Abstract
While the potential of biochar (BC) to immobilize potentially toxic elements (PTEs) in contaminated soils has been studied and reviewed, no review has focused on the potential use of BC for enhancing the phytoremediation efficacy of PTE-contaminated soils. Consequently, the overarching purpose in this study is to critically review the effects of BC on the mobilization, phytoextraction, phytostabilization, and bioremediation of PTEs in contaminated soils. Potential mechanisms of the interactions between BC and PTEs in soils are also reviewed in detail. We discuss the promises and challenges of various approaches, including potential environmental implications, of BC application to PTE-contaminated soils. The properties of BC (e.g., surface functional groups, mineral content, ionic content, and π-electrons) govern its impact on the (im)mobilization of PTEs, which is complex and highly element-specific. This review demonstrates the contrary effects of BC on PTE mobilization and highlights possible opportunities for using BC as a mobilizing agent for enhancing phytoremediation of PTEs-contaminated soils.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Soil is a basic resource, essential for the survival of humans and also the source of their wealth. Soils provide humanity with 98.8% of its food; therefore, global food security and safety are strongly affected by soil agriculture policies (Kopittke et al. 2019). The rapid growth of human population (from ca. 250 million in the year AD 1000, to ca. 7.7 billion in 2020-it may reach 9.8 billion by 2050 and 11.6 billion in 2100; UN 2017) and increasing food consumption are placing unprecedented pressure on soil resources. For example, food production needs to increase by up to 70% by 2050, to achieve global food security (ELD 2015). This requirement implies more intensive agricultural production, because soil is a finite resource (Tilman et al. 2011; FAO et al. 2017; Hunter et al. 2017; Ickowitz et al. 2019). The current intensification of agricultural practices—including the over-application of inorganic fertilizers may cause degradation of soils and ecosystem, as depicted in Fig. 1 (Kopittke et al. 2019).

(Reproduced from Kopittke et al. 2019, with permission of the publisher)
Impact of agricultural intensification on soils, including degradation by loss of soil organic matter and the release of greenhouse gasses, over-application of fertilizers, erosion, soil contamination, acidification, salinization, and loss of soil genetic diversity
Soil contamination is one of the major forms of soil degradation, which can adversely affect food safety and security and thus human health (Antoniadis et al. 2022). Soils are being increasingly exposed to potentially toxic element (PTE) pollution due to various industrialization, modernization, and other anthropogenic activities. Contamination of soils with PTEs such as As, Cd, Cr, Co, Cu, Hg, Sb, Se, Sn, Tl, V, and Zn represents a health risk to humans (Antoniadis et al. 2022). Therefore, remediation of PTE-contaminated soils has received considerable attention over the past few years. Among the different remediation approaches for the sustainable management of contaminated soils, green remediation is one of the cheapest and most environmentally friendly (Jeyasundar et al. 2021). Green remediation of PTE-contaminated soils can be achieved with various methods including metal immobilization, phytostabilization, and/or phytoextraction (e.g., Shaheen and Rinklebe 2015; Antoniadis et al. 2017, 2021; Ali et al. 2020a; Hou et al. 2020; Palansooriya et al. 2020). The combination of soil amendments with hyperaccumulators is an effective method for increasing the efficiency of green remediation of PTE-contaminated soils. Among the soil amendments, BC has received significant attention in the last fifteen years (since 2007; Lehmann 2007). The focus on BC as a soil amendment for management of degraded soils is likely due to the fact that its feedstocks are locally available and potentially recyclable or renewable (El-Naggar et al. 2019a).
Research on BC as promising materials for remediation of degraded soils and water has grown rapidly since 2007 (e.g., Bolan et al. 2014, 2022; El-Naggar et al. 2019a; Bandara et al. 2020; Ali et al. 2020a; Shaheen et al. 2019a, b, 2022a, b). Many studies have concluded that BC has viable potential for the immobilization of some PTEs in soils and for reducing their phytoavailability (Shaheen et al. 2022c; Yang et al. 2023a,b). However, other studies have reported that BC can rather increase the mobilization and uptake of some PTEs (Yang et al. 2023a,b). These paradoxical findings depend mainly on the types and properties of the studied element, BC, and soils, which are discussed in the subsequent sections.
The potential of BC for PTEs immobilization in soils has been studied, but no review has focused on the potential use of BC for enhancing the phytoremediation efficacy of PTE-contaminated soils. Here, we review the effects of BC on PTE mobilization, phytoextraction, phytostabilization, and bioremediation in contaminated soils. We also summarize potential mechanisms of the interactions between BC and PTEs in soils in in detail. The pros and cons of BC application to PTE-contaminated soils and the potential environmental implications are also reviewed and summarized.
2 Soil PTEs: Sources, Mobilization, and Potential Risks
Potentially toxic elements can be interred into soils from geogenic and/or anthropogenic sources (Fig. 2; Palansooriya et al. 2020). Metals sources govern their mobilization in soils; for example, the mobilization of PTEs in soils contaminated with anthropogenic sources is higher than that in geogenically contaminated soils (Khan et al. 2021; Shaheen et al. 2022a). Soil properties affect PTE sorption/availability and thus affect their mobilization (Shaheen et al. 2013; Antoniadis et al. 2017; Rinklebe et al. 2020). Moreover, the fractions and speciation of PTEs in soils affect their solubilization (Rinklebe and Shaheen 2017; Kumar et al. 2022). Therefore, understanding the soil and PTE properties is necessary before selecting appropriate soil amendments (Palansooriya et al. 2020; Kumar et al. 2022). Soil PTEs can be bio-accumulated by plants, thus entering the food chain and raising serious concerns over adverse risks to human health (Fig. 3; Ali et al. 2020b; Antoniadis et al. 2017; Isinkaye 2018; Ekoa Bessa et al. 2021). The toxicity of PTEs depends on an element’s speciation and corresponding bioavailability, which are governed by environmental conditions such as redox potential, soil minerals, soil solution chemistry, and soil microorganism communities (Palansooriya et al. 2020; Khan et al. 2021; Antoniadis et al. 2022). Therefore, it is important to understand soil PTE behavior and bioavailability to determine relevant environmental risks and adopt appropriate monitoring or remediation methods, as discussed in the following sections.

(Reproduced from Palansooriya et al. 2020, with a permission of the publisher)
Sources of toxic elements (TEs) in soil ecosystems

(Reproduced from Ali et al. 2020b, with a permission of the publisher)
A general overview of natural and anthropogenic sources of trace elements in environmental matrices, and associated human health toxicities
3 Remediation of PΤΕ-Contaminated Soils
Different physical, chemical, and biological ex situ and in situ trials can be employed for the remediation of PTE-contaminated soils (Fig. 4), which have been reported in previous studies (e.g., Ali et al. 2020a, b; Palansooriya et al. 2020; Khan et al. 2021; Kumar et al. 2022). The PTE clean-up techniques are categorized into containment-based (e.g., capping/encapsulation), transformation-based (e.g., stabilization/immobilization), and transport-based (e.g., extraction/removal), all employing various physical, chemical, electrical, thermal and biological methods (Khan et al. 2021; Kumar et al. 2022). However, each method has different preference criteria, since an intensive analysis of specific field conditions is required. In general, different polluted soils require different treatment methods (Khan et al. 2021). For instance, surface capping is only viable to prevent PTEs from infiltrating vertically through the soil profile; nevertheless, the capped area may lose its natural soil functions. Similarly, the main challenge for encapsulation is to construct impermeable walls at contaminated sites. Therefore, several well-established conventional methods for PTE remediation have often failed due to their high cost, technical complexity and the release of secondary pollutants.

(Reproduced from Ali et al. 2020b, with a permission of the publisher)
Comparison of soil management/remediation methods for trace elements-contaminated soil
Among remediation methods, bioremediation is a green strategy that recruits the biological mechanisms that are inherent in microbes, plants and other biological substances to clean-up PTE-polluted environments, rendering contaminated soil less polluted and free of recalcitrant and secondary pollutants (Singh and Gupta 2016; Nedjimi 2021; Zhang et al. 2023). It is operationally simple, esthetically non-destructive, solar energy-driven, economically viable and widely accepted; it also improves the physical, chemical and biological qualities of the contaminated site offering ecological restoration (Sarwar et al. 2017). Microbes may use PTEs as a source of nutrition and also can cause the oxidation, reduction and transition of PTEs; therefore, they do not incur any additional expense in meeting their nutritional demands (Verma and Kuila 2019). There are several advantages to adopting bioremediation strategies for PTE clean-up. They can enhance revenue while an ecosystem is being restored, a benefit which cannot be realized with any other method. The produced biomass may be considered as an alternative feedstock for sustainable biofuel production without affecting food security (Dastyar et al. 2019). Tailoring of microbes and plants may, therefore, add value of an ecosystem while simultaneously achieving enhanced decontamination efficiencies.
The efficiency of the remediation of PTE-contaminated soils depends on the type and properties of the soils and elements involved. The mobilization of PTEs in soil significantly affects the remediation process efficiency (Palansooriya et al. 2020). For example, increasing the PTE mobilization in soil may increase their absorption by hyperaccumulator plants and may thus enhance the rate of PTE phytoremediation or phytoextraction processes (Amin et al. 2018; Shaheen et al. 2019a, b; Wang et al. 2019). On the other hand, the immobilization of PTEs in soils aims to reduce their mobility via sorption, precipitation, and/or stabilization with various soil amendments (Fig. 4), to minimize ecosystem and human health risks.
Among the (im)mobilizing agents, BC has been used successfully for PTEs immobilization and reducing their phytoavailability; however, in some cases BC mobilized the PTEs and enhanced their phytoavailability, as discussed in details in the following sections. Therefore, understanding the factors and processes which govern PTE mobilization in BC-treated soils is necessary to develop an effective remediation plan.
4 Biochars for the Remediation of PTE-Contaminated Soils
4.1 Biochars for PTE Immobilization
4.1.1 Biochars and PTE Immobilization Efficiency
Biochar can be used effectively for PTE immobilization in soils. The efficiency of the immobilization is highly dependent upon multiple BC-related factors, including its application rate, source of feedstock, pyrolysis temperature, pyrolysis gas, ash content, and particle size. The physicochemical properties of BC derived from different feedstocks under varying pyrolysis temperatures are summarized in Table 1. Additionally, several indirect factors related to soil characteristics (e.g., soil pH, redox potential and particle size distribution) control how effectively BC can mitigate PTE phytotoxicity.
Several attempts have been made to identify the optimum application rate of BC in soil, which nevertheless remains uncertain. We extracted data from recent published investigations (36 studies, comprising 315 observations) to evaluate the most important factors underlying PTE immobilization in soil with emphasis on the most widely studied elements (As, Cd, Cr, Cu, Pb and Zn) (Fig. 5). Among the studied factors, there seems to be a consensus that application rate of BC is the most crucial factor for enhancing the immobilization efficiency of PTEs. According to the obtained data, immobilization efficiency of PTEs averaged 30.8% (i.e., if a 100 mg PTE L−1-solution was added to a soil-BC mixture, 30.8 mg L−1 was retained by the mixture and 69.2 mg L−1 remained in solution) when the application rate was < 2%. The retention rate rose to 44.6% at a dose of 2–5%, and to 48.1% at a dose of > 5.0% (Fig. 6a). Highly polluted sites (e.g., mining areas) might need even higher application rates of BC to achieve an optimal safeguard effect (Khan et al. 2020).
Effect of biochar application on immobilization efficiency of TEs (%). Data are extracted from 315 observations comprising 36 recent studies. The box chart presents median (centerline), mean (dot), lower quartiles (lower border of the box), upper quartiles (upper border of the box) and whiskers-error bars (the minimum and maximum observations)
Effect of biochar application rate (a), feedstock type (b), pyrolysis temperature (c) and ash content (d) on immobilization efficiency of TEs (%). Data are extracted from 315 observations comprising 36 recent studies. The box chart presents median (centerline), mean (dot), lower quartiles (lower border of the box), upper quartiles (upper border of the box) and whiskers-error bars (the minimum and maximum observations)
The effect of BC feedstock on its properties and on the ability of BC to immobilize PTE has also been widely investigated (Table 1). Data extracted from recent literature show that the immobilization efficiency of PTEs (As, Cd, Cr, Cu, Pb and Zn) as a function of feedstock type can be ranked as follows: weeds (49.6%) > sludge (37.8%) > crop residues (32.7%) > woods (29.2%) > bones (20.3%) (Fig. 6b). Pyrolysis temperature also has a pivotal role in controlling the functionality of BC and its stabilization capacity. Analysis of recent published data demonstrates that BC produced at high pyrolysis temperatures are more efficient in immobilizing PTEs than those produced at low temperatures. For instance, 32.57% immobilization was achieved when BC were produced at 300–499 °C, but 36.17% and 36.51% were achieved when BC were produced at 500–599 °C and 600–800 °C, respectively (Fig. 6c).
A possible explanation for this could be that BC produced at high pyrolysis temperature have higher persistence in soil as well as higher pH values, total carbon, specific surface area and porosity relative to those produced at low pyrolysis temperatures (El-Naggar et al. 2022; Tomczyk et al. 2020).
The ash content of BC depends not only on the feedstock type (Table 1), but also on the details of the thermochemical conversion process. From recently published data we found that the average efficiency of PTE immobilization increased slightly as the ash content increased from < 10 to 20% (with immobilization increased from 36 to 37.7%, respectively). This immobilization efficiency decreased sharply to 29.4% with ash contents above 20% (Fig. 6d). The particle size of BC often depends on the feedstock particle size, but it is likely smaller than the precursor due to shrinkage and attrition during pyrolysis (Panahi et al. 2020). Few investigations have studied the effect of particle size of BC on its stabilization efficiency. It is hypothesized that the efficiency of BC in stabilizing PTEs increases when size is decreased due to enhanced available surface area. In the view of this, Fahmi et al. (2018) recommended using fine particles of BC derived from empty fruit bunch oil palm (< 50 µm) to achieve the highest in situ immobilization of Cd2+ and Pb2+. They reported that such fine particles were more efficient compared to coarse particles of > 2 mm.
Several factors related to soil physicochemical properties underly the efficiency of BC in immobilizing PTEs. It is generally agreed that the value of soil pH is a crucial criterion for controlling the ability of BC to safeguard against the phytotoxicity of PTEs. Meta-analysis of PTE stabilization indicates that soil pH is the most important factor (Yuan et al. 2021). These authors showed that the bioavailability of PTEs decreased by about 28.7% in weakly acid coarse-textured soils and by 6.4% in medium-textured soils. This reduction in PTE bioavailability reached 149% and 121% in soils with a highly alkaline nature (pH > 8.0).
Soil texture can also play a pivotal role in controlling the mobility/accessibility of PTEs, since fine-textured soils possess high clay content that improves PTEs retention in the soil matrix (Mosa et al. 2021, 2022; Yin et al. 2019). Meta-analysis of PTE bioavailability in response to BC aging illustrated that the contribution of soil texture (18.6%) was the second most influential criterion, after soil pH (Yuan et al. 2021). In this regard, BC exhibits a remarkable effect in fine-textured soils relative to coarse-textured soils. For example, a sandy soil was more responsive to the lowest application rate (1%) of a 620 °C-wood BC; however, BC addition to a sandy loam soil only started to show a significant response at a 5% application rate (Verheijen et al. 2019).
Unlike most soil characteristics, the mutual interactions between BC and soil redox conditions have rarely been studied in respect to PTE decontamination. The effect of pig carcass-derived BC at 650 °C was studied for As immobilization in a contaminated paddy soil using an automated biogeochemical microcosm apparatus to control the redox potential (EH) (Yang et al. 2022a). The authors concluded that BC reduced the phytoavailability of As under moderately reducing conditions (38.7 and 35.4% reduction at EH = +100 and +200 mV, respectively). Meanwhile, BC application maximized the phytotoxic effect of As under oxidized (317.6% increment at EH = +250 mV) and highly reducing conditions (13.5% increment at EH = − 300 mV). The interactions between BC and PTEs in soils can be happen via different potential mechanisms as disused in detail in the following section.
4.1.2 Mechanistic Insights into the Role of BC in PTE Immobilization in Soil
The immobilization of PTEs in soil by the addition of BC depends on a variety of complex and intertwined mechanisms. Direct mechanisms include the interaction between biochar and PTEs, while indirect mechanisms include the modulation of soil physicochemical properties (Fig. 7).

(Reproduced from Bandara et al. (2020), with a permission of the publisher)
Mechanistic illustration of Cd, Cr, Hg, and Pb sorption (A) and As and Sb sorption (B) by biochars in soil. DOC: dissolved organic C
Physisorption is a weak secondary mechanism, which depends mainly on the porosity of BC (Yang et al. 2019). Physisorption often relies on the porosity of BC through Van der Waals forces, which increase with increasing specific surface area and BC pore size (Salam et al. 2019; Ahmad et al. 2021; El-Naggar et al. 2021; Shaheen et al. 2022a). As such, the higher specific surface area and pore volume of two phosphorus-supported BC were found to stimulate the physisorption mechanism for the effective amelioration of Pb-contaminated Udic ferrosols (Cui et al. 2022). Physical adsorption via pore filling onto modified BC (EDTA crosslinked β-cyclodextrin at 300 °C corn stalks) was also tested for the remediation of a Cd/Pb-contaminated soil (Qu et al. 2022). The involvement of cation-π electron interaction mechanisms in PTE sorption upon remediation of polluted soils has also been reported (Yi et al. 2022; Zhou et al. 2022). The electron-rich domains of S/Fe-modified corncob BC were found to have enhanced the active contribution of the cation-π interaction mechanism for Cd2+ and Pb2+ stabilization in a contaminated soil, as revealed by Shen et al. (2019). However, the elements sorbed by this mechanism could be exposed to leaching at low values of soil pH levels. Electrostatic interaction is another physisorption mechanism, which could function as the spark for PTE immobilization in contaminated soils (Sun et al. 2022).
On the other hand, several chemisorption mechanisms contribute mutually toward PTE immobilization. Among them, ion exchange involves the replacement of labile PTEs with similarly charged ion exchangers of BC. Metal cations initially on BC surfaces (e.g., Na+, K+, Ca2+ and Mg2+) could be exchanged with PTEs (Qi et al. 2022). Ion exchange might also occur between PTEs and oxygen-containing functional groups (Wu et al. 2021b). According to Cui et al. (2022), a gradual reduction in the pH value of the soil solution occurred during ion exchange with soil Pb2+ in Udic ferrosols according to the following reactions:
The active surfaces functional groups on BC can also bind PTEs to create multi-atom structures. For example, the grafting of thiol functional groups onto a 500 °C-pyrolyzed rice straw BC was the predominant mechanism for the remediation of a Pb-contaminated soil (Fan et al. 2020). The tendency to form surface complexes containing active functional groups differs among PTEs. As an example, the higher affinity of alcoholic, hydroxylic and carboxylic functional groups to Pb2+ than Zn2+ ions was found to be the main reason for the wide variation in their stabilization efficiency in a contaminated paddy soil (Chao et al. 2018).
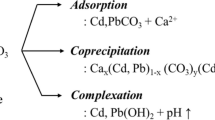
Precipitation is another important chemisorption mechanism for the stabilization of PTEs into mineral formations in the charosphere. The high ash and total phosphorus contents in reed straw BC-supported phosphate composite stimulated the remediation of a Pb-contaminated soil via the formation of insoluble cerussite, hydrocerussite and hydroxylpyromorphite precipitates (Cui et al. 2022). In yet in another study, the release of high concentrations of OH−, CO32−, and PO43− anions from a 500 °C-pyrolyzed rice straw BC was found to have promoted the formation of stable Zn(OH)2, ZnCO3 and Zn3(PO4)2 precipitates (Li et al. 2022a).
Biochar application as a green oxidant/reductant has been widely explored for the decontamination of redox elements [e.g., Cr(VI) and As(III)] by enhancing electron transfer between oxidants and reductants in soil (Ambika et al. 2022; Rafique et al. 2021; Shaheen et al. 2022b; Wang et al. 2022; Yang et al. 2022b) (Fig. 7). Sulfurization of a 700 °C-Eichhornia crassipes BC using sulfide Fe3O4 coating triggered As(III) redox conversion through the generation of dissolved O2 and Fe2+ ions (Wang et al. 2022). On the other hand, utilization of sewage sludge BC supported the action of ferrous sulfate as a green reductant for a Cr(VI)-contaminated soil, where passivation was considered from a mechanistic point of view (Li et al. 2020). Alterations in leachability/bioaccessibility of Cr(VI) in the soil matrix could be mainly attributed to its reduction into less soluble and toxic Cr(III) with the help of active functional groups of BC (OH, C=O, C–H and C–O), as well as the concurrent oxidation of grafted Fe2+ ions to their trivalent species. The description of the Cr reduction mechanism can be summarized as follows:
Note that BC plays roles in modulating soil physicochemical properties and regulating the phytotoxicity of PTEs. The liming effect of BC to raise the pH of acidic soil has been acknowledged as an indirect mechanism for PTE decontamination given the alkaline nature and high ash content of BC (Xu et al. 2022a). Biochar application often increases soil pH, which in turn helps to stabilize PTEs by accelerating the formation of precipitated hydroxide species (Chao et al. 2018). The applicability of BC for mitigating PTE phytotoxicity remains challenging when both cationic and anionic elements are involved. Nevertheless, the addition of a 500 °C-wood BC increased the pH value of soil, promoted the phytoavailability of Cr(VI) and enhanced the immobilization of Cd2+ and Pb2+ by 85.14% and 28.68%, respectively (Gong et al. 2022).
In addition, the phytoavailability of PTEs can be mitigated indirectly by BC through modulation of the redox state of soil. Yang et al. (Yang et al. 2022b) reported that original (650 °C Platanus orientalis L. branches) and Fe-modified BC were highly suitable for mitigating environmental risk of As in paddy soils. The plentiful redox-active moieties (O-containing functional groups and condensed aromatics) improved BC redox buffering capacity, changed soil EH and thus immobilized As by about 16.0–41.3% at EH < − 100 mV. A Fe-modified BC immobilized As to a higher extent, by 32.6–81.1% at EH < 0 mV due to the transformation of As-bound Fe (hydr)oxides into complexes (e.g., ternary As-Fe-DOC). In addition, the redox activity of dissolved organic substances derived from BC could also reduce the phytotoxicity of Cr and As [oxidation of As(III) and reduction of Cr(VI)] (Wu et al. 2021a) (Fig. 7).
The beneficial effect of BC application on soil fertility and buffering capacity can be expressed by increases in CEC (Cation Exchange Capacity), which improves the PTE immobilization efficiency in soil. For example, the addition of 5% and 10% sludge BC (400 °C) to soil collected from a mining area had a significant effect on its CEC (Kong et al. 2021): The CEC value of the untreated soil increased from 9.2 to 12.86 (at 5% of application) and to 13.04 cmolc kg−1 (at 10%). The beneficial effect of BC in regulating soil biological properties is another indirect mechanism for enhancing PTE remediation, since bacteria can adhere better to the charosphere instead of being leached out from the unamended rhizospheric layer (Lehmann et al. 2011). The significant contribution of active functional groups (i.e., –OH, –COOH, –NH– and PO43−) that exist on PTE-tolerant bacterial cells (Pseudomonas sp. NT-2) might be the predominant mechanism for immobilizing Cd2+ and Cu2+ in Cd- and Pb-contaminated soils (Tu et al. 2020). The enhancement of soil bacteria following BC application might also promote the swift reduction of phytoavailable Cr(IV) by accelerating extracellular electron transfer (Zheng et al. 2021). Biochar can also immobilize Hg and reduce its accumulation in rice plants by binding with thiols (e.g., cysteine), which are either BC- or soil-borne (Fig. 8; Xing et al. 2020).

(Reproduced from Xing et al. (2020), with a permission of the publisher
Effects of the application of rice hull-derived biochar (RHB) at 24 t ha−1 and 72 t ha−1 on total Hg (THg) and methylHg (MeHg) immobilization in a paddy field and their accumulation by rice plants
4.1.3 Designer/Functionalized BC for the Stabilization of PTEs in Soil
Recently, BC functionalization (or specialization) has shown a progressively increased effectiveness in the remediation of PTE-contaminated soils (Yang et al. 2023a,b). Analysis of recent literature shows enhancement in the immobilization efficiency of Cd by 2.38%, Cr by 19.27%, Cu by 24.47%, and Pb by 7.22%, following the functionalization process (Fig. 9). Acid and alkali treatments of pristine BC have been widely investigated to maximize their functionality. Alkali-treated BC (KOH-modified rice straw BC at 500 °C) exhibited higher efficiency than the pristine form for reducing Cd2+ solubility (30.3 vs. 27.4%) and biocaccesability (32.4 vs. 25.2%) in Cd-contaminated red acidic soils (Bashir et al. 2018; 2020). On the other hand, the modulating effect of vinegar residue BC (pH = 5.47) on a Pb-contaminated alkaline soil was studied by Li et al. (2022b). The authors reported that acidic BC reduced exchangeable & carbonate-bound Pb fractions and increased the residual and Fe/Mn–bound fractions.
Effect of biochar functionalization on immobilization efficiency of TEs (%). Data are extracted from 315 observations comprising 36 recent studies. The box chart presents median (centerline), mean (dot), lower quartiles (lower border of the box), upper quartiles (upper border of the box) and whiskers-error bars (the minimum and maximum observations)
Experiments have also been performed in an attempt to graft active functional groups (e.g., –OH, –C=O and –NH2) onto BC surfaces to improve its complexation potential. For instance, a cetyltrimethylammonium bromide-modified peanut-shell BC showed outstanding capacity for decreasing the bioavailability of Cr(VI) by 92%, its leachability by 100%, and its bioaccessibility by 97% compared to the untreated form (Murad et al. 2022). The authors argued that this greater passivation efficiency of the engineered BC was due to the grafting of carbonyl functional groups, which served as proton donors for the reduction of Cr(VI) ions. Gholami and Rahimi (2021) synthesized a modified thiourea-potato peel BC for the immobilization of Cd2+, Cu2+ and Zn2+ in an acidic contaminated soil. The application of the modified BC at a dose of 8% maximized the solidifying efficiency of Cd2+ (5142.63 mg kg−1), Cu2+ (4993.12 mg kg−1) and Zn2+ (3508.44 mg kg−1) due to the enrichment of BC surfaces with –OH, –C=O, –COOH and –C–O groups. Recent results about the impacts of functionalized BC on the mobilization and phytoavailability of As, Cd, and Pb in a paddy soil are published in Yang et al. (2023a,b).
Nano-scale fabricated BC have recently been tested for their ability to improve TE immobilization in soil. In this regard, ball milled BC-supported nanoscale red P particles were investigated for the immobilization of Cd2+ and Pb2+ in an alkaline soil (Zhang et al. 2022). The nano-sized BC showed outstanding performance for the decontamination of PTEs in soil by accelerating the conversion of red P into phosphorus oxides, (hydro)phosphates and phosphoric acid, following the interaction with alkaline minerals, carbonates and O-containing functional groups of ball milled-BC. Another study used a 700 °C-pine BC as a carrier for nano zirconia (ZrO2) for solidifying an As(V)-contaminated brown soil (Liu et al. 2021). Application of 2 wt% of a nano zirconia-loaded BC recorded outstanding stabilization efficiency (retaining 99.3% of the amount added) due to the abundance of –Zr–O functional groups that produce tetranuclear ions, which are rich in –OH ligands.
Biological activation of raw BC was also investigated by Ji et al. (2022). Several bacterial strains (Bacillus velezensis, Bacillus cereus and Bacillus amyloliquefaciens) were loaded onto a 500 °C-corn stalks BC and exploited for the decontamination of a polluted soil system. Results pointed to a mutual synergistic effect between bacterial strains and BC with a long-lasting passivation of PTEs into precipitated forms [i.e., Pb5(PO4)3OH, Ca2As2O7, CdCO3 and Cd3(AsO4)2]. Interestingly, application of BC-immobilized Cd-resistant bacteria (Arthrobacter sp. and Micrococcus sp.) significanly increased the accumulation of Cd in Chlorophytum laxum grown in a highly contaminated agricultural soil in Thailand (Chuaphasuk and Prapagdee 2019). Those authors explained this trend as being the result of the accumulation of Cd in tolerant bacterial strains (bacterial cell walls and exopolymers), leading to maximized Cd2+ bioavalability in the soil. This type of functionalized BC, therefore, can effectively promote Cd2+-phytoextraction efficiency by metallophytes.
Doping heteroatoms (e.g., P, N, S and Ni) into BC showed promising results for the PTE decontamination, owing to the electron-withdrawal effect. Phosphorus-based BC (reed straw-supported potassium dihydrogen phosphate or hydroxyapatite) showed higher Pb2+ stabilization than the original form (400 °C) through the active transformation of labile Pb2+ into Fe/Mn oxides and residual fractions (Cui et al. 2022). Similar observations were also reported by Ren et al. (2020) in stabilization of Cd and Pb in soils using pristine swine manure BC and P-enhanced engineered BC. Moreover, Luo et al. (2022) attributed the beneficial effect of incorporating P into BC to the enhancement of BC stability in soil by the chemical formation of C–O–P, C–PO3 and C2–PO2, thereby maximizing the immobilization of Pb2+ and Cd2+. Sulfur doping into a 550 °C-bamboo hardwoods BC greatly increased the formation of Fe plaque on the surfaces of rice roots and decreased the accumulation of Cd in grains below the standard soil maximum allowable limits (0.2 mg kg−1 as recommenended by the China National Standard) (Rajendran et al. 2019).
It is believed that BC have a greater capacity to stabilize PTEs after aging. The effect of dry–wet and freeze–thaw aging on the phytoavailability of Cd2+ and Cu2+ was studied in a contaminated soil near a Cu smelter in Jiangxi Province, China (Cui et al. 2021). Biochar aging resulted in the reduction of Cu2+ and Cd2+ phytoavailability; however, the ecotoxicological risk could be increased by aged BC rich in endogenous PTEs. The effect of three aging methods on the stabilization performance in a Pb-contaminated soil by BC was found to be significant; these methods were high-temperature, freeze–thaw, and natural aging, in order of decreasing effectiveness (Chen et al. 2022). In a study conducted by Rathnayake et al. (2021), it was found that when H2O2-aged BC (equivalent to a naturally aged BC of 100 years) was applied at 2 wt%, it resulted a higher Cd decontamination efficiency when compared to fresh BC; aged BC produced at high temperatures (500 and 600 °C) were superior to those produced at low temperature (400 °C).
Recent attempts at BC activation have also been directed toward generating hybrid magnetized forms through grafting ferromagnetic oxides into the carbonaceous matrix of the pristine BC (Shaheen et al. 2022a, b, c. For example, an Fe–Zn oxide-modified corn straw BC (at 500 °C) was more effective than its pristine form for reducing the extractability of Cd in both acidic and alkaline soils: the modified BC retained 35.1% of the initially added amount vs. 17.0% for the pristine BC in the acidic soil, while in the alkaline soil the modified BC retained 38.1 vs. 12.0% for the pristine BC (Yang et al. 2021a). The prominent safeguarding effect of the functionalized BC is related mainly to the incorporation of exchangeable Cd2+ into its Fe/Mn oxyhydroxide-bound fraction. The abundance and diversity of bacterial communities in the soil was significantly stimulated as well. To date, functionalization of effective BC composites for simultaneous immobilization of cationic and anionic pollutants remains challenging. In this regard, a novel sulfide Fe3O4 coated BC composite (derived at 700 °C from Eichhornia crassipes) was fabricated for the simultaneous removal of As(III) and Pb(II) (Wang et al. 2022). The excellent stabilization performance of the designed dual-purpose BC was attributed to the maximized BC functionality (specific surface area and anionic exchange capacity) followed by the stimulation of As(III) oxidation and Pb(II) precipitation.
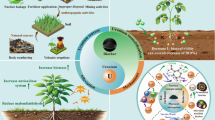
4.2 Biochars for Enhancing PTE Phytoremediation
Phytoremediation is a green, ecofriendly, and low-cost method for the remediation of PTE-contaminated soils (Fig. 10). Many hyperaccumulators such as Pteris vittata, Zea mays, and Brassica species have been employed for the phytoextraction of PTEs (Khalid et al. 2017). Plant growth in highly contaminated soils is severely affected, and biochar application in combination with phytoextractors can be an effective method for PTE remediation in such soils (Khalid et al. 2017; Pračke et al. 2021).

(adapted from Favas et al. 2014 and Reproduced from Huo et al. 2021, with a permission of the publisher)
Schematic representation of phytoremediation strategies
4.2.1 Biochars for Enhancing PTE Mobilization and Phytoextraction
Phytoextraction is a widely applied method for the reclamation of contaminated soils using hyperaccumulator plants to absorb PTEs. Phytoextraction is often used in agricultural soils to lower the concentrations of PTEs to ensure sustainable crop production. Preferably, plant species used for phytoextraction not only accumulate high concentrations of targeted PTEs but also exhibit high biomass yield, high tolerance capacity, resistant to pests and diseases, and are easy to cultivate (Khalid et al. 2017; Yadav et al. 2018). Although hyperaccumulators absorb high PTE concentrations, they are often characterized by slow growth, which may limit their efficiency. If this is the case, then in highly contaminated soils phytoextraction could last for hundreds of years. There is a considerable and ever-increasing bibliography regarding the use of BC for the immobilization of PTEs (El-Naggar et al. 2021). However, there are only few studies available focusing on the effect of BC on the phytoextraction of PTEs (Uchimiya et al. 2011).
PTEs mobility and bioavailability are dependent on the characteristics of both BC and the contaminated soil (Ghosh and Maiti 2021). The addition of BC to soil can affect soil pH and dissolved organic matter. Consequently, its application to soil influences the mobility and phytoextraction of PTEs (Fig. 11). Some studies have shown that in BC-amended soils the mobility of PTEs such as As, Cu, and Sb may increase (Beesley et al. 2010; Uchimiya et al. 2011). The possible mechanism for such mobilization is the electrostatic repulsion between anionic PTEs species and negatively charged BC surfaces, resulting in the desorption of PTEs (Sun et al. 2022). The resulting increase in phyto-available PTEs results in high uptake by plants (Fig. 11). Although most research findings indicate that the addition of BC reduces the bioavailability of PTEs, little is known about the use of BC to phytoremediate PTE-contaminated soils by increasing their bioavailability. Plants require high concentrations of soluble PTEs and any change of the added BC to this effect could accelerate phytoextraction, an approach not widely studied yet.
Potential effects of biochar on phytoextraction of trace element contaminated soils (adapted from Ghosh and Maiti 2021)
BC was indeed found to increase the dissolved concentrations of Cu, Cd, Ni, and Zn, especially the former, under oxic conditions was reported by El-Naggar et al. (2018). In another work, El-Naggar et al. (2019b) found that BC increased the dissolved and colloidal concentrations of As, Co, and Mo under oxidizing conditions. In the same soil, Rinklebe et al. (2020) found that BC mitigated Ag release, but promoted Sb, Sn, and Tl mobilization under a wide range of redox changes. In three studies (El-Naggar et al. 2018, 2019b; Rinklebe et al. 2020), the changes in soil redox potential (EH) affected the release of the studied PTEs, particularly the anions As and Mo. Oxic conditions can stimulate the formation of mobile pentavalent As species such as H2AsVO4− and HAsVO42−, as reported by Takeno (2005). Also, the BC surface functional groups may act as electron donors for PTE reduction reactions. For example, BC may play an important role in reducing TlIII to TlI, SbV to SbIII, and SnIV to SnII. During all these processes, the reduced state is more mobile than the oxidized, and so PTE availability increases.
The changes in soil EH may also cause changes in soil pH, which can affect the solubility of PTEs. For example, the EH-induced decrease in pH of BC-treated soils was found to increase the mobilization of metallic cations such as Cd, Cu, and Zn, while EH-induced increases in pH caused the mobilization of the metal anions As, Mo, and V (Shaheen et al. 2019a, b; El-Naggar et al. 2019b). Soil EH and pH may also affect the BC surface activity, which might decrease the original alkaline sites on BC surfaces and form new acidic functional groups; these may increase the dissolved concentration of the anionic PTEs like As in BC-treated soils. Hence the surface functionality of BC plays a vital role in its effects on PTE mobilization in soils. For example, El-Naggar et al. (2021) concluded that the application of rice-hull BC, which has a low density of oxygen-containing functional groups, increased V solubility and uptake. They also found that a BC with a high density of oxygen-containing functional groups immobilized V and reduced its solubility by 46% and its uptake by corn and sorghum by up to 86% as compared to the control. This can be attributed to the high reactive surface area, the acidity, the abundance of various oxygen-containing functional groups, and the hydrophilicity of the former BC.
4.2.2 Designer/Functionalized BC for Enhancing PTE Mobilization and Phytoextraction
Previous studies have reported the production and use of designer/functionalized (i.e., specialized) BC with superior functions to achieve an improved performance in reducing the mobilization and bioavailability of PTEs in soils (Chen et al. 2021; Sornhiran et al. 2022; Yang et al. 2022b). However, phytoremediation, including phytostabilization, phytoextraction, and phytostimulation (Antoniadis et al. 2017; Zhang et al. 2021), is generally restricted due to the bioavailability of PTEs. On the other hand, designer/functionalized BC could also assist in phytoremediation by enhancing the mobility and bioavailability of PTEs (Wang et al. 2019), being at the same time a source of both carbon and nutrients (Khudzari et al. 2019), improving microbial activity (Natasha et al. 2021), and regulating soil reaction (e.g., pH and redox potential; Yang et al. 2021b).
Several recent studies have proved the potential of designer/functionalized BC for the mobilization of PTEs in soils, as well as the enhancement of their uptake and translocation within plants (Abd El-Mageed et al. 2020; Zhang et al. 2020; Wen et al. 2021). For instance, Abd El-Mageed et al. (2020) reported that the uptake of Cu, Fe, Mn, and Zn by Capsicum annum increased by 6%, 16%, 8%, and 18%, respectively, following the application of a residual sulfur-enhanced citrus wood BC. Wen et al. (2021) conducted a rice cultivation experiment, where they found that the application of an iron-modified green waste BC alleviated As, while elevating Cd and Pb accumulation in rice plants (Oryza sativa L.), under both continuously flooded and alternatively wet and dry water management regimes. This could have been caused by the BC-induced change in soil pH. However, Zhang et al. (2020) reported that tripotassium phosphate-impregnated BC, regardless of feedstocks (i.e., bamboo offcuts, camphorwood chips, cornstalks, and rice husks), immobilized Cd(II) and Cu(II) through precipitation and complexation, while increasing the mobilization of As(V), because of the competition between phosphate and arsenate for the same binding sites. Similarly, through a redox incubation experiment, Yang et al. (2022a) found an increase of As mobilization after the addition of a phosphorus-rich BC derived from pig carcasses, under both strongly reducing and oxidizing conditions. Guo and Li (2019) observed an enhanced uptake of Cr (by 16%) and Pb (by 2%) by Senna occidentalis after the incorporation of an iron-modified coconut husk BC into the soil. It is well-know that PTE accumulators/hyperaccumulators are capable of absorbing higher-than-normal concentrations of PTEs from soils and can readily transport and accumulate those PTEs in their above-ground tissues (Antoniadis et al. 2017). Although many studies have paid attention to the bioremediation of PTE-contaminated soils using hyperaccumulators assisted with pristine BC (Gu et al. 2020; Ghosh and Maiti 2021; Rathika et al. 2021; Zhang et al. 2023), the role of designer/functionalized BC is scarcely studied. A schematic of phytoremediation mechanisms of designer/functionalized BC in PTE-contaminated soils is shown in Fig. 12, as a recommendation for future studies.
4.2.3 Biochars for Enhancing PTE Phytostabilization
Phytostabilization is another process widely used for decelerating the mobility of soil contaminants. Vegetative cover diminishes eolian dispersion and water erosion, while roots prevent leaching and contribute to the immobilization of PTEs (Sun et al. 2022). The mechanisms involved in phytostabilization include precipitation, root sorption, complexation, and metal valence reduction (Shahid 2021). Unlike phytoextraction, phytostabilization primarily focuses on PTE sequestration within the rhizosphere or sometimes in the roots. The low transfer of PTE in the aerial parts can be considered as a major plant defense mechanism that contributes to their phytostabilization efficiency (Ogundiran et al. 2018).
The primary mechanism of stabilization in soil is related to pH: soils with high pH keep PTs relatively immobilized and thus stabilized. However, changes to soil pH in the rhizosphere can potentially affect the effectiveness of BC to immobilize PTEs in soils (Wang et al. 2022). The immobilization of PTEs takes place through multiple ractions of BC with soil PTEs, including precipitation, complexation, electrostatic interaction, and ion exchange. However, some changes in physicochemical soil properties (pH, CEC, water retention) can also lead toward the immobilization of PTEs in soils (Ghosh and Maiti 2021; Natasha et al. 2021). A wide range of different studies have unanimously reported that BC can stabilize contaminants in soils to reduce their bioavailability and thus their concentrations in plants [e.g., (Visconti et al. 2020)].
4.2.4 Biochars for Enhancing PTE Bioremediation
Microbial bioremediation is the processes by which soil microbes can govern the amelioration of PTEs in soils (Tsezos 2009; Ahemad 2019; Huo et al. 2021; Zhang et al. 2023; Fig. 13). Microbes can affect PTEs mobilization in soils via different mechanisms including biosorption, biosynthesis or biodegradation, bioleaching, bioprecipitation, bioaccumulation, and bioassimilation (Fig. 13; Hou et al. 2020). Details about the microbe-PTE interactions can be found in other articles (e.g., Tsezos 2009; Ahemad 2019; Hou et al. 2020; Zhang et al. 2023).
Adding BC to soil strongly influences soil physiochemical and biological properties (Azeem et al. 2023). This is largely because BC is stable under microbial decomposition, which is mainly a consequence of the presence of aromatic carbons in its macromolecular structure (Yang et al. 2022a,b; Tomczyk et al. 2020). Biochar offers a wide range of environmental services, especially with respect to phytoremediation, by directly or indirectly shaping the microbial community structures (Xu et al. 2022a). Stimulations of plant potential primarily take place through changes in the BC-microbe-plant interface (Andrey et al. 2019).
Biochar can influence the microbially mediated phytoremediation in many ways (Fig. 14). A few notable interactions are: (i) enhancing the key soil factors shaping the rhizosphere; (ii) acting as a niche or microhabitat preventing predation; and (iii) impacting chemical signals to accelerate soil decontamination (Zhu et al. 2017). Many of these functional alterations induced by BC probably occur at a micron scale and thus only the immediate vicinity of BC is predominantly influenced (Gundale and DeLuca 2006; Azeem et al. 2023).

(Reproduced from Bandara et al. (2020), with a permission of the publisher)
Proposed immobilization mechanisms of potentially toxic elements (PTEs) by soil microorganisms in biocharamended soil. EPS, Extracellular polymeric substances
The modification of nutrient availability is a prerequisite for alleviating the inhibitions to microbial and plant growth in a highly stressed environment (Olmo et al. 2016; Azeem et al. 2023). Primarily, BC elevates the quantity of bioavailable nutrients, base cations and minerals (metallic and non-metallic alike) by altering soil pH and increasing cation exchange capacity (Hossain et al. 2020). Biochar also makes microbial mobility easier by decreasing the soil bulk density due to increased macroporosity, an effect that also improves soil aeration and water retention (Chang et al. 2021). Other BC components such as minerals, free radicals and volatile organic matters strongly controlmicrobial abundances (Spokas et al. 2011; Paz-Ferreiro et al. 2014).
By enhancing beneficial plant genera, including Pseudomonas, Bacillus, Rhizobia, and Trichoderma, BC stimulates plant performance by enhancing growth hormones, including IAA, ethylene, cytokinin and gibberellins (Jaiswal et al. 2020). This boost helps to alleviate growth restrictions and achieve a higher biomass, all of which are indispensable for phytoremediation (Kocsis et al. 2020). Biochar helps in reversible nutrient retention via sorption, either of cations or inorganic anions, onto its surface functional groups. It can thus act as a slow-release fertilizer in its own right, boosting overall nutrients efficiency (Mukherjee and Zimmerman 2013). By promoting soil fertility as well as by acting as a carbon source, BC also promotes plant growth-promoting rhizobacteria (PGPR) that are crucial for transforming sulfur, phosphorous and other such metallic and non-metallic minerals into phyto-available forms (Wu et al. 2019).
Plant growth-promoting microbes can resist metal pollutants and this reduces PTE availability to plants. This is achieved by various interactions, including secretion of metal-binding proteins (phytochelatins and metallothioneins), chelation, complexation, mobilization, and translocation (Wiangkham and Prapagdee 2018). Plant pathogens generally degrade plant health and reduce plant phytoextraction potential. However, by controlling the hormonal and nutrient equilibrium, PGPR can inhibit attacks by pathogens (Spence and Bais 2015). Biochar may even become toxic toward pathogens, due to the fact that it possesses microbial inhibitors in its structure, such as benzene, phenols, furans, VOCs (volatile organic carbons), and persistent free radicals (Truong et al. 2010). The inhibition is not species-specific, but at higher concentrations, these substances can cause toxic effects on pathogenic microbes.
Due to its porous nature, BC can provide more habitable pore volume per unit volume than soil (Quilliam et al. 2013). The porosity determines its surface activity; macro-pores can act as a habitat accommodating microbes, protecting them from predation, while capillary pores are mostly involved in molecule adsorption and transport (Atkinson et al. 2010). Moreover, BC provides a surface for microbial attachment by promoting niches. Recent studies (e.g., Hill et al. 2019) reported that smooth-surfaced BC may act as a substrate for the formation of microbial biofilm. Smooth surfaces (BC as well as plant roots) and quorum sensing molecules are the prerequisites for such biofilm establishment. Biofilms are dense communities composed of polymeric matrices, mostly of polysaccharides, protein complexes and extracellular DNA (Limoli et al. 2015). They thus offer protection to beneficial microbes against various stresses, toxins and pollutants. Biofilms can act as biosorbents or exopolymeric substances, due to their surfactant and emulsifying properties, to mitigate the toxic effects of metal ions (Ugya et al. 2021). Such natural transformation and/or protein interaction of BC is responsible for changes in the bioavailability of metal ions to plants, enhancing their phytoremediation potential (Sharma 2021).
The rhizosphere accommodates numerous interactions, secretions and signaling between plant roots and microbial biota (Li et al. 2022a, b, c). Biochar, by adsorbing the inhibitory phenolic contents of soil, promotes the beneficial microbial biomass in the rhizosphere, which may then actively take part in metallic mineral solubilization (Liu et al. 2022). The sorptive capacity of BC could act as signaling interference in the rhizosphere and could serve as a signal reservoir or sink. Due to the sorption capacity of BC, it may also inhibit certain components, including allelochemicals (Oni et al. 2019). For instance, Akiyama et al. (2005) demonstrated the ability of BC to adsorb and desorb strigolactone, a signaling compound that promotes arbuscular mycorrhizal colonization in plant roots. The adsorbed signaling molecules can thus act as a secondary source, which can easily be desorbed back into solution, making them available to stimulate microbe-plant interactions (Ding et al. 2016).
Biochar is also recognized for adsorbing and protecting chemical signaling molecules that are derived from plants, such as the nod factor; this is responsible for enhancing root nodulation by rhizhobia, a major determining factor for enhancing plant biomass (Thies and Rillig 2012). Biochar can alter signaling-dependent protein expression and can also modify microbe-plant interactions especially in the rhizosphere, diminishing pathogen attacks as well as triggering systemic plant defenses, including both SAR (systemic acquired resistance) and ISR (induced systemic resistance) systems (Harel et al. 2012). The SAR is related to the pathogenic protein-mediated salicylic response and the ISR occurs through plant beneficial microbial colonization (Elad et al. 2010). Apart from inducing systemic plant defense mechanisms, BC has priming effects on gene expression in plants once they become infected (Jaiswal et al. 2020). BC also promotes the colonization of arbuscular mycorrhizal fungi, some of which are known to be involved in interplant signaling in pathogen resistance through interconnected hyphal networks (Johnson and Gilbert 2015). By conferring resistance to plant pathogens and enhancing microbial abundance, BC stimulates biomass as well as the microbial-assisted metal uptake by plants (Zhang et al. 2017).
5 Conclusions
The pros and cons of the addition of BC to PTEs contaminated soils are reviewed in this article. In particular, BC-induced changes on the mobilization, phytoextraction, phytostabilization, and bioremediation of PTEs in contaminated soils have been discussed and summarized. We conclude that addition of BC to metal(loid)s polluted soils affect both their mobilization and phytoavailability, which mainly depends on the type and properties of the elements, soils, and feedstocks. The chemical and surface properties of BC strongly control their ability to (im)mobilize PTEs in soils. The addition alkaline BC to contaminated acidic soils can increase soil pH, and thus may increase the sorption and immobilization of metal cations (e.g., Cd, Cu, Zn); however, it may increase the mobilization of metal anions such as As, V, and Cr. The associated increase of soil alkalinity after addition of alkaline BC to soils can cause a competition between OH− and HAsO42− and thus increase As release and mobilization. The BC-induced increase of dissolved organic carbon may increase the mobilization of some PTEs including Cu, and the redox-mediated interactions between BC and PTEs greatly impact their mobilization. Biochar can donate or accept electrons via functional groups and thus can reduce or oxidize the soil environment, which impacts the reduction or oxidation of redox sensate PTEs such as As and Cr, changing their speciation and mobilization potential. Biochar may enhance the PTEs mobilization and thus increase phytoremediation efficiency. The BC induced enhances in roots growth may increase the roots ability for PTEs absorption (rhizoremediation). Addition of BC can also enhance the PTEs translocation from the roots to the above-ground biomass, where they can be lost by transpiration (phytovolatilization) or be removed from the field by harvesting the plant. Moreover, BC-induced changes on the microbial community and activity can affect the mobilization and bioremediation of PTEs in polluted soils. In turn, increasing the mobilization of PTEs using BC can enhance phytoextraction of these PTEs in soils and shorten the phytoremediation period, which is recommended from agro-environmental and economic points of view. This review demonstrates the potentiality of using BC as a promising effective material for the green remediation of PTEs contaminated soils. However, more attention is needed to verify the potentiality of engineered/designer BC as a mobilizing agent for enhancing the phytoavailability and phytoextraction of PTEs in soils. Also, more future research is needed to investigate the interactions between PTEs with BC using advanced spectroscopic techniques.
Data Availability
Not applicable.
Abbreviations
- PTE:
-
Potentially toxic elements
- BC:
-
Biochar
- UN:
-
United Nation
- FAO:
-
Food and Agriculture Organization
- EDTA:
-
Ethylenediamine- tetra-acetic acid
- CEC:
-
Cation Exchange Capacity
- SAR:
-
Systemic acquired resistance
- ISR:
-
Induced systemic resistance
References
Abd El-Mageed TA, Rady MM, Taha RS, Abd El Azeam S, Simpson CR, Semida WM (2020) Effects of integrated use of residual sulfur-enhanced biochar with effective microorganisms on soil properties, plant growth and short-term productivity of Capsicum annuum under salt stress. Sci Hortic 261:108930
Ahemad M (2019) Remediation of metalliferous soils through the heavy metal resistant plant growth promoting bacteria: paradigms and prospects. Arab J Chem 12:1365–1377
Ahmad Z, Mosa A, Zhan L, Gao B (2021) Biochar modulates mineral nitrogen dynamics in soil and terrestrial ecosystems: a critical review. Chemosphere 278:130378. https://doi.org/10.1016/j.chemosphere.2021.130378
Akiyama KK, Matsuzaki K, Hayashi H (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435(7043):824–827
Ali A, Guo D, Jeyasundar PGSA, Li Y, Xiao R, Du J, Li R, Zhang Z (2019) Application of wood biochar in polluted soils stabilized the toxic metals and enhanced wheat (Triticum aestivum) growth and soil enzymatic activity. Ecotoxicol Environ Saf 184:109635. https://doi.org/10.1016/j.ecoenv.2019.109635
Ali A, Shaheen SM, Guo D et al (2020a) Apricot shell- and apple tree-derived biochar affect the fractionation and bioavailability of Zn and Cd as well as the microbial activity in smelter contaminated soil. Environ Pollut 264:114773
Ali W, Mao K, Zhang H, Junaid M (2020b) Comprehensive review of the basic chemical behaviours, sources, processes, and endpoints of trace element contamination in paddy soil-rice systems in rice-growing countries. J Hazard Mater 397:122720
Ambika S, Kumar M, Pisharody L, Malhotra M, Kumar G, Sreedharan V, Singh L, Nidheesh PV, Bhatnagar A (2022) Modified biochar as a green adsorbent for removal of hexavalent chromium from various environmental matrices: mechanisms, methods, and prospects. Chem Eng J 439:135716. https://doi.org/10.1016/j.cej.2022.135716
Amin H, Arain BA, Abbasi MS, Jahangir TM, Amin F (2018) Potential for phytoextraction of Cu by Sesamum indicum L. and Cyamopsis tetragonoloba L.: a green solution to decontaminate soil. Earth Syst Environ 2:133–143
Andrey G, Rajput V, Tatiana M, Saglara M, Svetlana S, Igor K, Grigoryeva TV, Vasily C, Iraida A, Vladislav Z (2019) The role of biochar-microbe interaction in alleviating heavy metal toxicity in Hordeum vulgare L. grown in highly polluted soils. Appl Geochem 104:93–101
Antoniadis V, Levizou E, Shaheen SM, Ok YS, Sebastian A, Baum C, Prasad MNV, Wenzel WW, Rinklebe J (2017) Trace elements in the soil-plant interface: phytoavailability, translocation, and phytoremediation–a review. Earth-Sci Rev 171:621–645
Antoniadis V, Shaheen SM, Stärk HJ, Wennrich R, Levizou E, Merbach I, Rinklebe J (2021) Phytoremediation potential of twelve wild plant species for toxic elements in a contaminated soil. Environ Int. https://doi.org/10.1016/j.envint.2020.106233
Antoniadis V, Thalassinos G, Levizou E, Wang J et al (2022) Hazardous enrichment of toxic elements in soils and olives in the urban zone of Lavrio, Greece, a legacy, millennia-old silver/lead mining area and related health risk assessment. J Hazard Mater 434:128906
Atkinson CJ, Fitzgerald JD, Hipps NA (2010) Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant Soil 337(1):1–18
Azeem M, Jeyasundar PGSA, Ali A, Riaz L et al (2023) Cow bone-derived biochar enhances microbial biomass and alters bacterial community composition and diversity in a smelter contaminated soil. Environ Res 216(Part 1):114278
Bandara T, Franks A, Xu J et al (2020) Chemical and biological immobilization mechanisms of potentially toxic elements in biochar-amended soils. Crit Rev Env Sci Tec 50(9):903–978
Bashir S, Hussain Q, Shaaban M, Hu H (2018) Efficiency and surface characterization of different plant derived biochar for cadmium (Cd) mobility, bioaccessibility and bioavailability to Chinese cabbage in highly contaminated soil. Chemosphere 211:632–639
Bashir S, Hussain Q, Zhu J, Fu Q, Houben D, Hu H (2020) Efficiency of KOH-modified rice straw-derived biochar for reducing cadmium mobility, bioaccessibility and bioavailability risk index in red soil. Pedosphere 30:874–882. https://doi.org/10.1016/S1002-0160(20)60043-1
Beesley L, Moreno-Jiménez E, Gomez-Eyles JL (2010) Effects of biochar and greenwaste compost amendments on mobility, bioavailability and toxicity of inorganic and organic contaminants in a multi-element polluted soil. Environ Pollut 158(6):2282–2287
Bolan N, Kunhikrishnan A, Thangarajan R, Kumpiene J (2014) Remediation of heavy metal(loid)s contaminated soils—to mobilize or to immobilize? J Hazard Mater 266:141–166
Bolan N, Hoang SA, Beiyuan J, Gupta S, Hou D, Karakoti A, Joseph S, Jung S, Kim K-H, Kirkham MB, Kua HW, Kumar M, Kwon EE, Ok YS, Perera V, Rinklebe J, Shaheen SM, Sarkar B, Sarmah AK, Singh BP, Singh G, Tsang DCW, Vikrant K, Vithanage M, Vinu A, Wang H, Wijesekara H, Yan Y, Younis SA, van Zwieten L (2022) Multifunctional applications of biochar beyond carbon storage. Int Mater Rev 67(2):150–200. https://doi.org/10.1080/09506608.2021.1922047
Břendová K, Tlustoš P, Száková J (2015) Biochar immobilizes cadmium and zinc and improves phytoextraction potential of willow plants on extremely contaminated soil. Plant Soil Environ 61:303–308
Chang Y, Rossi L, Zotarelli L, Gao B, Shahid MA, Sarkhosh A (2021) Biochar improves soil physical characteristics and strengthens root architecture in Muscadine grape (Vitis rotundifolia L.). Chem Biol Technol Agric 8(1):7
Chao X, Qian X, Han-hua Z, Shuai W, Qi-hong Z, Dao-you H, Yang-zhu Z (2018) Effect of biochar from peanut shell on speciation and availability of lead and zinc in an acidic paddy soil. Ecotoxicol Environ Saf 164:554–561. https://doi.org/10.1016/j.ecoenv.2018.08.057
Chen H, Gao Y, El-Naggar A, Niazi NK, Sun C, Shaheen SM, Hou D, Yang X, Tang Z, Liu Z, Hou H, Chen W, Rinklebe J, Pohorely M, Wang H (2021) Enhanced sorption of trivalent antimony by chitosan-loaded biochar in aqueous solutions: characterization, performance and mechanisms. J Hazard Mater 425:127971
Chen D, Liu W, Wang Y, Lu P (2022) Effect of biochar aging on the adsorption and stabilization of Pb in soil. J Soils Sediments 22:56–66. https://doi.org/10.1007/s11368-021-03059-x
Chuaphasuk C, Prapagdee B (2019) Effects of biochar-immobilized bacteria on phytoremediation of cadmium-polluted soil. Environ Sci Pollut Res 26:23679–23688. https://doi.org/10.1007/s11356-019-05661-6
Cui H, Wang Q, Zhang X, Zhang S, Zhou J, Zhou D, Zhou J (2021) Aging reduces the bioavailability of copper and cadmium in soil immobilized by biochars with various concentrations of endogenous metals. Sci Total Environ 797:149136
Cui H, Dong T, Hu L, Xia R, Zhou J, Zhou J (2022) Adsorption and immobilization of soil lead by two phosphate-based biochars and phosphorus release risk assessment. Sci Total Environ 824:153957. https://doi.org/10.1016/j.scitotenv.2022.153957
Dastyar W, Raheem A, He J, Zhao M (2019) Biofuel production using thermochemical conversion of heavy metal-contaminated biomass (HMCB) harvested from phytoextraction process. Chem Eng J 358:759–785
Ding Y, Liu Y, Liu S, Li Z, Tan X, Huang X, Zeng G, Zhou L, Zheng B (2016) Biochar to improve soil fertility. A review. Agron Sustain Dev 36(2):36
Drzewiecka K, Gąsecka M, Magdziak Z, Budzyńska S, Szostek M, Niedzielski P, Budka A, Roszyk E, Doczekalska B, Górska M (2021) The possibility of using Paulownia elongata SY Hu× Paulownia fortunei hybrid for phytoextraction of toxic elements from post-industrial wastes with biochar. Plants 10:2049
Eissa MA (2019) Effect of compost and biochar on heavy metals phytostabilization by the halophytic plant old man saltbush [Atriplex Nummularia Lindl]. Soil Sediment Contam Int J 28:135–147. https://doi.org/10.1080/15320383.2018.1551325
Ekoa Bessa AZ, Ngueutchoua G, Kwewouo Janpou A et al (2021) Heavy metal contamination and its ecological risks in the beach sediments along the Atlantic Ocean (Limbe coastal fringes, Cameroon). Earth Syst Environ 5:433–444. https://doi.org/10.1007/s41748-020-00167-5
Elad Y, David DR, Harel YM, Borenshtein M, Kalifa HB, Silber A, Graber ER (2010) Induction of systemic resistance in plants by biochar, a soil-applied carbon sequestering agent. Phytopathology® 100(9):913–921
ELD (2015) Report for policy and decision makers: Reaping Economic and Environmental Benefits from Sustainable Land Management. Economics of Land Degradation (ELD) Initiative, Bonn, Germany
El-Naggar A, Shaheen SM, Ok YS, Rinklebe J (2018) Biochar affects the dissolved and colloidal concentrations of Cd, Cu, Ni, and Zn and their phytoavailability and potential mobility in a mining soil under dynamic redox-conditions. Sci Total Environ 624:1059–1071
El-Naggar A, Shaheen SM, Hseu Z-Y, Wang S-L, Ok YS, Rinklebe J (2019a) Release dynamics of As Co, and Mo in a biochar treated soil under pre-defined redox conditions. Sci Total Environ 657:686–695
El-Naggar A, Lee SS, Rinklebe J, Muhammad Farooq M et al (2019b) Biochar application to low fertility soils: a review of current status, and future prospects. Geoderma 337:536–554
El-Naggar A, Shaheen SM, Chang SX, Hou D, Ok YS, Rinklebe J (2021) Biochar surface functionality plays a vital role in (Im)mobilization and phytoavailability of soil vanadium. ACS Sustainable Chem Eng 9:6864–6874
El-Naggar A, Mosa A, Ahmed N, Niazi NK, Yousaf B, Sarkar B, Rinklebe J, Cai Y, Chang SX (2022) Modified and pristine biochars for remediation of chromium contamination in soil and aquatic systems. Chemosphere. https://doi.org/10.1016/j.chemosphere.2022.134942
Fahmi AH, Samsuri AW, Jol H, Singh D (2018) Bioavailability and leaching of Cd and Pb from contaminated soil amended with different sizes of biochar. R Soc Open Sci 5:181328
Fan J, Cai C, Chi H, Reid BJ, Coulon F, Zhang Y, Hou Y (2020) Remediation of cadmium and lead polluted soil using thiol-modified biochar. J Hazard Mater 388:122037. https://doi.org/10.1016/j.jhazmat.2020.122037
FAO, IFAD, UNICEF, WFP, WHO (2017) The State of Food Security and Nutrition in the World 2017. Building Resilience for Peace and Food Security. FAO, Rome.
Favas PJC, Pratas J, Varun M, D’Souza R, Paul MS (2014). Phytoremediation of Soil Contaminated with Metals and Metalloids at Mining Areas: Potential of Native Flora. In: Environmental Risk Assessment of Soil Contamination (ed. Hernandez Soriano, M.C.). PP 485–518. DOI: https://doi.org/10.5772/57469
Gascó G, Álvarez ML, Paz-Ferreiro J, Méndez A (2019) Combining phytoextraction by Brassica napus and biochar amendment for the remediation of a mining soil in Riotinto (Spain). Chemosphere 231:562–570. https://doi.org/10.1016/j.chemosphere.2019.05.168
Gholami L, Rahimi G (2021) Efficiency of CH4N2S−modified biochar derived from potato peel on the adsorption and fractionation of cadmium, zinc and copper in contaminated acidic soil. Environ Nanotechnol Monit Manag 16:100468. https://doi.org/10.1016/j.enmm.2021.100468
Ghosh D, Maiti SK (2021) Biochar assisted phytoremediation and biomass disposal in heavy metal contaminated mine soils: a review. Int J Phytoremediation 23:559–576
Gong H, Zhao L, Rui X, Hu J, Zhu N (2022) A review of pristine and modified biochar immobilizing typical heavy metals in soil: applications and challenges. J Hazard Mater 432:128668. https://doi.org/10.1016/j.jhazmat.2022.128668
Gu P, Zhang Y, Xie H, Wei J, Zhang X, Huang X, Wang J, Lou X (2020) Effect of cornstalk biochar on phytoremediation of Cd-contaminated soil by Beta vulgaris var. cicla L. Ecotoxicol Environ Saf 205:111144
Gundale MJ, DeLuca TH (2006) Temperature and source material influence ecological attributes of ponderosa pine and Douglas-fir charcoal. For Ecol Manag 231(1–3):86–93
Guo X, Li H (2019) Effects of iron-modified biochar and AMF inoculation on the growth and heavy metal uptake of senna occidentalis in heavy metal-contaminated soil. Pol J Environ Stud 28:2611–2621
Harel M, Elad Y, Rav-David D, Borenstein M, Shulchani R, Lew B, Graber ER (2012) Biochar mediates systemic response of strawberry to foliar fungal pathogens. Plant Soil 357(1):245–257
Hill RA, Hunt J, Sanders E, Tran M, Burk GA, Mlsna TE, Fitzkee NC (2019) Effect of biochar on microbial growth: a metabolomics and bacteriological investigation in E. coli. Environ Sci Technol 53(5):2635–2646
Hossain MZ, Bahar MM, Sarkar B, Donne SW, Ok YS, Palansooriya KN, Kirkham MB, Chowdhury S, Bolan N (2020) Biochar and its importance on nutrient dynamics in soil and plant. Biochar 2(4):379–420
Hou D, O’Connor D, Igalavithana AD et al (2020) Metal contamination and bioremediation of agricultural soils for food safety and sustainability. Nat Rev Earth Environ 1:366–381. https://doi.org/10.1038/s43017-020-0061-y
Hunter M, Smith RG, Schipanski M, Atwood LW, Mortensen DA (2017) Agriculture in 2050: recalibrating targets for sustainable intensification. Bioscience 67:386–391
Ickowitz A, Powellb B, Rowland D, Jones A, Sunderland T (2019) Agricultural intensification, dietary diversity, and markets in the global food security narrative. Glob Food Sec 20:9–16
Isinkaye OM (2018) Distribution and multivariate pollution risks assessment of heavy metals and natural radionuclides around abandoned iron-ore mines in north central nigeria. Earth Syst Environ 2:331–343
Jaiswal AK, Alkan N, Elad Y, Sela N, Philosoph AM, Graber ER, Frenkel O (2020) Molecular insights into biochar-mediated plant growth promotion and systemic resistance in tomato against Fusarium crown and root rot disease. Sci Rep 10(1):13934
Jeyasundar PGSA, Ali A, Azeem M, Li Y, Guo D, Sikdar A, Abdelrahman H, Kwon E, Antoniadis V, Mani VM, Shaheen SM, Rinklebe J, Zhang Z (2021) Green remediation of toxic metals contaminated mining soil using bacterial consortium and Brassica juncea. Environ Pollut 277:116789
Ji X, Wan J, Wang X, Peng C, Wang G, Liang W, Zhang W (2022) Mixed bacteria-loaded biochar for the immobilization of arsenic, lead, and cadmium in a polluted soil system: effects and mechanisms. Sci Total Environ 811:152112. https://doi.org/10.1016/j.scitotenv.2021.152112
Johnson D, Gilbert L (2015) Interplant signalling through hyphal networks. New Phytol 205(4):1448–1453
Khalid S, Shahid M, Niazi NK, Murtaza B, Bibi I, Dumat C (2017) A comparison of technologies for remediation of heavy metal contaminated soils. J Geochem Explor 182:247–268. https://doi.org/10.1016/j.gexplo.2016.11.021
Khan AZ, Khan S, Ayaz T, Brusseau ML, Khan MA, Nawab J, Muhammad S (2020) Popular wood and sugarcane bagasse biochars reduced uptake of chromium and lead by lettuce from mine-contaminated soil. Environ Pollut 263:114446
Khan S, Naushad M, Lima EC, Zhang SX, Shaheen SM, Rinklebe J (2021) Global soil pollution by toxic elements: current status and future perspective on the risk assessment and remediation strategies—a review. J Hazard Mater 417:126039
Khudzari Md, Gariépy J, Kurian Y, Tartakovsky J, Raghavan B, Raghavan GSV (2019) Effects of biochar anodes in rice plant microbial fuel cells on the production of bioelectricity, biomass, and methane. Biochem Eng J 141:190–199
Kocsis T, Kotroczó Z, Kardos L, Biró B (2020) Optimization of increasing biochar doses with soil–plant–microbial functioning and nutrient uptake of maize. Environ Technol Innov 20:101191
Kong S, Tang J, Ouyang F, Chen M (2021) Research on the treatment of heavy metal pollution in urban soil based on biochar technology. Environ Technol Innov 23:101670. https://doi.org/10.1016/j.eti.2021.101670
Kopittke PM, Menzies NWW, P, McKenna, BA, Lombi E, (2019) Soil and the intensification of agriculture for global food security- Review article. Environ Int 132:105078
Kowitwiwat A, Sampanpanish P (2020) Phytostabilization of arsenic and manganese in mine tailings using Pennisetum purpureum cv. Mott supplemented with cow manure and acacia wood-derived biochar. Heliyon 6:04552. https://doi.org/10.1016/j.heliyon.2020.e04552
Kumar M, Bolan N, Jasemizad T, Padhye LP, Sridharan S, Singh L, Bolan S, O'Connor J, Zhao H, Shaheen SM, Song H, Siddique KHM, Wang H, Kirkham MB, Rinklebe J (2022) Mobilization of contaminants: Potential for soil remediation and unintended consequences. Sci Total Environ 839:156373. https://doi.org/10.1016/j.scitotenv.2022.156373
Lebrun M, Miard F, Nandillon R, Hattab-Hambli N, Scippa GS, Bourgerie S, Morabito D (2018a) Eco-restoration of a mine technosol according to biochar particle size and dose application: study of soil physico-chemical properties and phytostabilization capacities of Salix viminalis. J Soils Sediments 18:2188–2202. https://doi.org/10.1007/s11368-017-1763-8
Lebrun M, Miard F, Nandillon R, Léger J-C, Hattab-Hambli N, Scippa GS, Bourgerie S, Morabito D (2018b) Assisted phytostabilization of a multicontaminated mine technosol using biochar amendment: early stage evaluation of biochar feedstock and particle size effects on As and Pb accumulation of two Salicaceae species (Salix viminalis and Populus euramericana). Chemosphere 194:316–326. https://doi.org/10.1016/j.chemosphere.2017.11.113
Lehmann J (2007) A handful of carbon. Nature 447:143–144. https://doi.org/10.1038/447143a
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota—a review. Soil Biol Biochem 43:1812–1836
Li X, Zhang X, Wang X, Cui Z (2019) Phytoremediation of multi-metal contaminated mine tailings with Solanum nigrum L. and biochar/attapulgite amendments. Ecotoxicol Environ Saf 180:517–525. https://doi.org/10.1016/j.ecoenv.2019.05.033
Li Y-Y, Zhang T-T, Ning Z, Chen J-H (2020) Characteristics and applications of sewage sludge biochar modified by ferrous sulfate for remediating cr (vi)-contaminated soils. Adv Civil Eng. https://doi.org/10.1155/2020/6521638
Li H, Li Z, Xie S, Huang Y, Chen M, Xie T, Wang G (2022) Accumulation and distribution of zinc in rice plants (Oryza sativa L.) growing in zinc contaminated paddy soils with biochar. J Environ Chem Eng 10:106811. https://doi.org/10.1016/j.jece.2021.106811
Li Y, Pei G, Zhu Y, Liu W, Li H (2022b) Vinegar residue biochar: a possible conditioner for the safe remediation of alkaline Pb-contaminated soil. Chemosphere 293:133555. https://doi.org/10.1016/j.chemosphere.2022.133555
Li W, Li Y, Lv J, He X, Wang J, Teng D, Jiang L, Wang H, Lv G (2022c) Rhizosphere effect alters the soil microbiome composition and C, N transformation in an arid ecosystem. Appl Soil Ecol 170:104296
Limoli DH, Jones CJ, Wozniak DJ (2015) Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol Spectr. https://doi.org/10.1128/microbiolspec.MB-0011-2014
Liu M, Sun F, Lv Y, Xu Y, Li M, Wang Y, Yin X, Jiang H (2021) Remediation of arsenic-contaminated soil by nano-zirconia modified biochar. Environ Sci Pollut Res 28:68792–68803. https://doi.org/10.1007/s11356-021-15362-8
Liu M, Almatrafi E, Zhang Y, Xu P, Song B, Zhou C, Zeng G, Zhu Y (2022) A critical review of biochar-based materials for the remediation of heavy metal contaminated environment: applications and practical evaluations. Sci Total Environ 806:150531
Lu H, Li Z, Fu S, Méndez A, Gascó G, Paz-Ferreiro J (2014) Can biochar and phytoextractors be jointly used for cadmium remediation? PLoS One 9:e95218. https://doi.org/10.1371/journal.pone.0095218
Lu H, Li Z, Fu S, Méndez A, Gascó G, Paz-Ferreiro J (2015) Combining phytoextraction and biochar addition improves soil biochemical properties in a soil contaminated with Cd. Chemosphere 119:209–216. https://doi.org/10.1016/j.chemosphere.2014.06.024
Luo Y, Zhao L, Li Z, Xu X, Xu H, Qiu H, Cao X (2022) Development of phosphorus composite biochar for simultaneous enhanced carbon sink and heavy metal immobilization in soil. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2022.154845
Moradi N, Rasouli-Sadaghiani M, Sepehr E (2021) Biochar application improves lettuce (Lactuca sativa L.) growth in a lead-contaminated calcareous soil. Arab J Geosci 14:1642. https://doi.org/10.1007/s12517-021-07982-8
Mosa A, Taha AA, Elsaeid M (2021) In-situ and ex-situ remediation of potentially toxic elements by humic acid extracted from different feedstocks: experimental observations on a contaminated soil subjected to long-term irrigation with sewage effluents. Environ Technol Innov 23:101599
Mosa A, Selim E-MM, El-Kadi SM, Khedr AA, Elnaggar AA, Hefny WA, Abdelhamid AS, El Kenawy AM, El-Naggar A, Wang H (2022) Ecotoxicological assessment of toxic elements contamination in mangrove ecosystem along the Red Sea coast. Egypt Marine Pollution Bulletin 176:113446
Mukherjee A, Zimmerman AR (2013) Organic carbon and nutrient release from a range of laboratory-produced biochars and biochar–soil mixtures. Geoderma 193:122–130
Murad HA, Ahmad M, Bundschuh J, Hashimoto Y, Zhang M, Sarkar B, Ok YS (2022) A remediation approach to chromium-contaminated water and soil using engineered biochar derived from peanut shell. Environ Res 204:112125. https://doi.org/10.1016/j.envres.2021.112125
Natasha N, Shahid M, Khalid S, Bibi I, Naeem MA, Niazi NK, Tack FMG, Ippolito JA, Rinklebe J (2021) Influence of biochar on trace element uptake, toxicity and detoxification in plants and associated health risks: a critical review. Crit Rev Environ Sci Technol. https://doi.org/10.1080/10643389.2021.1894064
Nedjimi B (2021) Phytoremediation: a sustainable environmental technology for heavy metals decontamination. SN Appl Sci 3(3):286
Ogundiran MB, Mekwunyei NS, Adejumo SA (2018) Compost and biochar assisted phytoremediation potentials of Moringa oleifera for remediation of lead contaminated soil. J Environ Chem Eng 6(2):2206–2213. https://doi.org/10.1016/j.jece.2018.03.025
Olmo M, Villar R, Salazar P, Alburquerque JA (2016) Changes in soil nutrient availability explain biochar’s impact on wheat root development. Plant Soil 399(1):333–343
Oni BA, Oziegbe O, Olawole OO (2019) Significance of biochar application to the environment and economy. Ann Agric Sci 64(2):222–236
Palansooriya KN, Shaheen SM, Chen SS, Tsang DCW, Hashimoto Y, Hou D, Bolan NS, Rinklebe J, Ok YS (2020) Soil amendments for immobilization of potentially toxic elements in contaminated soils: a critical review. Environ Int 134:105046. https://doi.org/10.1016/j.envint.2019.105046
Panahi HKS, Dehhaghi M, Ok YS, Nizami A-S, Khoshnevisan B, Mussatto SI, Aghbashlo M, Tabatabaei M, Lam SS (2020) A comprehensive review of engineered biochar: production, characteristics, and environmental applications. J Clean Prod 270:122462
Paz-Ferreiro J, Lu H, Fu S, Mendez A, Gasco G (2014) Use of phytoremediation and biochar to remediate heavy metal polluted soils: a review. Solid Earth 5(1):65–75
Pračke K, Száková J, Tlustoš P (2021) Biochar applications enhance the phytoextraction potential of Salix smithiana [Willd.] (willow) in heavily contaminated soil: potential for a sustainable remediation method? J Soils Sediments. https://doi.org/10.1007/s11368-021-03104-9
Qi X, Yin H, Zhu M, Yu X, Shao P, Dang Z (2022) MgO-loaded nitrogen and phosphorus self-doped biochar: High-efficient adsorption of aquatic Cu2+, Cd2+, and Pb2+ and its remediation efficiency on heavy metal contaminated soil. Chemosphere 294:133733
Qu J, Dong M, Bi F, Tao Y, Wang L, Jiang Z, Zhang G, Zhang B, Zhang Y (2022) Microwave-assisted one-pot synthesis of β-cyclodextrin modified biochar for stabilization of Cd and Pb in soil. J Clean Prod 346:131165. https://doi.org/10.1016/j.jclepro.2022.131165
Quilliam RS, Glanville HC, Wade SC, Jones DL (2013) Life in the ‘charosphere’—does biochar in agricultural soil provide a significant habitat for microorganisms? Soil Biol Biochem 65:287–293
Rafique MI, Usman ARA, Ahmad M, Al-Wabel MI (2021) Immobilization and mitigation of chromium toxicity in aqueous solutions and tannery waste-contaminated soil using biochar and polymer-modified biochar. Chemosphere 266:129198
Rajendran M, Shi L, Wu C, Li W, An W, Liu Z, Xue S (2019) Effect of sulfur and sulfur-iron modified biochar on cadmium availability and transfer in the soil–rice system. Chemosphere 222:314–322. https://doi.org/10.1016/j.chemosphere.2019.01.149
Rathika R, Srinivasan P, Alkahtani J, Al-Humaid LA, Alwahibi MS, Mythili R, Selvankumar T (2021) Influence of biochar and EDTA on enhanced phytoremediation of lead contaminated soil by Brassica juncea. Chemosphere 271:129513
Rathnayake D, Rego F, Van Poucke R, Bridgwater AV, Mašek O, Meers E, Wang J, Yang Y, Ronsse F (2021) Chemical stabilization of Cd-contaminated soil using fresh and aged wheat straw biochar. Environ Sci Pollut Res 28:10155–10166. https://doi.org/10.1007/s11356-020-11574-6
Rees F, Sterckeman T, Morel JL (2020) Biochar-assisted phytoextraction of Cd and Zn by Noccaea caerulescens on a contaminated soil: a four-year lysimeter study. Sci Total Environ 707:135654
Ren J, Zhao Z, Ali A, Guan W, Xiao R, Wang JJ, Ma S, Guo D, Zhou B, Zhang Z, Li R (2020) Characterization of phosphorus engineered biochar and its impact on immobilization of Cd and Pb from smelting contaminated soils. J Soils Sediments 20:3041–3052. https://doi.org/10.1007/s11368-019-02403-6
Rinklebe J, Shaheen SM (2017) Geochemical distribution of Co, Cu, Ni, and Zn in soil profiles of Fluvisols, Luvisols, Gleysols, and Calcisols originating from Germany and Egypt. Geoderma 307:122–138
Rinklebe J, Shaheen SM, El-Naggar A, Wang H, Laing GD, Alessi DS, Ok YS (2020) Redox-induced mobilization of Ag, Sb, Sn, and Tl in the dissolved, colloidal and solid phase of a biochar-treated and un-treated mining soil. Enviro Int 140:105754
Rue M, Rees F, Simonnot M-O, Morel JL (2019) Phytoextraction of Ni from a toxic industrial sludge amended with biochar. J Geochem Explor 196:173–181. https://doi.org/10.1016/j.gexplo.2018.10.007
Salam A, Shaheen SM, Bashir S et al (2019) Rice straw- and rapeseed residue-derived biochars affect the geochemical fractions and phytoavailability of Cu and Pb to maize in a contaminated soil under different moisture content. J Environ Manag 237:5–14
Sarwar N, Imran M, Shaheen MR, Ishaque W, Kamran MA, Matloob A, Rehim A, Hussain S (2017) Phytoremediation strategies for soils contaminated with heavy metals: modifications and future perspectives. Chemosphere 171:710–721
Shaheen SM, Rinklebe J (2015) Impact of emerging and low cost alternative amendments on the (im) mobilization and phytoavailability of Cd and Pb in a contaminated floodplain soil. Ecol Eng 74:319–326
Shaheen SM, Tsadilas CD, Rinklebe J (2013) A review of the distribution coefficient of trace elements in soils: influence of sorption system, element characteristics, and soil colloidal properties. Adv Colloid Interface Sci 201–202:43–56
Shaheen SM, El-Naggar A, Wang J, Hassan NE, Niazi NK, Wang H, Tsang DC, Ok YS, Bolan N, Rinklebe J (2019) Biochar as an (Im) mobilizing agent for the potentially toxic elements in contaminated soils. Biochar from biomass and waste. Elsevier, pp 255–274. https://doi.org/10.1016/B978-0-12-811729-3.00014-5
Shaheen SM, Niazi NK, Hassan NEE, Bibi I, Wang H, Tsang DC, Ok YS, Rinklebe J (2019b) Wood-based biochar for removal of potentially toxic elements in water and wastewater: a critical review. Int Mater Rev 64(4):216–247
Shaheen SM, Natasha MA, El-Naggar A, Hossain MF, Abdelrahman H, Niazi NK, Shahid M, Zhang T, Fai Tsang Y, Trakal L, Wang S, Rinklebe J (2022a) Manganese oxide-modified biochar: production, characterization and applications for the removal of pollutants from aqueous environments—a review. Biores Technol 346:126581. https://doi.org/10.1016/j.biortech.2021.126581
Shaheen SM, Mosa A, Natasha, Abdelrahma H, Niazi NK, Antoniadis V, Shahid M, Song H, Kwon EE, Rinklebe J (2022) Removal of toxic elements from aqueous environments using nano zero-valent iron- and iron oxide-modified biochar: a review. Biochar. https://doi.org/10.1007/s42773-022-00149-y
Shaheen SM, Antoniadis V, Shahid M, Yang Y, Abdelrahman H, Zhang T, Hassan NE, Bibi I, Niazi NK, Younis SA, Almazroui M, Tsang Y, Sarmah A, Kim K-H, Rinklebe J (2022c) Sustainable applications of rice feedstock in agro-environmental and construction sectors: a global perspective. Renew Sustain Energy Rev 153:111791. https://doi.org/10.1016/j.rser.2021.111791
Shahid M (2021) Effect of soil amendments on trace element-mediated oxidative stress in plants: meta-analysis and mechanistic interpretations. J Hazard Mater 407:124881
Sharma P (2021) Efficiency of bacteria and bacterial assisted phytoremediation of heavy metals: an update. Biores Technol 328:124835
Shen Z, Zhang J, Hou D, Tsang DCW, Ok YS, Alessi DS (2019) Synthesis of MgO-coated corncob biochar and its application in lead stabilization in a soil washing residue. Environ Int 122:357–362
Sigua GC, Novak JM, Watts DW, Ippolito JA, Ducey TF, Johnson MG, Spokas KA (2019) Phytostabilization of Zn and Cd in mine soil using corn in combination with biochars and manure-based compost. Environments 6:69
Singh S, Gupta VK (2016) Biodegradation and bioremediation of pollutants: perspectives strategies and applications. Int J Pharmacol Biol Sci 10(1):53
Sornhiran N, Aramrak S, Prakongkep N, Wisawapipat W (2022) Silicate minerals control the potential uses of phosphorus-laden mineral-engineered biochar as phosphorus fertilizers. Biochar 4:2
Spence C, Bais H (2015) Role of plant growth regulators as chemical signals in plant–microbe interactions: a double edged sword. Curr Opin Plant Biol 27:52–58
Spokas KA, Novak JM, Stewart CE, Cantrell KB, Uchimiya M, DuSaire MG, Ro KS (2011) Qualitative analysis of volatile organic compounds on biochar. Chemosphere 85(5):869–882
Sun F, Chen J, Chen F, Wang X, Liu K, Yang Y, Tang M (2022) Influence of biochar remediation on Eisenia fetida in Pb-contaminated soils. Chemosphere 295:133954
Takeno N (2005) Atlas of Eh-pH diagrams. Geological survey of Japan open file report 419, 102
Thies JE, Rillig MC (2012) Characteristics of biochar: biological properties. Biochar for environmental management. Routledge, pp 117–138
Tilman D, Balzer C, Hill J, Befort BL (2011) Global food demand and the sustainable intensification of agriculture. Proc Natl Acad Sci USA 108:20260–20264
Tomczyk A, Sokołowska Z, Boguta P (2020) Biochar physicochemical properties: pyrolysis temperature and feedstock kind effects. Rev Environ Sci Bio/technol 19:191–215. https://doi.org/10.1007/s11157-020-09523-3
Truong H, Lomnicki S, Dellinger B (2010) Potential for misidentification of environmentally persistent free radicals as molecular pollutants in particulate matter. Environ Sci Technol 44(6):1933–1939
Tsezos M (2009) Metal–microbes interactions: beyond environmental protection. Adv Mater Res 71–72:527–532
Tu C, Wei J, Guan F, Liu Y, Sun Y, Luo Y (2020) Biochar and bacteria inoculated biochar enhanced Cd and Cu immobilization and enzymatic activity in a polluted soil. Environ Int 137:105576. https://doi.org/10.1016/j.envint.2020.105576
Ugya AY, Ajibade FO, Hua X (2021) The efficiency of microalgae biofilm in the phycoremediation of water from River Kaduna. J Environ Manag 295:113109
Uchimiya M, Chang S, Klasson KT (2011) Screening biochars for heavy metal retention in soil: role of oxygen functional groups. J Hazard Mater 190(1–3):432–441
United Nations (2017) Department of Economics and Social affairs. https://www.un.org/development/desa/en/news/population/world-population-prospects-2017.html. Downlaoded in 28 Aug 2022
Verheijen FGA, Zhuravel A, Silva FC, Amaro A, Ben-Hur M, Keizer JJ (2019) The influence of biochar particle size and concentration on bulk density and maximum water holding capacity of sandy vs sandy loam soil in a column experiment. Geoderma 347:194–202. https://doi.org/10.1016/j.geoderma.2019.03.044
Verma S, Kuila A (2019) Bioremediation of heavy metals by microbial process. Environ Technol Innov 14:100369
Visconti D, Álvarez-Robles MJ, Fiorentino N, Fagnano M, Clemente R (2020) Use of Brassica juncea and Dactylis glomerata for the phytostabilization of mine soils amended with compost or biochar. Chemosphere 260:127661. https://doi.org/10.1016/j.chemosphere.2020.127661
Wang J, Shaheen SM, Swertz AC, Rennert T, Feng X, Rinklebe J (2019) Sulfur-modified organoclay promotes plant uptake and affects geochemical fractionation of mercury in a polluted floodplain soil. J Hazard Mater 371:687–693
Wang G, Peng C, Tariq M, Lin S, Wan J, Liang W, Zhang W, Zhang L (2022) Mechanistic insight and bifunctional study of a sulfide Fe3O4 coated biochar composite for efficient As(III) and Pb(II) immobilization in soils. Environ Pollut 293:118587. https://doi.org/10.1016/j.envpol.2021.118587
Wen E, Yang X, Chen H, Shaheen SM, Sarkar B, Xu S, Song H, Liang Y, Rinklebe J, Hou D, Li Y, Wu F, Pohorely M, Wong JWC, Wang H (2021) Iron-modified biochar and water management regime-induced changes in plant growth, enzyme activities, and phytoavailability of arsenic, cadmium and lead in a paddy soil. J Hazard Mater 407:124344
Wiangkham N, Prapagdee B (2018) Potential of Napier grass with cadmium-resistant bacterial inoculation on cadmium phytoremediation and its possibility to use as biomass fuel. Chemosphere 201:511–518
Wu B, Wang Z, Zhao Y, Gu Y, Wang Y, Yu J, Xu H (2019) The performance of biochar-microbe multiple biochemical material on bioremediation and soil micro-ecology in the cadmium aged soil. Sci Total Environ 686:719–728
Wu H, Chen Z, Sheng F, Wang C, Jin X, Gu C (2021a) Mechanisms for the dissolved biochar promoted iron dissolution and consequential chromium release. Sci Total Environ 796:148923. https://doi.org/10.1016/j.scitotenv.2021.148923
Wu J, Wang T, Wang J, Zhang Y, Pan W-P (2021b) A novel modified method for the efficient removal of Pb and Cd from wastewater by biochar: enhanced the ion exchange and precipitation capacity. Sci Total Environ 754:142150
Xing Y, Wang J, Shaheen SM, Feng X, Chen Z, Zhang H, Rinklebe J (2020) Mitigation of mercury accumulation in rice using rice hull-derived biochar as soil amendment: a field investigation. J Hazard Mater 388:121747
Xu Q, Xu Q, Zhu H, Li H, Yin W, Feng K, Wang S, Wang X (2022a) Does biochar application in heavy metal-contaminated soils affect soil micronutrient dynamics? Chemosphere 290:133349. https://doi.org/10.1016/j.chemosphere.2021.133349
Yadav KK, Gupta N, Kumar A, Reece LM, Singh N, Rezania S, Khan SA (2018) Mechanistic understanding and holistic approach of phytoremediation: a review on application and future prospects. Ecol Eng 120:274–298. https://doi.org/10.1016/j.ecoleng.2018.05.039
Yang X, Wan Y, Zheng Y, He F, Yu Z, Huang J, Wang H, Ok YS, Jiang Y, Gao B (2019) Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: a critical review. Chem Eng J 366:608–621
Yang T, Xu Y, Huang Q, Sun Y, Liang X, Wang L, Qin X, Zhao L (2021a) An efficient biochar synthesized by iron-zinc modified corn straw for simultaneously immobilization Cd in acidic and alkaline soils. Environ Pollut 291:118129. https://doi.org/10.1016/j.envpol.2021.118129
Yang X, Pan H, Shaheen SM, Wang H, Rinklebe J (2021b) Immobilization of cadmium and lead using phosphorus-rich animal-derived and iron-modified plant-derived biochars under dynamic redox conditions in a paddy soil. Environ Int 156:106628
Yang X, Hinzmann M, Pan H, Wang J, Bolan N, Tsang DCW, Ok YS, Wang S-L, Shaheen SM, Wang H (2022a) Pig carcass-derived biochar caused contradictory effects on arsenic mobilization in a contaminated paddy soil under fluctuating controlled redox conditions. J Hazard Mater 421:126647
Yang X, Shaheen SM, Wang J, Hou D, Ok YS, Wang S-L, Wang H, Rinklebe J (2022b) Elucidating the redox-driven dynamic interactions between arsenic and iron-impregnated biochar in a paddy soil using geochemical and spectroscopic techniques. J Hazard Mater 422:126808. https://doi.org/10.1016/j.jhazmat.2021.126808
Yang X, Wen E, Ge C, El-Naggar A, Yu H, Wang S, Kwon EE, Song H, Shaheen SM, Wang H, Rinklebe J (2023a) Iron-modified phosphorus- and silicon-based biochars exhibited various influences on arsenic, cadmium and lead accumulation in rice and enzyme activities in a paddy soil. J Hazard Mater 443:130203. https://doi.org/10.1016/j.jhazmat.2022.130203
Yang X, Dai Z, Ger C, Huamei Yu H, Nanthi Bolan N et al (2023) Multiple-functionalized biochar affects rice yield and quality via regulating arsenic and lead redistribution and bacterial community structure in soils under different hydrological conditions. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2022.130308
Yi S, Li F, Wu C, Wei M, Tian J, Ge F (2022) Synergistic leaching of heavy metal-polycyclic aromatic hydrocarbon in co-contaminated soil by hydroxamate siderophore: role of cation-π and chelation. J Hazard Mater 424:127514. https://doi.org/10.1016/j.jhazmat.2021.127514
Yin X, Yu L, Luo X, Zhang Z, Sun H, Mosa AA, Wang N (2019) Sorption of Pb (II) onto< 1 μm effective diameter clay minerals extracted from different soils of the Loess Plateau, China. Geoderma 337:1058–1066
Yuan C, Gao B, Peng Y, Gao X, Fan B, Chen Q (2021) A meta-analysis of heavy metal bioavailability response to biochar aging: importance of soil and biochar properties. Sci Total Environ 756:144058. https://doi.org/10.1016/j.scitotenv.2020.144058
Zhang C, Lin Y, Tian X, Xu Q, Chen Z, Lin W (2017) Tobacco bacterial wilt suppression with biochar soil addition associates to improved soil physiochemical properties and increased rhizosphere bacteria abundance. Appl Soil Ecol 112:90–96
Zhang M, Wang J, Bai SH, Zhang Y, Teng Y, Xu Z (2019) Assisted phytoremediation of a co-contaminated soil with biochar amendment: contaminant removals and bacterial community properties. Geoderma 348:115–123
Zhang H, Shao J, Zhang S, Zhang X, Chen H (2020) Effect of phosphorus-modified biochars on immobilization of Cu (II), Cd (II), and As (V) in paddy soil. J Hazard Mater 390:121349
Zhang X, Yu J, Huang Z, Li H, Liu X, Huang J, Zhuo R, Wu Z, Qin X, Gao Y, Wang M, Zhu Y (2021) Enhanced Cd phytostabilization and rhizosphere bacterial diversity of Robinia pseudoacacia L. by endophyte Enterobacter sp. YG-14 combined with sludge biochar. Sci Total Environ 787:147660–7
Zhang P, Xue B, Jiao L, Meng X, Zhang L, Li B, Sun H (2022) Preparation of ball-milled phosphorus-loaded biochar and its highly effective remediation for Cd- and Pb-contaminated alkaline soil. Sci Total Environ 813:152648. https://doi.org/10.1016/j.scitotenv.2021.152648
Zhang S, Li Y, Wang P, Zhang H, Ali EF, Li R et al (2023) Lactic acid bacteria promoted soil quality and enhanced phytoextraction of Cd and Zn by mustard: a trial for bioengineering of toxic metal contaminated mining soils. Environ Res 216:114646. https://doi.org/10.1016/j.envres.2022.114646
Zheng C, Yang Z, Si M, Zhu F, Yang W, Zhao F, Shi Y (2021) Application of biochars in the remediation of chromium contamination: fabrication, mechanisms, and interfering species. J Hazard Mater 407:124376. https://doi.org/10.1016/j.jhazmat.2020.124376
Zhou C, Song X, Wang Y, Wang H, Ge S (2022) The sorption and short-term immobilization of lead and cadmium by nano-hydroxyapatite/biochar in aqueous solution and soil. Chemosphere 286:131810. https://doi.org/10.1016/j.chemosphere.2021.131810
Zhu X, Chen B, Zhu L, Xing B (2017) Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: a review. Environ Pollut 227:98–115
Acknowledgements
Not applicable.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
SMS: creating the idea, scientific concept, visualization, investigation, collecting review of literature, creating tables and figures, writing the original draft, coordination, and corresponding author. AM: writing—review and editing, particularly Sect. 4.1 and creating Figs. 5, 6, and 9. N: Creating and discussing Table 1. PJ: writing Sect. 4.2.4. NH: creating figures, editing, and listing the references. XY: writing—review and editing, particularly Sect. 4.2 and creating Fig. 12. VA: writing—review and editing, particularly Sect. 2. RL, JW, TZ, NKN, MS, GS, DSA, MV, Z-YH, AKS, BS, ZZ, DH, BG, HW, NB, JR: visualization, writing—review and editing the entire article.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no potential conflict of interest.
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Consent to Publish
Authors are responsible for correctness of the statements provided in the manuscript.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shaheen, S.M., Mosa, A., Natasha et al. Pros and Cons of Biochar to Soil Potentially Toxic Element Mobilization and Phytoavailability: Environmental Implications. Earth Syst Environ 7, 321–345 (2023). https://doi.org/10.1007/s41748-022-00336-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41748-022-00336-8