Abstract
Acute respiratory distress syndrome (ARDS) has a ~ 40% mortality rate with an increasing prevalence exacerbated by the COVID-19 pandemic. Mechanical ventilation is the primary means for life-saving support to buy time for lung healing in ARDS patients, however, it can also lead to ventilator-induced lung injury (VILI). Effective strategies to reduce or prevent VILI are necessary but are not currently delivered. Therefore, we aim at evaluating the current imaging technologies to visualize where pressure and volume being delivered to the lung during mechanical ventilation; and combining plasma biomarkers to guide management of mechanical ventilation. We searched PubMed and Medline using keywords and analyzed the literature, including both animal models and human studies, to examine the independent use of computed tomography (CT) to evaluate lung mechanics, electrical impedance tomography (EIT) to guide ventilation, ultrasound to monitor lung injury, and plasma biomarkers to indicate status of lung pathophysiology. This investigation has led to our proposal of the combination of imaging and biomarkers to precisely deliver mechanical ventilation to improve patient outcomes in ARDS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
1.1 Complications of Mechanical Ventilation in ARDS
Acute respiratory distress syndrome (ARDS) has a complex and uncontrolled inflammatory response caused by multiple insults such as pneumonia [25], COVID-19 [50], sepsis [25], acid aspiration [25], inhalation injuries [25] and trauma [25]. An influx of fluid and protein into the interstitial spaces and alveoli leads to heterogenous diffuse lung damage, resulting in pulmonary edema and subsequent fibrosis [17]. The ARDS mortality rate is as high as 30–50% [43].
ARDS patients receive life-saving support with mechanical ventilation (MV). MV is a significant confounder to improving patient outcomes, yet it remains the most valuable treatment [32]. A complication from MV is ventilator-induced lung injury (VILI). VILI is acute lung injury that results from or is aggravated by MV treatment and has physiological and biochemical mechanisms that lead to adverse patient outcomes [6, 32].
There are several mechanisms of injury: atelectrauma which results from the cyclic opening and closing of alveoli in collapsed lung areas; volutrauma from high tidal volumes putting excessive stress on the lungs; barotrauma due to high transpulmonary pressures that overinflate the lungs; and biotrauma that is a combination of atelectrauma and volutrauma, which increases the inflammatory response through the activation of neutrophils, macrophages and alveolar epithelial cells, and releases biomarkers [6].
Clinical guidelines have been developed to try to reduce VILI: positive end-expiratory pressure (PEEP) to keep alveoli open to reduce atelectrauma; low-tidal volumes (4–8 mL/kg of predicted body weight) to minimize volutrauma; and plateau pressure < 30 cmH2O to avoid barotrauma [20]. However, these strategies require precise individualization to protect patients’ heterogeneous lung injury and individual responses to MV. Therefore, the accuracy and effectiveness of the current delivery of MV for treatment are limited.
2 Approaches for the Evaluation of Lung Mechanics and Mechanical Ventilation
The Pressure–Volume (P–V) curve is used to diagnose and monitor ARDS. It shows the relationship between the stage and severity of acute lung injury, assesses the volume recruited by PEEP, and the slope represents the compliance of the respiratory system [7]. However, the information provided by the P–V curve is evaluated under static conditions and may not be representative of the dynamic changes during MV [7].
The classic tool for diagnosis is the X-Ray, but it is very limited. The 2-D images do not account for gravity on the pleural pressure gradient and the ventro-dorsal distribution of lesions, and the consolidation of the dorsal lung can be hidden by the ventral aspect [62]. There is also a lack of assessment of dynamic lung aeration and function, and agreement between readers is low [62].
Advances in technology have led to tools that better assess lung mechanics and the effect of volume and pressure on the lungs. Computed Tomography (CT) delivers X-Ray imaging in 3-D. It can quantitatively assess the gravitational influence of alveolar aeration compared to atelectasis and lung tissue weight [24, 62]. It can evaluate lung mechanics, show the recruitment of collapsed or consolidated areas, and provides the highest resolution available to visualize the lung parenchyma for injury assessment [15, 62].
Electrical Impedance Tomography (EIT) shows real-time MV and monitors individual lung dynamics [33]. Ventilation-perfusion mismatch (V/Q) occurs when non-ventilated alveoli are perfused with pulmonary capillaries (shunt) or ventilated alveoli do not have sufficient blood flow to pulmonary capillaries (dead space), which can be assessed by EIT [3, 33]. Ventilation and perfusion maps show changes in quadrants to guide MV.
Lung ultrasound (LUS) can confirm and monitor the evolution of ARDS at the bedside by assessing changes in aeration, tissue density and PEEP-induced recruitment [2]. It can determine the efficacy of treatment and the ultrasound waves can also penetrate injured lung areas leading to targeted delivery of therapeutics [35, 46].
These tools each contribute to evaluating important components of the delivery of mechanical ventilation. Therefore, there are limitations in applying CT, EIT and LUS individually during MV. The gold standard for visualizing lung injury is CT and thus it is the most accurate tool for this purpose. However, CT requires patient transport and exposure to radiation, compared to LUS, which does not have as high spatial resolution, but can be easily applied at the bedside for monitoring injury progression. Detailed structural changes can be viewed on CT in contrast to EIT which has low spatial resolution, although EIT compensates for CT in its ability to visualize detailed functional changes and allows for the titration of ventilation settings for personalization. EIT can be used at the bedside, but it is an expensive technology.
Overall, these new technologies allow physicians to visualize MV distribution, assess individual responses to MV, and monitor measurements that are currently not examined at the bedside. This review will evaluate the current application of CT, EIT, LUS and plasma biomarkers in the context of dynamic management of MV, aiming to provide future direction on the best use of these tools to improve clinical outcomes in ARDS with precise treatment.
An electronic database search of PubMed and Medline was conducted using keywords such as ARDS, VILI, LUS, CT, EIT, mechanical ventilation patient outcomes, ventilation perfusion, COVID-19, plasma biomarkers, delivery of therapeutics, animal models and human studies (Fig. 1).
3 CT for Diagnosis and Evaluation of Lung Mechanics in ARDS and EIT to Monitor Lung Mechanics and Guide Ventilation
3.1 Animal Models for CT
CT scans can quantitatively assess alveolar strain and lung tissue weight [41] as well as ventilation distribution in the lung [40]. Quantifying pulmonary fibrosis visualized on micro-CT using the Ashcroft scale (0–8) scored lung tissue with mild fibrotic changes between 0 to 3, and fibrotic masses and collagen from 4 to 5 [45]. The fibrosis measured by micro-CT correlated with the Ashcroft score, percentage of collagen content and alveolar air (r2 = 0.91, 0.77 and 0.94, respectively) [45]. The CT number categorized injury by a normal aerated range of − 1000 to − 500 or a poorly aerated range of − 500 to − 100 [54]. A shift in the CT number to − 300 correlated to the development of inflammation and fibrosis [31].
Mechanical power, defined as transpulmonary pressure, tidal volume and respiratory rate, was used to identify a VILI threshold. CT showed changes from isolated densities to whole-lung edema, indicating VILI above a threshold of 12 J/min [16]. The ratio between tidal volume, functional residual capacity and transpulmonary pressure determined a strain threshold. CT scans showed increased lung weight, stipulating VILI at a point between 1.5 and 2 [42].
3.2 Human Studies for CT
The extent of the atelectatic lung on a CT scan classified patients with high and low recruitability with a sensitivity of 0.96, specificity of 0.76 and diagnostic accuracy of 0.86 [11]. Opacification in the anterior lung in 37.5% of patients represented fibrosis due to VILI [48]. Cross-sectional imaging identified opacification in 100% of ARDS patients, with consolidation in 75% of cases [48].
The percentage of alveolar fibrocytes was an independent predictor of mortality, associated with prolonged ventilation, and an indicator of poor prognosis in the early stages of ARDS [8]. Scoring pathology on a scale of 1 to 6, with a greater score indicating worse lung injury, showed that a CT score < 230 enabled survival prediction with a sensitivity of 73% and specificity of 75% [29]. Survivors had an overall smaller CT score (p = 0.002), a lower incidence of barotrauma (p = 0.013), and a greater number of ventilation-free days (p = 0.018) [29].
3.3 Animal Models for EIT
There was an excellent correlation (r2 = 0.98) between the tidal volume and air delivered from the ventilator with < 6% mean error [30]. As PEEP was increased from 5 to 20 cmH2O, targeted re-aeration reached a lung volume of 87.1% of its normal value [30]. Air distribution was viewed with the estimated center of ventilation and anterior to posterior ventilation ratio. As PEEP was accurately titrated, the center of ventilation increased to 48.3%, almost reaching its normal value of 50%, anterior to posterior ratio was restored to 91% of its normal value, and the global inhomogeneity index decreased to its normal value [30].
EIT-tracked lung volumes maintained oxygenation, alveolar architecture, and lung mechanics better than low-tidal volume ventilation, while also decreasing histological evidence of VILI through a significant decrease in the presence of airway fibrin and hyaline membrane [49, 58]. PEEP determined by the global inhomogeneity index and hyperdistention indices led to the use of different FiO2 and PEEP combinations than the ones suggested by the ARDS network [28]. Identification of the onset of collapse and regional lung recruitment before changes in global pulmonary mechanics, occurred during PEEP titration [36].
3.4 Human Studies for EIT
Individualization of PEEP and tidal volumes improved oxygenation (p = 0.0002) while reducing alveolar cycling without causing global overdistension (p = 0.0007, p = 0.015, stress and strain, respectively) [4]. Evaluation of early individualized PEEP in ARDS led to a 6% absolute reduction in mortality [27]. Guided PEEP titration was associated with higher weaning success, improved oxygenation and compliance, and an 18.3% increase in hospital survival in severe ARDS patients [61].
COVID-19 patients had a median value of 34% pixels with V/Q mismatch, with 6/7 patients having values > 30% [34]. The percentage of unmatched units in ARDS patients (% only ventilated units + % only perfused units) was significantly higher in non-survivors (p < 0.001) and an independent predictor of mortality (p = 0.004) with a sensitivity, specificity and negative predictive value of 77%, 87% and 91%, respectively [50].
4 Assessment of Agreement between CT and EIT
4.1 Animal Models
There were significant correlations (r = 0.98–0.99) between EIT and CT in assessing end-expiratory gas volumes, with a slightly lower correlation (r = 0.88) in tidal volumes [36]. Tidal recruitment on CT was strongly correlated to regional-ventilation-delay on EIT (r = 0.90–0.99, p < 0.05) [37]. Lung density measured by CT and EIT regional ventilation at increasing tidal volumes during PEEP titration had the strongest correlation in the dependent lung (r2 = 0.86) [22].
4.2 Human Studies
In people with ARDS, when global CT-derived strain (StrainCT) was compared with simultaneous changes in electrical impedance (ΔZ) measured using EIT at end-inspiration and end-expiration, it revealed that ΔZ provides a real-time assessment of global cyclic strain at bedside, where the optimum PEEP with smallest lung strain is the PEEP where ΔZ is minimized, making EIT a potential surrogate for cyclic lung strain measured by CT (StrainCT) [12]. EIT regional ventilation changes in impedance showed a strong correlation (r2 = 0.92) to changes in lung density assessed by air content on CT [53]. Recruitable collapse estimated by EIT and CT showed good agreement at all levels of PEEP, with hyperinflation measured by CT and hyperdistention by EIT having good correlations (r2 = 0.85 and 0.95 in patients 1 and 2, respectively) [13]. A simulated EIT image with corrected lung areas based on CT, enhanced the global inhomogeneity index calculation, and increased sensitivity to alveolar collapse and overdistension [60]. EIT image reconstruction with CT showed that the ventilation distribution on EIT could be correlated to the underlying morphological information on CT [47].
5 LUS for Diagnostics and Therapeutics
Management of patients on MV with LUS identified alveolar consolidation with a sensitivity, specificity and diagnostic accuracy of 100%, 78%, and 95%, respectively [59]. Classification of lung morphology (focal vs. non-focal) in ARDS during MV, using the Amsterdam method, had a specificity of 100% and sensitivity of 77% [39]. In patients receiving MV, the ventral LUS score was the most predictive of non-focal ARDS, with 100% specificity and 94% sensitivity [14]. The LUS total and ventral scores had an area under the ROC curve of 0.89 and 0.958, respectively [14]. Daily assessment of LUS scores indicated that a progressive reduction in the score was due to lung re-aeration and recovery, with a persistently high score corresponding to mortality [38].
Compared to normally aerated lung tissue that scatters LUS waves, injured lung areas can be penetrated by ultrasound due to the loss of aeration and fluid in the alveoli [46]. This allows for targeted delivery of therapeutics to injured lung areas [46]. Once LUS identifies the site of injury, therapeutics can be directly delivered to these areas, improving uptake, and reducing systemic effects [46].
6 The Application of Plasma Biomarkers to MV
There are two lung epithelial biomarkers: Receptor for Advanced Glycation End-products and its soluble form (RAGE/sRAGE) and Surfactant Protein D (SP-D), which have been used for diagnosis and mortality in ARDS [55, 57]. Patients with high levels of RAGE benefitted from low-tidal volumes (p = 0.02), increases in SP-D indicated injurious ventilation and may be a marker of VILI (p = 0.02), with low tidal volumes attenuating these levels (0.0006) [10, 18, 19].
Angiopoietin-2 (Ang-2), a lung endothelial marker aided in the determination of ARDS severity and mortality, improved the ability of the Lung Injury Prediction score (area under-ROC = 0.84, p = 0.05) and augmented clinical scores by identifying patients who were developing or at high risk of lung injury during treatment [1, 21]. Interleukin-8 (IL-8) is a chemoattract immune cell and augmented diagnosis and morality in ARDS [9, 56]. IL-8 decreased during lung-protective ventilation (p < 0.05) [43].
A two-biomarker panel using Ang-2 and RAGE performed well across multiple patient cohorts of ARDS, with IL-8 and SP-D being the most frequently used biomarkers and having a higher predictive value in combination with clinical variables [55, 56]. Overall, these biomarkers indicate different aspects of the injurious and inflammatory mechanisms, and their combination improved clinical predictors [21, 23].
7 Prospective
Using CT to visualize individual lung injury is important because understanding individual patient lung mechanics is linked to choosing ventilation strategies and patient outcomes. Most patients admitted to the Intensive Care Unit already have a CT scan, thus it is a feasible tool for this purpose. EIT is valuable for monitoring lung volumes and titrating ventilation settings to reach precisely determined values, but can also measure V/Q mismatch, which is not routinely assessed at the bedside. The current application of MV is not precise due to PEEP guidelines from the ARDS network not being adequate for all patients and with lung protective ventilation strategies being underused in ~ 30% of patients [5]. Therefore, there is a need for EIT to precisely guide ventilation according to validated indices, which would improve the current challenges of MV to potentially enhance patient outcomes.
CT and EIT are valuable tools independently, but also strongly correlate and have been validated against each other, with the structural capabilities of CT augmenting the functional aspects of EIT. Therefore, we propose their combination to precisely deliver MV by evaluating the lungs’ response to ventilation on EIT based on lung injury that can be visualized with CT. This approach considers the heterogeneous and individual lung responses in real-time, and the complexity of ARDS, but also provides clinicians with strategies that can accurately and effectively tailor MV compared to the current delivery. Although the studies used to inform this conclusion have not combined CT and EIT simultaneously, the analysis of their individual power and correlation between their measures shows their strong potential.
Regular monitoring of injury with LUS can be easily applied at the bedside to identify lung morphology in ARDS during MV. The loss of aeration in injured lungs also has utilization for therapeutics during MV. Advancements in using LUS at the bedside during MV are recent and the increase in patients during the COVID-19 pandemic led to its’ use to complement CT and monitor ARDS patients [52]. Treatment of MV includes using LUS to identify injury, followed by the precise delivery of therapeutics to the injured lung area. Therapeutics are currently not precisely distributed, leading to low treatment efficacy, which cannot be adequately evaluated due to a lack of routine injury assessment. Therefore, applying LUS to visualize injury and its’ progression, and for targeted delivery of therapeutics is another necessary component to precisely deliver treatment during MV.
Plasma biomarkers have prognostic value in ARDS and represent the underlying pathophysiology in the lung. They also augment clinical scores and MV strategies with statistical significance [1, 21, 55, 56]. We recommend the application of plasma biomarkers to elucidate the biochemical pathways in the lung that contribute to injury and patient outcomes during MV. Biomarkers could guide MV by indicating alterations in the lungs before they can be visualized. The biochemical contributors to VILI and ARDS are not currently assessed in the Intensive Care Unit, however, they are valuable because they could provide clinicians with a biological understanding of the efficacy of MV, which could lead to early changes in treatment.
8 Conclusions
Our evaluation of the literature assessing the utility of imaging and biochemical markers individually during MV has led to the proposal of their combination to provide precise management for ARDS patients (Fig. 2). Each imaging tool explored in this review provides vital information to guide and inform treatment with MV. Together, they have the potential to ameliorate the disadvantages of the current delivery of MV through precision medicine. A holistic understanding of the contributors to ARDS, VILI, and treatment during MV is imperative. Therefore, we have suggested the potential application of imaging and biomarkers to improve the delivery of MV, assess the progression of injury and to understand the pathophysiology in the lung. A summary of the value of each tool for key parameters are outlined (Table 1).
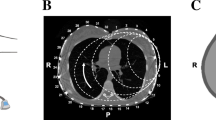
Proposed approach to manage mechanical ventilation (MV) in ARDS with precision medicine. The current MV approach is blinded regarding where pressure and volume is delivered into the lung by a ventilator. Systemic biomarkers maybe used as a result for evaluation of appropriate ventilatory strategies. The proposed approach is to use real-time bioimaging techniques, such as CT scan, electric impedance tomography (EIT) and lung ultrasound (LUS) to visualize and guide the pressure and volume delivered during MV in addition to biomarkers. EIT images are adopted [12], CT images [40] and LUC images (Galderisi 25)
An evaluation of the individual components of our proposed approach has been and continues to be tested to better understand the capability of each element. Further studies should be completed to test the simultaneous use of CT and EIT in animal models and then human studies, followed by the inclusion of plasma biomarkers to augment treatment decisions. LUS has strong evidence for identifying lung injury, but more research on its’ potential for delivering therapeutics in animals and then humans is necessary. Finally, these treatments should be combined and compared to the current delivery of MV to understand their full potential in improving patient outcomes in ARDS.
Availability of Data and Material
Not applicable.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- CT:
-
Computed tomography
- EIT:
-
Electrical impedance tomography
- LUS:
-
Lung ultrasound
- MV:
-
Mechanical ventilation
- VILI:
-
Ventilator-induced lung injury
- PEEP:
-
Positive end-expiratory pressure
- V/Q mismatch:
-
Ventilation perfusion mismatch
- RAGE:
-
Receptor for advanced glycation end-products
- sRAGE:
-
Soluble receptor for advanced glycation end-products
- SP-D:
-
Surfactant protein-D
- Ang-2:
-
Angiopoietin-2
- IL-8:
-
Interleukin-8
References
Agrawal A, Matthay MA, Kangelaris KN, Stein J, Chu JC, Imp BM, et al. Plasma angiopoietin-2 predicts the onset of acute lung injury in critically ill patients. Am J Respir Crit Care Med. 2013;187:736–42. https://doi.org/10.1164/rccm.201208-1460OC.
Arbelot C, Ferrari F, Bouhemad B, Rouby JJ. Lung ultrasound in acute respiratory distress syndrome and acute lung injury. Curr Opin Crit Care. 2008;14:70–4. https://doi.org/10.1097/MCC.0b013e3282f43d05.
Bachmann MC, Morais C, Guillermo B, Bruhn A, Morales A, Borges JB, et al. Electrical impedance tomography in acute respiratory distress syndrome. Crit Care. 2018;22:263–74. https://doi.org/10.1186/s13054-018-2195.
Becher T, Buchholz V, Hassel D, Meinel T, Schädler D, Frerichs I, et al. Individualization of PEEP and tidal volume in ARDS patients with electrical impedance tomography: a pilot feasibility study. Ann Intensive Care. 2021;11:89. https://doi.org/10.1186/s13613-021-00877-7.
Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. https://doi.org/10.1001/jama.2016.0291.
Beitler JR, Malhotra A, Thompson BT. Ventilator-induced lung injury. Clin Chest Med. 2016;37:633–46. https://doi.org/10.1016/j.ccm.2016.07.004.
Brochard L. What is a pressure-volume curve? Crit Care. 2006;10:156. https://doi.org/10.1186/cc502.
Burnham EL, Janssen WJ, Riches DWH, Moss M, Downey GP. The fibroproliferative response in acute respiratory distress syndrome: mechanisms and clinical significance. Eur Respir J. 2014;43:276–85. https://doi.org/10.1183/09031936.00196412.
Calfee CS, Ware LB, Glidden DV, Eisner MD, Parsons PE, Thompson BT, et al. Use of risk reclassification with multiple biomarkers improves mortality prediction in acute lung injury. Crit Care Med. 2011;39:711–7. https://doi.org/10.1097/CCM.0b013e318207ec3c.
Calfee CS, Ware LB, Eisner MD, Parsons PE, Thompson BT, Wickersham N, et al. Plasma receptor for advanced glycation end products and clinical outcomes in acute lung injury. Thorax. 2008;63:1083–9. https://doi.org/10.1136/thx.2008.095588.
Chiumello D, Marino A, Brioni M, Menga F, Cigada I, Lazzerini M, et al. Visual anatomical lung CT scan assessment of lung recruitability. Intensive Care Med. 2012;39:66–73. https://doi.org/10.1007/s00134-012-2707-9.
Cornejo R, Iturrieta P, Olegário T, Kajiyama C, Arellano D, Guiñez D, et al. Estimation of changes in cyclic lung strain by electrical impedance tomography: proof-of-concept study. Acta Anaesthesiol Scand. 2021;65:228–35. https://doi.org/10.1111/aas.13723.
Costa ELV, Borges JB, Melo A, Suarez-Sipmann F, Toufen C Jr, Bohm SH, et al. Bedside estimation of recruitable alveolar collapse and hyperdistention by electrical impedance tomography. Intensive Care Med. 2009;35:1132–7. https://doi.org/10.1007/s00134-009-1447-y.
Costamagna A, Pivetta E, Goffi A, Steinberg I, Arina P, Mazzeo AT, et al. Clinical performance of lung ultrasound in predicting ARDS morphology. Ann Intensive Care. 2021;11:51. https://doi.org/10.1186/s13613-021-00837-1.
Coxson HO. Lung parenchyma density and airwall thickness in airway diseases. Breathe. 2012;9:36–45. https://doi.org/10.1183/20734735/018912.
Cressoni M, Gotti M, Chiurazzi C, Massari D, Algieri I, Amini M, et al. Mechanical power and development of ventilator-induced lung injury. Anesthesiology. 2016;124:1100–8. https://doi.org/10.1097/ALN.0000000000001056.
Crimi E, Slutsky AS. Inflammation and the acute respiratory distress syndrome. Best Pract Res Clin Anaesthesiol. 2004;18:477–92. https://doi.org/10.1016/j.bpa.2003.12.007.
Determann RM, Royakkers AA, Haitsma JJ, Zhang H, Slutsky AS, Ranieri VM, et al. Plasma levels of surfactant protein D and KL-6 for evaluation of lung injury in critically ill mechanically ventilated patients. BMC Pulm Med. 2010;10:6. https://doi.org/10.1186/1471-2466-10-6.
Eisner M, Parsons P, Matthay M, Ware L, Greene K. Plasma surfactant protein levels and clinical outcomes in patients with lung injury. Thorax. 2003;58:983–8. https://doi.org/10.1136/thorax.58.11.983.
Fan E, Del Sorbo L, Goligher EC, Hodgson CL, Munshi L, Walkey AJ, et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit. 2017;195:1253–63. https://doi.org/10.1164/rccm.201703-0548ST.
Fremont RD, Koyama T, Calfee CS, Wu W, Dossett LA, Bossert FR, et al. Acute lung injury in patients with traumatic injuries: utility of a panel of biomarkers for diagnosis and pathogenesis. J Trauma. 2010;68:1121–7. https://doi.org/10.1097/TA.0b013e3181c40728.
Frerichs I, Hinz J, Herrmann P, Weisser G, Hahn G, Dudykevych T, et al. Detection of local lung air content by electrical impedance tomography compared with electron beam CT. J Appl Physiol. 1985;93:660–6. https://doi.org/10.1152/japplphysiol.0081.2002.
García-Laorden MI, Lorente JA, Flores C, Slutsky AS, Villar J. Biomarkers for the acute respiratory distress syndrome: how to make the diagnosis more precise. Ann Transl Med. 2017;5:283. https://doi.org/10.21037/atm.2017.06.49.
Gattinoni L, Taccone P, Carlesso E, Marini JJ. Prone position in acute respiratory distress syndrome. Rationale, indications, and limits. Am J Respir Crit. 2013;188:1286–93. https://doi.org/10.1164/rccm.201308-1532CI.
Galderisi M. Lung ultrasound. European Society of Cardiology. 2019. https://www.escardio.org/staticfile/Escardio/Subspecialty/EACVI/Education/Teaching%20courses/2019/Lung%20ultrasound.pdf.
Han S, Mallampalli R. The acute respiratory distress syndrome: from mechanism to translation. J Immunol. 2015;194:855–60. https://doi.org/10.4049/jimmunol.1402513.
He H, Chi Y, Yang Y, Yuan S, Long Y, Zhao P, et al. Early individualized positive end-expiratory pressure guided by electrical impedance tomography in acute respiratory distress syndrome: a randomized controlled clinical trial. Crit Care. 2021;25:230. https://doi.org/10.1186/s13054-021-03645-y.
Hochhausen N, Biener I, Rossaint R, Follman A, Bleilevens C, Braunschweig T, et al. Optimizing PEEP by electrical impedance tomography in a porcine animal model of ARDS. Respir Care. 2017;62(3):340–9. https://doi.org/10.4187/respcare.05060.
Ichikado K, Suga M, Muranaka H, Gushima Y, Miyakawa H, Tsubamoto M, et al. Prediction of prognosis for acute respiratory distress syndrome with thin-section CT: validation in 44 cases. Radiology. 2006;238:321–9. https://doi.org/10.1148/radiol.2373041515.
Jang GY, Ayoub G, Kim YE, Oh TI, Chung CR, Suh GY, et al. Integrated EIT system for functional lung ventilation imaging. BioMed Eng OnLine. 2019. https://doi.org/10.1186/s12938-019-0701-y.
Johnson KA. Imaging techniques for small animal imaging models pulmonary disease: micro-CT. Toxicol Pathol. 2007;35:59–64. https://doi.org/10.1080/01926230601184262.
Katira BH. Ventilator-induced lung injury: classic and novel concepts. Respir Care. 2019;64:629–37. https://doi.org/10.4187/respcare.07055.
Maciejewski D, Putowski Z, Czok M, Krzych L. Electrical impedance tomography as a tool formonitoring mechanical ventilation. An introduction to the technique. Adv Med Sci. 2021;66:388–95. https://doi.org/10.1016/j.advms.2021.07.010.
Mauri T, Spinelli E, Scotti E, Colussi G, Basile MC, Crotti S, et al. Potential for lung recruitment and ventilation-perfusion mismatch in patients with the acute respiratory distress syndrome from coronavirus disease 2019. Crit Care Med. 2020;48:1129–34. https://doi.org/10.1097/CCM.0000000000004386.
Mojoli F, Bouhemad B, Mongodi S, Lichtenstein D. Lung ultrasound for critically ill patients. Am J Respir Crit. 2018;199:701–14. https://doi.org/10.1164/rccm.201802-0235CI.
Meier T, Luepschen H, Karsten J, Leibecke T, Grossherr M, Gehring H, et al. Assessment of a lung recruitment and derecruitment during a PEEP trial based on electricalimpedance tomography. Intensive Care Med. 2008;34:543–50. https://doi.org/10.1007/s00134-007-0786-9.
Muders T, Luepschen H, Zinserling J, Greschus S, Fimmers R, Guenther U, et al. Tidal recruitment assessed by electrical impedance tomography and computed tomography in a porcine model of lung injury. Crit Care Med. 2012;40:903–11. https://doi.org/10.1097/CCM0b013e318236f452.
Mongodi S, Pozzi M, Orlando A, Bouhemad B, Stella A, Tavazzi G, et al. Lung ultrasound for daily monitoring of ARDS patients on extracorporeal membrane oxygenation: preliminary experience. Intensive Care Med. 2017;44:123–4. https://doi.org/10.1007/s00134-017-4941-7.
Pierrakos C, Smit MR, Pisani L, Paulus F, Schultz MJ, Constantin JM, et al. Lung ultrasound assessment of focal and non-focal lung morphology in patients with acute respiratory distress syndrome. Front Physiol. 2021;12:730857. https://doi.org/10.3389/fphys.2021.730857.
Porra L, Bayat S, Malaspinas I, Albu G, Doras C, Broche L, et al. Pressure-regulated volume control vs. volume control ventilation in healthy and injured rabbit lung: an experimental study. Eur J Anaesthesiol. 2016;33:767–75. https://doi.org/10.1097/EJA.0000000000000485.
Protti A, Massimo C, Santini A, Langer T, Mietto C, Febres D, et al. Lung stress and strain during mechanical ventilation: any safe threshold? Am J Respir Crit Care Med. 2011;15:1354–62. https://doi.org/10.1164/rccm.201010-1757OC.
Protti A, Andreis DT, Milesi M, Iapichino GE, Monti M, Comini B, et al. Lung anatomy, energy load, and ventilator-induced lung injury. Intensive Care Med Exp. 2015;3:34. https://doi.org/10.1186/s40635-015-0070-1.
Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, et al. Effect of mechanical ventilation on inflammatory mediates in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1999;282:54–61. https://doi.org/10.1001/jama.282.1.54.
Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–93. https://doi.org/10.1056/NEJMoa050333.
Ruscitti F, Ravanetti F, Essers J, Ridwan Y, Belenkov S, Vos W, et al. Longitudinal assessment of bleomycin-induced lung fibrosis by micro-CT correlates with histological evaluation in mice. Multidiscip Respir Med. 2017. https://doi.org/10.1186/s404248-017-0089-0.
Sanwal R, Joshi K, Ditmans M, Tsai SSH, Lee WL. Ultrasound and microbubbles for targeted drug delivery to the lung endothelium in ARDS: cellular mechanisms and therapeutic opportunities. Biomedicines. 2021;9(7):803. https://doi.org/10.3390/biomedicines9070803.
Schullcke B, Gong B, Krueger-Ziolek S, Soleimani M, Mueller-Lisse U, Moeller K. Structural-functional lung imaging using a combined CT-EIT and discrete cosine transformation reconstruction method. Sci Rep. 2016. https://doi.org/10.1038/srep25951.
Sheard S, Rao P, Devaraj A. Imaging of acute respiratory distress syndrome. Respir Care. 2012;57:607–12. https://doi.org/10.4187/resspcare.01731.
Shono A, Kotani T. Clinical implication of monitoring regional ventilation using electrical impedance tomography. J Intensive Care. 2019. https://doi.org/10.1186/s40560-019-0358-4.
Spinelli E, Kircher M, Stender B, Ottaviani I, Basile MC, Marongiu I, et al. Unmatched ventilation and perfusion measured by electrical impedance tomography predicts the outcome of ARDS. Crit Care. 2021;25:192. https://doi.org/10.1186/s13054-021-03615-4.
Tzotzos SJ, Fischer B, Fischer H, Zeitlinger M. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: a global literature survey. Crit Care. 2020;24:516. https://doi.org/10.1186/s13054-020-03240-7.
Vertrugno L, Baciarello M, Bignami E, Bonetti A, Saturno F, Orso D, et al. The “pandemic” increase in lung ultrasound use in response to Covid-19: can we complement computed tomography findings? A narrative review. Ultrasound J. 2020. https://doi.org/10.1186/s13089-020-00185-4.
Victorino JA, Borges JB, Okamoto VN, Matos GFJ, Tucci MR, Caramez MPR, et al. Imbalances in regional lung ventilation a validation study on electrical impedance tomography. Am J Respir Crit. 2003;169:791–800. https://doi.org/10.1164/rccm.200301-133OC.
Vieira SR, Puybasset L, Richecoeur J, Lu Q, Cluzel P, et al. A lung computed tomographic assessment of positive end-expiratory pressure-induced lung overdistension. Am J Respir Crit. 1998;158:1571–7. https://doi.org/10.1164/ajrccm.158.5.9802101.
Ware LB, Koyama T, Billheimer DD, Wu W, Bernard GR, Thompson BT, et al. Prognostic and pathogenetic value of combining clinical and biochemical indices in patients with acute lung injury. Chest. 2010;137:288–96. https://doi.org/10.1378/chest.09-1484.
Ware LB, Zhao Z, Koyama T, Brown RM, Semler MW, Janz DR, et al. Derivation and validation of a two-biomarker panel for diagnosis of ARDS in patients with severe traumatic injuries. Trauma Surg Acute Care Open. 2017;2:e000121. https://doi.org/10.1136/tsaco-2017-000121.
Ware LB, Koyama T, Zhao Z, Janz DR, Wickersham N, Bernard GR, et al. Biomarkers of lung epithelial injury and inflammation distinguish severe sepsis patients with acute respiratory distress syndrome. Crit Care. 2013;17:R253. https://doi.org/10.1186/cc13080.
Wolf GK, Gómez-Laberge C, Rettig JS, Vargas SO, Smallwood CD, Prabhu SP, et al. Mechanical ventilation guided by electrical impedance tomography in experimental acute lung injury. Crit Care Med. 2013;41:1296–304. https://doi.org/10.1097/CCM.0b013e3182771516.
Xirouchaki N, Magkanas E, Vaporidi K, Kondili E, Plataki M, Patrianakos A, et al. Lung ultrasound in critically ill patients: comparison with bedside chest radiography. Intensive Care Med. 2011;37:1488–93. https://doi.org/10.1007/s00134-011-2317-y.
Yang L, Dai M, Möller K, Frerichs I, Adler A, Fu F, et al. Lung regions identified with CT improve the value of global inhomogeneity index measured with electrical impedance tomography. Quant Imaging Med Surg. 2021;11:1209–19. https://doi.org/10.21037/qims-20-682.
Zhao Z, Chang MY, Chang MY, Gow CH, Zhang JH, Hsu YL, et al. Positive end-expiratory pressure titration with electrical impedance tomography and pressure-volume curve in severe acute respiratory distress syndrome. Ann Intensive Care. 2019;9:7. https://doi.org/10.1186/s13613-019-0484-0.
Zompatori M, Ciccarese F, Fasano L. Overview of current lung imaging in acute respiratory distress syndrome. Eur Respir Rev. 2014;23:519–30. https://doi.org/10.1183/09059180.00001314.
Acknowledgements
This review is dedicated to my father, Neil Abbott, who passed away from respiratory complications. We thank Professor Patricia Brubaker, Department of Physiology for her critical comments that have helped improve the paper.
Funding
The work is supported by the Canadian Institutes of Health Research (CIHR) 202210PJT to H.Z.
Author information
Authors and Affiliations
Contributions
MA and HZ helped conceptualize the project, MA evaluated all studies and prepared the review paper. YL and HZ made illustration, HZ guided the project, MA, YL, LB and HZ contributed to the editing of the review paper.
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Yes.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abbott, M., Li, Y., Brochard, L. et al. Precision Medicine Using Simultaneous Monitoring and Assessment with Imaging and Biomarkers to Manage Mechanical Ventilation in ARDS. Intensive Care Res 3, 195–203 (2023). https://doi.org/10.1007/s44231-023-00045-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s44231-023-00045-4