 84:218–243
Bhattacharyya A, Schmidt MP, Stavitski E, Azimzadeh B, Martínez CE (2019) Ligands representing important functional groups of natural organic matter facilitate Fe redox transformations and resulting binding environments. Geochim Cosmochim Acta 251:157–175
Biagioni C, D'Orazio M, Lepore GO, d’Acapito F, Vezzoni S (2017) Thallium-rich rust scales in drinkable water distribution systems: a case study from northern Tuscany, Italy. Sci Total Environ 587-588:491–501
Boland DD, Collins RN, Miller CJ, Glover CJ, Waite TD (2014) Effect of solution and solid-phase conditions on the fe(ii)-accelerated transformation of ferrihydrite to lepidocrocite and goethite. Environ Sci Technol 48(10):5477–5485
Chen C, Kukkadapu R, Sparks DL (2015) Influence of coprecipitated organic matter on fe2+(aq)-catalyzed transformation of ferrihydrite: implications for carbon dynamics. Environ Sci Technol 49(18):10927–10936
Chen C, Thompson A (2021) The influence of native soil organic matter and minerals on ferrous iron oxidation. Geochim Cosmochim Acta 292:254–270
Chen K-Y, Tzou Y-M, Hsu L-C, Guo J-W, Cho Y-L, Teah H-Y, Hsieh Y-C, Liu Y-T (2022) Oxidative removal of thallium(I) using Al beverage can waste with amendments of Fe: Tl speciation and removal mechanisms. Chem Eng J 427:130846
Chen N, Fu Q, Wu T, Cui P, Fang G, Liu C, Chen C, Liu G, Wang W, Wang D, Wang P, Zhou D (2021) Active iron phases regulate the abiotic transformation of organic carbon during redox fluctuation cycles of paddy soil. Environ Sci Technol 55(20):14281–14293
Chen N, Huang Y, Hou X, Ai Z, Zhang L (2017) Photochemistry of hydrochar: reactive oxygen species generation and sulfadimidine degradation. Environ Sci Technol 51(19):11278–11287
Chen Y, Zhang X, Feng S (2018) Contribution of the excited triplet state of humic acid and superoxide radical anion to generation and elimination of phenoxyl radical. Environ Sci Technol 52(15):8283–8291
Cheng S, Zhao Y, Pan Y, Yu J, Lei Y, Lei X, Ouyang G, Yang X (2022) Role of antioxidant moieties in the quenching of a purine radical by dissolved organic matter. Environ Sci Technol 56(1):546–555
Cismasu AC, Williams KH, Nico PS (2016) Iron and carbon dynamics during aging and reductive transformation of biogenic ferrihydrite. Environ Sci Technol 50(1):25–35
Cruz-Hernández Y, Villalobos M, Marcus MA, Pi-Puig T, Zanella R, Martínez-Villegas N (2019) Tl(I) sorption behavior on birnessite and its implications for mineral structural changes. Geochim Cosmochim Acta 248:356–369
Cuong DV, Wu P-C, Chen L-I, Hou C-H (2021) Active MnO2/biochar composite for efficient As(III) removal: insight into the mechanisms of redox transformation and adsorption. Water Res 188:116495
Daugherty EE, Gilbert B, Nico PS, Borch T (2017) Complexation and redox buffering of iron(ii) by dissolved organic matter. Environ Sci Technol 51(19):11096–11104
Deng G, Ma T, Tanaka K, Ohnuki T, Qiu X, Yu Q (2020) Saccharide-mediated transformation of Ce during the formation of manganese (hydr)oxide. Geochim Cosmochim Acta 286:143–158
Di Palma L, Verdone N, Vilardi G (2018) Kinetic Modeling of Cr(VI) Reduction by nzvi in soil: the influence of organic matter and manganese oxide. Bull Environ Contam Toxicol 101(6):692–697
Ding X, Pu Y, Tang M, Zhang T (2022a) Environmental and health effects of graphene-family nanomaterials: potential release pathways, transformation, environmental fate and health risks. Nano Today 42:101379
Ding Z, Ding Y, Liu F, Yang J, Li R, Dang Z, Shi Z (2022b) Coupled sorption and oxidation of soil dissolved organic matter on manganese oxides: nano/sub-nanoscale distribution and molecular transformation. Environ Sci Technol 56(4):2783–2793
Dong X, Ma LQ, Gress J, Harris W, Li Y (2014) Enhanced Cr(VI) reduction and As(III) oxidation in ice phase: important role of dissolved organic matter from biochar. J Hazard Mater 267:62–70
Donham JE, Rosenfeldt EJ, Wigginton KR (2014) Photometric hydroxyl radical scavenging analysis of standard natural organic matter isolates. Environ Sci Process Impacts 16(4):764–769
Du H, Cao Y, Li Z, Li L, Xu H (2021) Formation and mechanisms of hydroxyl radicals during the oxygenation of sediments in Lake Poyang, China. Water Res 202:117442
Duran K, van den Dikkenberg M, van Erven G, Baars JJP, Comans RNJ, Kuyper TW, Kabel MA (2022) Microbial lignin degradation in an industrial composting environment. Bioresour Technol Rep 17:100911
Fang G, Zhu C, Dionysiou DD, Gao J, Zhou D (2015) Mechanism of hydroxyl radical generation from biochar suspensions: implications to diethyl phthalate degradation. Bioresour Technol 176:210–217
Farid IM, Siam HS, Abbas MHH, Mohamed I, Mahmoud SA, Tolba M, Abbas HH, Yang X, Antoniadis V, Rinklebe J, Shaheen SM (2022) Co-composted biochar derived from rice straw and sugarcane bagasse improved soil properties, carbon balance, and zucchini growth in a sandy soil: a trial for enhancing the health of low fertile arid soils. Chemosphere 292:133389
Fei Y-h, Li M, Ye Z, Guan J, Huang Z, Xiao T, Zhang P (2022) The pH-sensitive sorption governed reduction of Cr(VI) by sludge derived biochar and the accelerating effect of organic acids. J Hazard Mater 423:127205
Frankl AL, Maxbauer DP, Savina ME (2022) Linkages between soil organic matter and magnetic mineral formation in agricultural fields in southeastern Minnesota, USA. Geoderma 406:115466
Fu H, Liu H, Mao J, Chu W, Li Q, Alvarez PJJ, Qu X, Zhu D (2016) Photochemistry of dissolved black carbon released from biochar: reactive oxygen species generation and phototransformation. Environ Sci Technol 50(3):1218–1226
Fu L, Shozugawa K, Matsuo M (2018) Oxidation of antimony (III) in soil by manganese (IV) oxide using X-ray absorption fine structure. J Environ Sci 73:31–37
Fulda B, Voegelin A, Maurer F, Christl I, Kretzschmar R (2013) Copper redox transformation and complexation by reduced and oxidized soil humic acid. 1. X-ray absorption spectroscopy study. Environ Sci Technol 47(19):10903–10911
Gao Y, Jiang J, Zhou Y, Pang S-Y, Jiang C, Guo Q, Duan J-B (2018) Does soluble mn(iii) oxidant formed in situ account for enhanced transformation of triclosan by mn(vii) in the presence of ligands? Environ Sci Technol 52(8):4785–4793
Gong H, Zhao L, Rui X, Hu J, Zhu N (2022) A review of pristine and modified biochar immobilizing typical heavy metals in soil: applications and challenges. J Hazard Mater 432:128668
Gubler R, ThomasArrigo LK (2021) Ferrous iron enhances arsenic sorption and oxidation by non-stoichiometric magnetite and maghemite. J Hazard Mater 402:123425
Han Y-S, Park J-H, Kim S-J, Jeong HY, Ahn JS (2019) Redox transformation of soil minerals and arsenic in arsenic-contaminated soil under cycling redox conditions. J Hazard Mater 378:120745
He X-S, Yang C, You S-H, Zhang H, Xi B-D, Yu M-D, Liu S-J (2019) Redox properties of compost-derived organic matter and their association with polarity and molecular weight. Sci Total Environ 665:920–928
Hoffland E, Kuyper TW, Comans RNJ, Creamer RE (2020) Eco-functionality of organic matter in soils. Plant Soil 455(1–2):1–22
Hong R, Zhang L, Zhu W, Gu C (2019) Photo-transformation of atrazine in aqueous solution in the presence of Fe3+−montmorillonite clay and humic substances. Sci Total Environ 652:224–233
Hou S, Ling L, Dionysiou DD, Wang Y, Huang J, Guo K, Li X, Fang J (2018) Chlorate formation mechanism in the presence of sulfate radical, chloride, bromide and natural organic matter. Environ Sci Technol 52(11):6317–6325
Huang C, Zhang C, Huang D, Wang D, Tian S, Wang R, Yang Y, Wang W, Qin F (2021) Influence of surface functionalities of pyrogenic carbonaceous materials on the generation of reactive species towards organic contaminants: A review. Chem Eng J 404:127066
Huang Y-N, Qian T-T, Dang F, Yin Y-G, Li M, Zhou D-M (2019) Significant contribution of metastable particulate organic matter to natural formation of silver nanoparticles in soils. Nat Commun 10(1):3775
Hubova P, Tejnecky V, Ash C, Boruvka L, Drabek O (2017) Low-molecular-mass organic acids in the forest soil environment. Mini Rev Org Chem 14(1):75–84
Jiang Z, Li X, Li M, Zhu Q, Li G, Ma C, Li Q, Meng J, Liu Y, Li Q (2021) Impacts of red mud on lignin depolymerization and humic substance formation mediated by laccase-producing bacterial community during composting. J Hazard Mater 410:124557
Jing F, Sun Y, Liu Y, Wan Z, Chen J, Tsang DCW (2022) Interactions between biochar and clay minerals in changing biochar carbon stability. Sci Total Environ 809:151124
Johnston SG, Bennett WW, Burton ED, Hockmann K, Dawson N, Karimian N (2018) Rapid arsenic(V)-reduction by fire in schwertmannite-rich soil enhances arsenic mobilisation. Geochim Cosmochim Acta 227:1–18
Jones ME, LaCroix RE, Zeigler J, Ying SC, Nico PS, Keiluweit M (2020) Enzymes, manganese, or iron? Drivers of oxidative organic matter decomposition in soils. Environ Sci Technol 54(21):14114–14123
Kim C, Chin Y-P, Son H, Hwang I (2022) Activation of persulfate by humic substances: stoichiometry and changes in the optical properties of the humic substances. Water Res 212:118107
Kim H-B, Kim J-G, Choi J-H, Kwon EE, Baek K (2019) Photo-induced redox coupling of dissolved organic matter and iron in biochars and soil system: enhanced mobility of arsenic. Sci Total Environ 689:1037–1043
Klüpfel L, Keiluweit M, Kleber M, Sander M (2014a) Redox properties of plant biomass-derived black carbon (biochar). Environ Sci Technol 48(10):5601–5611
Klüpfel L, Piepenbrock A, Kappler A, Sander M (2014b) Humic substances as fully regenerable electron acceptors in recurrently anoxic environments. Nat Geosci 7(3):195–200
LaCroix RE, Walpen N, Sander M, Tfaily MM, Blanchard JL, Keiluweit M (2021) Long-term warming decreases redox capacity of soil organic matter. Environ Sci Technol Lett 8(1):92–97
Lafferty BJ, Ginder-Vogel M, Zhu M, Livi KJT, Sparks DL (2010) Arsenite oxidation by a poorly crystalline manganese-oxide. 2. Results from X-ray absorption spectroscopy and X-ray diffraction. Environ Sci Technol 44(22):8467–8472
Lamichhane S, Kumar L, Wilson B (2019) Digital soil mapping algorithms and covariates for soil organic carbon mapping and their implications: a review. Geoderma 352:395–413
Lehmann J (2007) A handful of carbon. Nature 447(7141):143–144
Lehmann J, Cowie A, Masiello CA, Kammann C, Woolf D, Amonette JE, Cayuela ML, Camps-Arbestain M, Whitman T (2021) Biochar in climate change mitigation. Nat Geosci 14(12):883–88+
Lehmann J, Gaunt J, Rondon M (2006) Bio-char sequestration in terrestrial ecosystems – a review. Mitig Adapt Strateg Glob Chang 11(2):403–427
Lehmann J, Kleber M (2015) The contentious nature of soil organic matter. Nature 528(7580):60–68
Li B, Zhu Q-H, Zhang Q, Zhu H-H, Huang D-Y, Su S-M, Wang Y-N, Zeng X-B (2021a) Cadmium and arsenic availability in soil under submerged incubation: the influence of humic substances on iron speciation. Ecotox Environ Safe 225:112773
Li H, Li X, Long J, Li K, Chen Y, Jiang J, Chen X, Zhang P (2019) Oxidation and removal of thallium and organics from wastewater using a zero-valent-iron-based Fenton-like technique. J Clean Prod 221:89–97
Li H, Santos F, Butler K, Herndon E (2021b) A critical review on the multiple roles of manganese in stabilizing and destabilizing soil organic matter. Environ Sci Technol 55(18):12136–12152
Li S, Shao L, Zhang H, He P, Lü F (2020) Quantifying the contributions of surface area and redox-active moieties to electron exchange capacities of biochar. J Hazard Mater 394:122541
Li X, Mu L, Hu X (2018) Integrating proteomics, metabolomics and typical analysis to investigate the uptake and oxidative stress of graphene oxide and polycyclic aromatic hydrocarbons. Environ Sci Nano 5(1):115–129
Liang J, Huang X, Yan J, Li Y, Zhao Z, Liu Y, Ye J, Wei Y (2021) A review of the formation of Cr(VI) via Cr(III) oxidation in soils and groundwater. Sci Total Environ 774:145762
Liao P, Liang Y, Shi Z (2019a) Impact of divalent cations on dark production of hydroxyl radicals from oxygenation of reduced humic acids at anoxic–oxic interfaces. ACS Earth Space Chem 3(4):484–494
Liao W, Ye Z, Yuan S, Cai Q, Tong M, Qian A, Cheng D (2019b) Effect of coexisting fe(iii) (oxyhydr)oxides on cr(vi) reduction by fe(ii)-bearing clay minerals. Environ Sci Technol 53(23):13767–13775
Liu X, Dong H, Zeng Q, Yang X, Zhang D (2019) Synergistic effects of reduced nontronite and organic ligands on cr(vi) reduction. Environ Sci Technol 53(23):13732–13741
Lü F, Lu X, Li S, Zhang H, Shao L, He P (2022) Dozens-fold improvement of biochar redox properties by KOH activation. Chem Eng J 429:132203
Luo Z, Chatterjee N (2010) Kinetics of oxidation of Cr(III)-organic complexes by H2O2. Chem Speciat Bioavailab 22(1):25–34
Luther GW, Thibault de Chanvalon A, Oldham VE, Estes ER, Tebo BM, Madison AS (2018) Reduction of manganese oxides: thermodynamic, kinetic and mechanistic considerations for one- versus two-electron transfer steps. Aquat Geochem 24(4):257–277
Lv X, Hu Y, Tang J, Sheng T, Jiang G, Xu X (2013) Effects of co-existing ions and natural organic matter on removal of chromium (VI) from aqueous solution by nanoscale zero valent iron (nZVI)-Fe3O4 nanocomposites. Chem Eng J 218:55–64
Ma D, Wu J, Yang P, Zhu M (2020) Coupled manganese redox cycling and organic carbon degradation on mineral surfaces. Environ Sci Technol 54(14):8801–8810
Maestrini B, Nannipieri P, Abiven S (2015) A meta-analysis on pyrogenic organic matter induced priming effect. Glob Change Biol Bioenergy 7(4):577–590
Marafatto FF, Dähn R, Grolimund D, Göttlicher J, Voegelin A (2021) Thallium sorption by soil manganese oxides: insights from synchrotron X-ray micro-analyses on a naturally thallium-rich soil. Geochim Cosmochim Acta 302:193–208
Marcińczyk M, Oleszczuk P (2022) Biochar and engineered biochar as slow- and controlled-release fertilizers. J Clean Prod 339:130685
Mazarji M, Minkina T, Sushkova S, Mandzhieva S, Bidhendi GN, Barakhov A, Bhatnagar A (2021) Effect of nanomaterials on remediation of polycyclic aromatic hydrocarbons-contaminated soils: a review. J Environ Manag 284:112023
Merino C, Matus F, Kuzyakov Y, Dyckmans J, Stock S, Dippold MA (2021) Contribution of the Fenton reaction and ligninolytic enzymes to soil organic matter mineralisation under anoxic conditions. Sci Total Environ 760:143397
Navalon S, Dhakshinamoorthy A, Alvaro M, Antonietti M, García HJCSR (2017) Active sites on graphene-based materials as metal-free catalysts. Chem Soc Rev 46(15):4501–4529
Oldham VE, Mucci A, Tebo BM, Luther GW (2017) Soluble Mn(III)–L complexes are abundant in oxygenated waters and stabilized by humic ligands. Geochim Cosmochim Acta 199:238–246
Ondrasek G, Bakić Begić H, Zovko M, Filipović L, Meriño-Gergichevich C, Savić R, Rengel Z (2019) Biogeochemistry of soil organic matter in agroecosystems & environmental implications. Sci Total Environ 658:1559–1573
Oze C, Sleep NH, Coleman RG, Fendorf SJG (2016) Anoxic oxidation of chromium. Geology 44(7):543–546
Page SE, Sander M, Arnold WA, McNeill K (2012) Hydroxyl radical formation upon oxidation of reduced humic acids by oxygen in the dark. Environ Sci Technol 46(3):1590–1597
Pan C, Jiao Y, Kersting AB, Zavarin M (2021) Plutonium redox transformation in the presence of iron, organic matter, and hydroxyl radicals: kinetics and mechanistic insights. Environ Sci Technol 55(3):1800–1810
Pan C, Troyer LD, Liao P, Catalano JG, Li W, Giammar DE (2017) Effect of humic acid on the removal of chromium(vi) and the production of solids in iron electrocoagulation. Environ Sci Technol 51(11):6308–6318
Pang L, Gao Z, Feng H, Wang S, Wang Q (2019) Cellulose based materials for controlled release formulations of agrochemicals: a review of modifications and applications. J Control Release 316:105–115
Park CM, Chu KH, Heo J, Her N, Jang M, Son A, Yoon Y (2016) Environmental behavior of engineered nanomaterials in porous media: a review. J Hazard Mater 309:133–150
Patel KF, Tejnecký V, Ohno T, Bailey VL, Sleighter RL, Hatcher PG (2021) Reactive oxygen species alter chemical composition and adsorptive fractionation of soil-derived organic matter. Geoderma 384:114805
Paul EA (2016) The nature and dynamics of soil organic matter: plant inputs, microbial transformations, and organic matter stabilization. Soil Biol Biochem 98:109–126
Pham DM, Kasai T, Yamaura M, Katayama A (2021) Humin: no longer inactive natural organic matter. Chemosphere 269:128697
Pham DM, Oji H, Yagi S, Ogawa S, Katayama A (2022) Sulfur in humin as a redox-active element for extracellular electron transfer. Geoderma 408:115580
Pignatello JJ, Mitch WA, Xu W (2017) Activity and reactivity of pyrogenic carbonaceous matter toward organic compounds. Environ Sci Technol 51(16):8893–8908
Polak F, Urik M, Matus P (2019) Low molecular weight organic acids in soil environment. Chem List 113(5):307–314
Qiao J, Li X, Li F, Liu T, Young LY, Huang W, Sun K, Tong H, Hu M (2019) Humic substances facilitate arsenic reduction and release in flooded paddy soil. Environ Sci Technol 53(9):5034–5042
Qiao W, Guo H, He C, Shi Q, Zhao B (2021) Unraveling roles of dissolved organic matter in high arsenic groundwater based on molecular and optical signatures. J Hazard Mater 406:124702
Qin J, Li Q, Liu Y, Niu A, Lin C (2020) Biochar-driven reduction of As(V) and Cr(VI): effects of pyrolysis temperature and low-molecular-weight organic acids. Ecotox Environ Safe 201:110873
Rangarajan G, Jayaseelan A, Farnood R (2022) Photocatalytic reactive oxygen species generation and their mechanisms of action in pollutant removal with biochar supported photocatalysts: a review. J Clean Prod 346:131155
Rashid M, Hussain Q, Khan KS, Alwabel MI, Hayat R, Akmal M, Ijaz SS, Alvi S, Obaid ur R (2021) Carbon-based slow-release fertilizers for efficient nutrient management: synthesis, applications, and future research needs. J Soil Sci Plant Nutr 21(2):1144–1169
Rinklebe J, Antoniadis V, Shaheen SM, Rosche O, Altermann M (2019) Health risk assessment of potentially toxic elements in soils along the Central Elbe River, Germany. Environ Int 126:76–88
Rinklebe J, Shaheen SM, El-Naggar A, Wang H, Du Laing G, Alessi DS, Sik Ok Y (2020) Redox-induced mobilization of Ag, Sb, Sn, and Tl in the dissolved, colloidal and solid phase of a biochar-treated and un-treated mining soil. Environ Int 140:105754
Ruan X, Sun Y, Du W, Tang Y, Liu Q, Zhang Z, Doherty W, Frost RL, Qian G, Tsang DCW (2019) Formation, characteristics, and applications of environmentally persistent free radicals in biochars: a review. Bioresour Technol 281:457–468
Saad EM, Sun J, Chen S, Borkiewicz OJ, Zhu M, Duckworth OW, Tang Y (2017) Siderophore and organic acid promoted dissolution and transformation of Cr(III)-Fe(III)-(oxy)hydroxides. Environ Sci Technol 51(6):3223–3232
Sarkar B, Naidu R, Krishnamurti GSR, Megharaj M (2013) Manganese(II)-catalyzed and clay-minerals-mediated reduction of chromium(vi) by citrate. Environ Sci Technol 47(23):13629–13636
Sheng A, Li X, Arai Y, Ding Y, Rosso KM, Liu J (2020) Citrate controls fe(ii)-catalyzed transformation of ferrihydrite by complexation of the labile fe(iii) intermediate. Environ Sci Technol 54(12):7309–7319
Shi Y, Zhang C, Liu J, Dai Q, Jiang Y, Xi M, Jia H (2021) Distribution of persistent free radicals in different molecular weight fractions from peat humic acids and their impact in reducing goethite. Sci Total Environ 797:149173
Smreczak B, Ukalska-Jaruga A (2021) Dissolved organic matter in agricultural soils. Soil Sci Annu 72(1):132234
Song Z, Wang M, Wang Z, Wang Y, Li R, Zhang Y, Liu C, Liu Y, Xu B, Qi F (2019) Insights into heteroatom-doped graphene for catalytic ozonation: active centers, reactive oxygen species evolution, and catalytic mechanism. Environ Sci Technol 53(9):5337–5348
Sun TR, Levin BDA, Guzman JJL, Enders A, Muller DA, Angenent LT, Lehmann J (2017) Rapid electron transfer by the carbon matrix in natural pyrogenic carbon. Nat Commun 8(1):1–2
Sun TR, Levin BDA, Schmidt MP, Guzman JJL, Enders A, Martinez CE, Muller DA, Angenent LT, Lehmann J (2018) Simultaneous quantification of electron transfer by carbon matrices and functional groups in pyrogenic carbon. Environ Sci Technol 52(15):8538–8547
Tang W-W, Zeng G-M, Gong J-L, Liang J, Xu P, Zhang C, Huang B-B (2014) Impact of humic/fulvic acid on the removal of heavy metals from aqueous solutions using nanomaterials: a review. Sci Total Environ 468-469:1014–1027
ThomasArrigo LK, Byrne JM, Kappler A, Kretzschmar R (2018) Impact of organic matter on iron(ii)-catalyzed mineral transformations in ferrihydrite–organic matter coprecipitates. Environ Sci Technol 52(21):12316–12326
ThomasArrigo LK, Kaegi R, Kretzschmar R (2019) Ferrihydrite growth and transformation in the presence of ferrous iron and model organic ligands. Environ Sci Technol 53(23):13636–13647
Trainer EL, Ginder-Vogel M, Remucal CK (2021) Selective reactivity and oxidation of dissolved organic matter by manganese oxides. Environ Sci Technol 55(17):12084–12094
Trusiak A, Treibergs LA, Kling GW, Cory RM (2018) The role of iron and reactive oxygen species in the production of CO2 in arctic soil waters. Geochim Cosmochim Acta 224:80–95
Usman M, Jellali S, Anastopoulos I, Charabi Y, Hameed BH, Hanna K (2022) Fenton oxidation for soil remediation: a critical review of observations in historically contaminated soils. J Hazard Mater 424:127670
Verbeeck M, Thiry Y, Smolders E (2020) Soil organic matter affects arsenic and antimony sorption in anaerobic soils. Environ Pollut 257:113566
Walpen N, Getzinger GJ, Schroth MH, Sander M (2018) Electron-donating phenolic and electron-accepting quinone moieties in peat dissolved organic matter: quantities and redox transformations in the context of peat biogeochemistry. Environ Sci Technol 52(9):5236–5245
Wan D, Wang H, Sharma VK, Selvinsimpson S, Dai H, Luo F, Wang C, Chen Y (2021a) Mechanistic investigation of enhanced photoreactivity of dissolved organic matter after chlorination. Environ Sci Technol 55(13):8937–8946
Wan Z, Xu Z, Sun Y, He M, Hou D, Cao X, Tsang DCW (2021b) Critical impact of nitrogen vacancies in nonradical carbocatalysis on nitrogen-doped graphitic biochar. Environ Sci Technol 55(10):7004–7014
Wang D, Huang D, Wu S, Fang G, Zhu F, Chen N, Liu S, Zhu C, Zhou D (2021a) Pyrogenic carbon initiated the generation of hydroxyl radicals from the oxidation of sulfide. Environ Sci Technol 55(9):6001–6011
Wang F, Liu X, Li X, Jiang C, Zhang T, Chen W (2020a) Sulfide and ferrous iron preferentially target specific surface O-functional groups of graphene oxide: implications for accumulation of contaminants. Environ Sci Nano 7(2):462–471
Wang H, Ge D, Cheng Z, Zhu N, Yuan H, Lou Z (2020b) Improved understanding of dissolved organic matter transformation in concentrated leachate induced by hydroxyl radicals and reactive chlorine species. J Hazard Mater 387:121702
Wang J, Wang S (2019) Preparation, modification and environmental application of biochar: a review. J Clean Prod 227:1002–1022
Wang L, O’Connor D, Rinklebe J, Ok YS, Tsang DCW, Shen Z, Hou D (2020c) Biochar aging: mechanisms, physicochemical changes, assessment, and implications for field applications. Environ Sci Technol 54(23):14797–14814
Wang M, Liu Y, Shi H, Li S, Chen S (2022) Yielding hydroxyl radicals in the Fenton-like reaction induced by manganese (II) oxidation determines Cd mobilization upon soil aeration in paddy soil systems. Environ Pollut 292:118311
Wang X, Pu L, Liu C, Gao J, Gu C (2021b) Enhanced and selective phototransformation of chlorophene on aluminum hydroxide-humic complexes. Water Res 193:116904
Wang X, Xiang W, Wang S, Ge J, Qu R, Wang Z (2020d) Oxidative oligomerization of phenolic endocrine disrupting chemicals mediated by Mn(III)-L complexes and the role of phenoxyl radicals in the enhanced removal: experimental and theoretical studies. Environ Sci Technol 54(3):1573–1582
Wang Y, Stone AT (2006) Reaction of MnIII,IV (hydr)oxides with oxalic acid, glyoxylic acid, phosphonoformic acid, and structurally-related organic compounds. Geochim Cosmochim Acta 70(17):4477–4490
Wang YH, Shi YM, Sun GD, Li JT, Chen H, Chow AT, Yang ZB, Majidzadeh H, Wang JJ (2020e) Soil organic carbon signature under impervious surfaces. ACS Earth Space Chem 4(10):1785–1792
Wei X, Wang J, She J, Sun J, Liu J, Wang Y, Yang X, Ouyang QE, Lin Y, Xiao T, Tsang DCW (2021) Thallium geochemical fractionation and migration in Tl-As rich soils: the key controls. Sci Total Environ 784:146995
Wei X, Zhou Y, Tsang DCW, Song L, Zhang C, Yin M, Liu J, Xiao T, Zhang G, Wang J (2020) Hyperaccumulation and transport mechanism of thallium and arsenic in brake ferns (Pteris vittata L.): a case study from mining area. J Hazard Mater 388:121756
Wen P, Wu Z, Han Y, Cravotto G, Wang J, Ye B-C (2017) Microwave-assisted synthesis of a novel biochar-based slow-release nitrogen fertilizer with enhanced water-retention capacity. ACS Sustain Chem Eng 5(8):7374–7382
Weng Z, Lehmann J, Van Zwieten L, Joseph S, Archanjo BS, Cowie B, Thomsen L, Tobin MJ, Vongsvivut J, Klein A, Doolette CL, Hou H, Mueller CW, Lombi E, Kopittke PM (2021) Probing the nature of soil organic matter. Crit Rev Environ Sci Technol. https://doi.org/10.1080/10643389.2021.1980346.
Wick S, Baeyens B, Marques Fernandes M, Göttlicher J, Fischer M, Pfenninger N, Plötze M, Voegelin A (2020) Thallium sorption and speciation in soils: role of micaceous clay minerals and manganese oxides. Geochim Cosmochim Acta 288:83–100
Wick S, Peña J, Voegelin A (2019) Thallium sorption onto manganese oxides. Environ Sci Technol 53(22):13168–13178
Woolet J, Whitman T (2020) Pyrogenic organic matter effects on soil bacterial community composition. Soil Biol Biochem 141:107678
Wu C, Mahandra H, Radzinski R, Ghahreman A (2020) Green catalytic process for in situ oxidation of arsenic(III) in concentrated streams using activated carbon and oxygen gas. Chemosphere 261:127688
Wu J, Qi H, Huang X, Wei D, Zhao Y, Wei Z, Lu Q, Zhang R, Tong T (2018) How does manganese dioxide affect humus formation during bio-composting of chicken manure and corn straw? Bioresour Technol 269:169–178
Wu T, Cui P, Huang M, Liu C, Dang F, Wang Z, Alves ME, Zhou D, Wang Y (2022) Oxidative dissolution of Sb2O3 mediated by surface Mn redox cycling in oxic aquatic systems. Water Res 217:118403
Wu X, Liu P, Gong Z, Wang H, Huang H, Shi Y, Zhao X, Gao S (2021) Humic acid and fulvic acid hinder long-term weathering of microplastics in lake water. Environ Sci Technol 55(23):15810–15820
Xie T, Reddy KR, Wang CW, Yargicoglu E, Spokas K (2015) Characteristics and applications of biochar for environmental remediation: a review. Crit Rev Environ Sci Technol 45(9):939–969
Xie Y, Lu G, Tao X, Wen Z, Dang Z (2022) A collaborative strategy for elevated reduction and immobilization of Cr(VI) using nano zero valent iron assisted by schwertmannite: removal performance and mechanism. J Hazard Mater 422:126952
Xin D, Saha N, Reza MT, Hudson J, Chiu PC (2021) Pyrolysis creates electron storage capacity of black carbon (biochar) from lignocellulosic biomass. ACS Sustain Chem Eng 9(19):6821–6831
Xing G, Garg S, Miller CJ, Pham AN, Waite TD (2020) Effect of chloride and suwannee river fulvic acid on cu speciation: implications to cu redox transformations in simulated natural waters. Environ Sci Technol 54(4):2334–2343
Xu H, Luo Y, Wang P, Zhu J, Yang Z, Liu Z (2019a) Removal of thallium in water/wastewater: A review. Water Res 165:114981
Xu J, Dai Y, Shi Y, Zhao S, Tian H, Zhu K, Jia H (2020a) Mechanism of Cr(VI) reduction by humin: role of environmentally persistent free radicals and reactive oxygen species. Sci Total Environ 725:138413
Xu W, Walpen N, Keiluweit M, Kleber M, Sander M (2021a) Redox properties of pyrogenic dissolved organic matter (pydom) from biomass-derived chars. Environ Sci Technol 55(16):11434–11444
Xu X, Huang H, Zhang Y, Xu Z, Cao X (2019b) Biochar as both electron donor and electron shuttle for the reduction transformation of Cr(VI) during its sorption. Environ Pollut 244:423–430
Xu Z, Wan Z, Sun Y, Cao X, Hou D, Alessi DS, Ok YS, Tsang DCW (2021b) Unraveling iron speciation on Fe-biochar with distinct arsenic removal mechanisms and depth distributions of As and Fe. Chem Eng J 425:131489
Xu Z, Wan Z, Sun Y, Gao B, Hou D, Cao X, Komárek M, Ok YS, Tsang DCW (2022a) Electroactive Fe-biochar for redox-related remediation of arsenic and chromium: distinct redox nature with varying iron/carbon speciation. J Hazard Mater 430:128479
Xu Z, Xu X, Tao X, Yao C, Tsang DCW, Cao X (2019c) Interaction with low molecular weight organic acids affects the electron shuttling of biochar for Cr(VI) reduction. J Hazard Mater 378:120705
Xu Z, Xu X, Tsang DCW, Yang F, Zhao L, Qiu H, Cao X (2020b) Participation of soil active components in the reduction of Cr(VI) by biochar: differing effects of iron mineral alone and its combination with organic acid. J Hazard Mater 384:121455
Xu Z, Xu X, Yu Y, Yao C, Tsang DCW, Cao X (2021c) Evolution of redox activity of biochar during interaction with soil minerals: effect on the electron donating and mediating capacities for Cr(VI) reduction. J Hazard Mater 414:125483
Xu Z, Xu X, Zhang Y, Yu Y, Cao X (2020c) Pyrolysis-temperature depended electron donating and mediating mechanisms of biochar for Cr(VI) reduction. J Hazard Mater 388:121794
Xu Z, Yu Y, Xu X, Tsang DCW, Yao C, Fan J, Zhao L, Qiu H, Cao X (2022b) Direct and indirect electron transfer routes of chromium(VI) reduction with different crystalline ferric oxyhydroxides in the presence of pyrogenic carbon. Environ Sci Technol 56(3):1724-1735
Yaashikaa PR, Senthil Kumar P, Karishma S (2022) Review on biopolymers and composites – evolving material as adsorbents in removal of environmental pollutants. Environ Res 212:113114
Yang F, Xu Z, Yu L, Gao B, Xu X, Zhao L, Cao X (2018) Kaolinite enhances the stability of the dissolvable and undissolvable fractions of biochar via different mechanisms. Environ Sci Technol 52(15):8321–8329
Yang F, Zhao L, Gao B, Xu X, Cao X (2016) The interfacial behavior between biochar and soil minerals and its effect on biochar stability. Environ Sci Technol 50(5):2264–2271
Yang JJ, Li AY, Yang YF, Li GH, Zhang F (2020) Soil organic carbon stability under natural and anthropogenic-induced perturbations. Earth Sci Rev 205:103199
Yang Z, Sun TR, Kleindienst S, Straub D, Kretzschmar R, Angenent LT, Kappler A (2021) A coupled function of biochar as geobattery and geoconductor leads to stimulation of microbial Fe(III) reduction and methanogenesis in a paddy soil enrichment culture. Soil Biol Biochem 163:108446
Yu C, Zhang Y, Lu Y, Qian A, Zhang P, Cui Y, Yuan S (2021) Mechanistic insight into humic acid-enhanced hydroxyl radical production from Fe(II)-bearing clay mineral oxygenation. Environ Sci Technol 55(19):13366–13375
Yu G-H, Kuzyakov Y (2021) Fenton chemistry and reactive oxygen species in soil: abiotic mechanisms of biotic processes, controls and consequences for carbon and nutrient cycling. Earth Sci Rev 214:103525
Zeng Q, Wang X, Liu X, Huang L, Hu J, Chu R, Tolic N, Dong H (2020) Mutual interactions between reduced fe-bearing clay minerals and humic acids under dark, oxygenated conditions: hydroxyl radical generation and humic acid transformation. Environ Sci Technol 54(23):15013–15023
Zhang B, Zhou S, Zhou L, Wen J, Yuan Y (2019a) Pyrolysis temperature-dependent electron transfer capacities of dissolved organic matters derived from wheat straw biochar. Sci Total Environ 696:133895
Zhang J, McKenna AM, Zhu M (2021) Macromolecular characterization of compound selectivity for oxidation and oxidative alterations of dissolved organic matter by manganese oxide. Environ Sci Technol 55(11):7741–7751
Zhang S, Chen S, Liu F, Li J, Liang X, Chu S, Xiang Q, Huang C, Yin H (2018a) Effects of Mn average oxidation state on the oxidation behaviors of As(III) and Cr(III) by vernadite. Appl Geochem 94:35–45
Zhang Y, Xu X, Cao L, Ok YS, Cao X (2018b) Characterization and quantification of electron donating capacity and its structure dependence in biochar derived from three waste biomasses. Chemosphere 211:1073–1081
Zhang Y, Xu X, Zhang P, Ling Z, Qiu H, Cao X (2019b) Pyrolysis-temperature depended quinone and carbonyl groups as the electron accepting sites in barley grass derived biochar. Chemosphere 232:273–280
Zhang Y, Yin M, Sun X, Zhao J (2020) Implication for adsorption and degradation of dyes by humic acid: light driven of environmentally persistent free radicals to activate reactive oxygen species. Bioresour Technol 307:123183
Zhao H-Q, Huang S-Q, Xu W-Q, Wang Y-R, Wang Y-X, He C-S, Mu Y (2019) Undiscovered mechanism for pyrogenic carbonaceous matter-mediated abiotic transformation of Azo dyes by sulfide. Environ Sci Technol 53(8):4397–4405
Zhao N, Yin Z, Liu F, Zhang M, Lv Y, Hao Z, Pan G, Zhang J (2018a) Environmentally persistent free radicals mediated removal of Cr(VI) from highly saline water by corn straw biochars. Bioresour Technol 260:294–301
Zhao W, Cheng H, Tao S (2020) Structure–reactivity relationships in the adsorption and degradation of substituted phenylarsonic acids on Birnessite (δ-MnO2). Environ Sci Technol 54(3):1475–1483
Zhao Y, Wang M, Hu S, Zhang X, Ouyang Z, Zhang G, Huang B, Zhao S, Wu J, Xie D, Zhu B, Yu D, Pan X, Xu S, Shi X (2018b) Economics- and policy-driven organic carbon input enhancement dominates soil organic carbon accumulation in Chinese croplands. Proc Natl Acad Sci U S A 115(16):4045–4050
Zhao Z, Zhou W (2019) Insight into interaction between biochar and soil minerals in changing biochar properties and adsorption capacities for sulfamethoxazole. Environ Pollut 245:208–217
Zheng G, Liu C, Deng Z, Wei Z, Zhao Y, Qi H, Xie X, Wu D, Zhang Z, Yang H (2021) Identifying the role of exogenous amino acids in catalyzing lignocellulosic biomass into humus during straw composting. Bioresour Technol 340:125639
Zheng W, Demers JD, Lu X, Bergquist BA, Anbar AD, Blum JD, Gu B (2019) Mercury stable isotope fractionation during abiotic dark oxidation in the presence of thiols and natural organic matter. Environ Sci Technol 53(4):1853–1862
Zheng W, Liang L, Gu B (2012) Mercury reduction and oxidation by reduced natural organic matter in anoxic environments. Environ Sci Technol 46(1):292–299
Zhong D, Jiang Y, Zhao Z, Wang L, Chen J, Ren S, Liu Z, Zhang Y, Tsang DCW, Crittenden JC (2019) pH dependence of arsenic oxidation by rice-husk-derived biochar: roles of redox-active moieties. Environ Sci Technol 53(15):9034–9044
Zhong D, Zhao Z, Jiang Y, Yang X, Wang L, Chen J, Guan C-Y, Zhang Y, Tsang DCW, Crittenden JC (2020) Contrasting abiotic As(III) immobilization by undissolved and dissolved fractions of biochar in Ca2+−rich groundwater under anoxic conditions. Water Res 183:116106
Zhong Q, Qi J, Liu J, Wang J, Lin K, Ouyang Qe, Zhang X, Wei X, Xiao T, El-Naggar A, Rinklebe J (2022) Thallium isotopic compositions as tracers in environmental studies: a review. Environ Int 162:107148
Zhou Y, Wu Y, Lei Y, Pan Y, Cheng S, Ouyang G, Yang X (2021) Redox-active moieties in dissolved organic matter accelerate the degradation of nitroimidazoles in SO4•–-based oxidation. Environ Sci Technol 55(21):14844–14853
Zhou Z, Latta DE, Noor N, Thompson A, Borch T, Scherer MM (2018) Fe(II)-catalyzed transformation of organic matter–ferrihydrite coprecipitates: a closer look using fe isotopes. Environ Sci Technol 52(19):11142–11150
Zhu S, Huang X, Yang X, Peng P, Li Z, Jin C (2020) Enhanced transformation of cr(vi) by heterocyclic-N within nitrogen-doped biochar: impact of surface modulatory persistent free radicals (PFRs). Environ Sci Technol 54(13):8123–8132
Zou J, Li G, Zheng D, Wang Y, Feng C, Sun Y, Juan M, Bai X, Wu M (2021) Humic acid promotes volatile fatty acids production from excess sludge in anaerobic fermentation. ACS Sustain Chem Eng 9(37):12540–12547
Acknowledgements
We appreciate the financial support from the Hong Kong Environment and Conservation Fund (ECF Project 101/2020) and Hong Kong Research Grants Council (PolyU 15222020) for this study.
Funding
This work was supported by the Hong Kong Environment and Conservation Fund (ECF Project 101/2020) and Hong Kong Research Grants Council (PolyU 15222020).
Author information
Authors and Affiliations
Contributions
Zibo Xu: Conceptualization, Methodology, Literature collection and analysis, Writing; Daniel C.W. Tsang: Supervision, Conceptualization, Resources, Project administration, Review and Editing. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Daniel C.W. Tsang is an editorial board member of Carbon Research and was not involved in the editorial review or the decision to publish this article. All authors declare that there are no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, Z., Tsang, D.C. Redox-induced transformation of potentially toxic elements with organic carbon in soil. carbon res 1, 9 (2022). https://doi.org/10.1007/s44246-022-00010-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44246-022-00010-8