Abstract
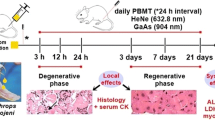
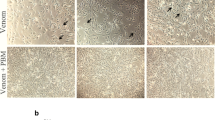
Envenoming caused by snakebites is a very important neglected tropical disease worldwide. The myotoxic phospholipases present in the bothropic venom disrupt the sarcolemma and compromise the mechanisms of energy production, leading to myonecrosis. Photobiomodulation therapy (PBMT) has been used as an effective tool to treat diverse cases of injuries, such as snake venom-induced myonecrosis. Based on that, the aim of this study was to analyze the effects of PBMT through low-level laser irradiation (904 nm) on the muscle regeneration after the myonecrosis induced by Bothrops jararacussu snake venom (Bjssu) injection, focusing on myogenic regulatory factors expression, such as Pax7, MyoD, and Myogenin (MyoG). Male Swiss mice (Mus musculus), 6–8-week-old, weighing 22 ± 3 g were used. Single sub-lethal Bjssu dose or saline was injected into the right mice gastrocnemius muscle. At 3, 24, 48, and 72 h after injections, mice were submitted to PBMT treatment. When finished the periods of 48 and 72 h, mice were euthanized and the right gastrocnemius were collected for analyses. We observed extensive inflammatory infiltrate in all the groups submitted to Bjssu injections. PBMT was able to reduce the myonecrotic area at 48 and 72 h after envenomation. There was a significant increase of MyoG mRNA expression at 72 h after venom injection. The data suggest that beyond the protective effect promoted by PBMT against Bjssu-induced myonecrosis, the low-level laser irradiation was able to stimulate the satellite cells, thus enhancing the muscle repair by improving myogenic differentiation.






Similar content being viewed by others
Availability of data and materials
The data sets generated and analyzed during the current study are available from the corresponding author (MACH; hofling@unicamp.br) upon request.
References
Chippaux, J. P. (2017). Snakebite envenomation turns again into a neglected tropical disease! Journal of Venomous Animals and Toxins Including Tropical Diseases. https://doi.org/10.1186/s40409-017-0127-6
Gutiérrez, J. M., Calvete, J. J., Habib, A. G., Harrison, R. A., Williams, D. J., & Warrell, D. A. (2017). Snakebite envenoming. Nature Reviews Disease Primers. https://doi.org/10.1038/nrdp.2017.63
Fan, H. W., & Cardoso, J. L. (1995). Clinical toxicology of snake bites in South America. In J. Meier & J. White (Eds.), Handbook of clinical toxicology of animal venoms and poisons.CRC Press. https://doi.org/10.1201/9780203719442
Gutiérrez, J. M. (1995). Clinical toxicology of snakebite in Central America. In J. Meier & J. White (Eds.), Handbook of clinical toxicology of animal venoms and poisons.CRC Press. https://doi.org/10.1201/9780203719442
Schaffazick, N., Amaral, L. S., Fonseca, T. F., Tomaz, M. A., Gaban, G. A., Borges, P. A., Calil-Elias, S., Nöel, F., Melo, P. A., Quintas, L. E., & Cunha, V. M. (2010). Effect of heparin treatment on the expression and activity of different ion-motive P-type ATPase isoforms from mouse extensor digitorum longus muscle during degeneration and regeneration after Bothrops jararacussu venom injection. Toxicon. https://doi.org/10.1016/j.toxicon.2009.06.032
Hamsi-Brandeburgo, M. I., Queiroz, L. S., Santo-Neto, H., Rodrigues-Simioni, L., & Giglio, J. R. (1988). Fractionation of Bothrops jararacussu snake venom: Partial chemical characterization and biological activity of bothropstoxin. Toxicon. https://doi.org/10.1016/0041-0101(88)90244-9
Andrião-Escarso, S. H., Soares, A. M., Rodrigues, V. M., Angulo, Y., Díaz, C., Lomonte, B., Gutiérrez, J. M., & Giglio, J. R. (2000). Myotoxic phospholipases A(2) in bothrops snake venoms: Effect of chemical modifications on the enzymatic and pharmacological properties of bothropstoxins from Bothrops jararacussu. Biochimie. https://doi.org/10.1016/s0300-9084(00)01150-0
De-Simone, S. G., Napoleão-Pego, P., Teixeira-Pinto, L. A. L., Santos, J. D. L., De-Simone, T. S., Melgarejo, A. R., Aguiar, A. S., & Marchi-Salvador, D. P. (2013). Linear B-cell epitopes in BthTX-1, BthTX-II and BthA-1, phospholipase A2’s from Bothrops jararacussu snake venom, recognized by therapeutically neutralizing commercial horse antivenom. Toxicon. https://doi.org/10.1016/j.toxicon.2013.06.004
Gutiérrez, J. M., & Lomonte, B. (1989). Local tissue damage induced by Bothrops snake venoms. A review. Memórias do Instituto Butantan, 51(4), 211–223
Gutiérrez, J. M., & Ownby, C. L. (2003). Skeletal muscle degeneration induced by venom phospholipases A2: Insights into the mechanisms of local and systemic myotoxicity. Toxicon. https://doi.org/10.1016/j.toxicon.2003.11.005
Kenzo-Kagawa, B., Vieira, W. F., Cogo, J. C., & da Cruz-Höfling, M. A. (2020). Muscle proteolysis via ubiquitin-proteasome system (UPS) is activated by BthTx-I Lys49 PLA2 but not by BthTx-II Asp49 PLA2 and Bothrops jararacussu venom. Toxicology and Applied Pharmacology. https://doi.org/10.1016/j.taap.2020.115119
WHO. (2010). Guidelines for the production, control and regulation of snake antivenom immunoglobulins. World Health Organization; 2010. http://www.who.int/bloodproducts/snake_antivenoms/snakeantivenomguideline.pdf. Accessed 08 Jan 2019.
Brazil, V. (1911). A defesa contra o ofidismo. (p. 152). Pocai & Weiss.
Rosenfeld, G. (1971). Symptomatology, pathology, and treatment of snake bites in South America, p. 345–384. In W. Bucherl, E. E. Buckley, & V. Deulofeu (Eds.), Venomous animals and their venoms. (pp. 345–384). Academic Press.
Hurme, T., & Kalimo, H. (1992). Activation of myogenic precursor cells after muscle Injury. Medicine and Science in Sports and Exercise, 24(2), 197–205 PMID: 1549008.
Holterman, C. E., & Rudnicki, M. A. (2005). Molecular regulation of satellite cell function. Seminars in Cell and Developmental Biology. https://doi.org/10.1016/j.semcdb.2005.07.004
Filippin, L. I., Cuevas, M. J., Lima, E., Marroni, N. P., Gonzalez-Gallego, J., & Xavier, R. M. (2011). Nitric oxide regulates the repair of injured skeletal muscle. Nitric Oxide. https://doi.org/10.1016/j.niox.2010.11.003
Chal, J., & Pourquié, O. (2017). Making muscle: Skeletal myogenesis in vivo and in vitro. Development. https://doi.org/10.1242/dev.151035
Addicks, G. C., Brun, C. E., Sincennes, M.-C., Saber, J., Porter, C. J., Stewart, A. F., Ernst, P., & Rudnicki, M. A. (2019). MLL1 is required for PAX7 expression and satellite cell self-renewal in mice. Nature Communications. https://doi.org/10.1038/s41467-019-12086-9
Relaix, F., Rocancourt, D., Mansouri, A., & Buckingham, M. (2005). A Pax3/Pax7-dependent population of skeletal muscle progenitor cells. Nature. https://doi.org/10.1038/nature03594
Asfour, H. A., Allouh, M. Z., & Said, R. S. (2018). Myogenic regulatory factors: The orchestrators of myogenesis after 30 years of discovery. Experimental Biology and Medicine. https://doi.org/10.1177/1535370217749494
Hyatt, J. P., McCall, G. E., Kander, E. M., Zhong, H., Roy, R. R., & Huey, K. A. (2008). PAX3/7 expression coincides with MyoD during chronic skeletal muscle overload. Muscle and Nerve. https://doi.org/10.1002/mus.21006
Wang, Y., Zhang, R. P., Zhao, Y. M., Li, Q. Q., Yan, X. P., Liu, J. Y., Gou, H., & Li, L. (2015). Effects of Pax3 and Pax7 expression on muscle mass in the Pekin duck (Anas platyrhynchos domestica). Genetics and Molecular Research. https://doi.org/10.4238/2015.September.28.1
Hernández-Hernández, J. M., García-González, E. G., Brun, C. E., & Rudnicki, M. A. (2017). The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Seminars in Cell and Developmental Biology. https://doi.org/10.1016/j.semcdb.2017.11.010
Zammit, P. S. (2017). Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Seminars in Cell and Developmental Biology. https://doi.org/10.1016/j.semcdb.2017.11.011
Ganassi, M., Badodi, S., Quiroga, H. P. O., Zammit, P. S., Hinits, Y., & Hughes, S. M. (2018). Myogenin promotes myocyte fusion to balance fibre number and size. Nature Communications. https://doi.org/10.1038/s41467-018-06583-6
Karu, T. I., Pyatibrat, L. V., Kolyakov, S. F., & Afanasyeva, N. I. (2005). Absorption measurements of a cell monolayer relevant to phototherapy: Reduction of cytochrome c oxidase under near IR radiation. Journal of Photochemistry and Photobiology B: Biology. https://doi.org/10.1016/j.jphotobiol.2005.07.002
Karu, T. I. (2010). Multiple roles of cytochrome c oxidase in mammalian cells under action of red and IR-A radiation. IUBMB Life. https://doi.org/10.1002/iub.359
Mason, M. G., Nicholls, P., & Cooper, C. E. (2010). Re-evaluation of the near infrared spectra of mitochondrial cytochrome c oxidase: Implications for non-invasive in vivo monitoring of tissues. Biochimica et Biophysica Acta. https://doi.org/10.1016/j.bbabio.2014.08.005
Hamblin, M. R. (2017). Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophysics. https://doi.org/10.3934/biophy.2017.3.337
de Freitas, L. F., & Hamblin, M. R. (2016). Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE Journal of Selected Topics in Quantum Electronics. https://doi.org/10.1109/JSTQE.2016.2561201
Dourado, D. M., Fávero, S., Baranauskas, V., & da Cruz-Höfling, M. A. (2003). Effects of the Ga–As laser irradiation on myonecrosis caused by Bothrops moojeni snake venom. Lasers in Surgery and Medicine. https://doi.org/10.1002/lsm.10237
Barbosa, A. M., Villaverde, A. B., Guimarães-Souza, L., Ribeiro, W., Cogo, J. C., & Zamunér, S. R. (2008). Effect of low-level laser therapy in the inflammatory response induced by Bothrops jararacussu snake venom. Toxicon. https://doi.org/10.1016/j.toxicon.2008.02.007
Doin-Silva, R., Baranauskas, V., Rodrigues-Simioni, L., & da Cruz-Höfling, M. A. (2009). The ability of low level laser therapy to prevent muscle tissue damage induced by snake venom. Photochemistry and Photobiology. https://doi.org/10.1111/j.1751-1097.2008.00397.x
Nadur-Andrade, N., Barbosa, A. M., Carlos, F. P., Lima, C. J., Cogo, J. C., & Zamunér, S. R. (2012). Effects of photobiostimulation on edema and hemorrhage induced by Bothrops moojeni venom. Lasers in Medical Science. https://doi.org/10.1007/s10103-011-0914-1
Vieira, W. F., Kenzo-Kagawa, B., Cogo, J. C., Baranauskas, V., & da Cruz-Höfling, M. A. (2016). Low-level laser therapy (904 nm) counteracts motor deficit of mice hind limb following skeletal muscle injury caused by snakebite-mimicking intramuscular venom injection. PLoS ONE. https://doi.org/10.1371/journal.pone.0158980
Dourado, D. M., Matias, R., Barbosa-Ferreira, M., Silva, B. A. K., Muller, J. A. I., Vieira, W. F., & da Cruz-Höfling, M. A. (2017). Effects of photobiomodulation therapy on Bothrops moojeni snake-envenomed gastrocnemius of mice using enzymatic biomarkers. Lasers in Medical Science. https://doi.org/10.1007/s10103-017-2252-4
Vieira, W. F., Kenzo-Kagawa, B., Britto, M. H. M., Ceragioli, H. J., Sakane, K. K., Baranauskas, V., & da Cruz-Höfling, M. A. (2018). Vibrational spectroscopy of muscular tissue intoxicated by snake venom and exposed to photobiomodulation therapy. Lasers in Medical Science. https://doi.org/10.1007/s10103-017-2389-1
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. https://doi.org/10.1006/meth.2001.1262
Pfaffl, M. W., Tichopad, A., Prgomet, C., & Neuvians, T. P. (2004). Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—EXCEL-based tool using pair-wise correlations. Biotechnology Letters. https://doi.org/10.1023/B:BILE.0000019559.84305.47
Allbrook, D. (1981). Skeletal muscle regeneration. Muscle and Nerve. https://doi.org/10.1002/mus.880040311
Grounds, M. D., Garret, K. L., Lai, M. C., Wright, W. E., & Beilharz, M. W. (1992). Identification of skeletal muscle precursor cells in vivo by use of MyoD1 and MyoG probes. Cell and Tissue Research. https://doi.org/10.1007/BF00318695
Chargé, S. B. P., & Rudnicki, M. A. (2004). Cellular and molecular regulation of muscle regeneration. Physiological Reviews. https://doi.org/10.1152/physrev.00019.2003
Kharraz, Y., Guerra, J., Mann, C. J., Serrano, A. L., & Muñoz-Cánoves, P. (2013). Macrophage plasticity and the role of inflammation in skeletal muscle repair. Mediators of Inflammation. https://doi.org/10.1155/2013/491497
Teixeira, C. F. P., Zamuner, S. R., Zuliani, J. P., Fernandes, C. M., da Cruz-Höfling, M. A., Fernandes, I., Chaves, F., & Gutiérrez, J. M. (2003). Neutrophils do not contribute to local tissue damage, but play a key role in skeletal muscle regeneration, in mice injected with Bothrops asper snake venom. Muscle and Nerve. https://doi.org/10.1002/mus.10453
Teixeira, C. F. P., Chaves, F., Zamunér, S. R., Fernandes, C. M., Zuliani, J. P., da Cruz-Höfling, M. A., Fernandes, I., & Gutiérrez, J. M. (2005). Effects of neutrophil depletion in the local pathological alterations and muscle regeneration in mice injected with Bothrops jararaca snake venom. International Journal of Experimental Pathology. https://doi.org/10.1111/j.0959-9673.2005.00419.x
Boeno, C. N., Paloschi, M. V., Lopes, J. A., Pires, W. L., Setúbal, S. S., Evangelista, J. R., Soares, A. M., & Zuliani, J. P. (2020). Inflammasome activation induced by a snake venom Lys49-phospholipase A2 homologue. Toxins. https://doi.org/10.3390/toxins12010022
Zheng, D., Liwinski, T., & Elinav, E. (2020). Inflammasome activation and regulation: Toward a better understanding of complex mechanisms. Cell Discov. https://doi.org/10.1038/s41421-020-0167-x
Gouveia, V. A., Pisete, F. R. F. S., Wagner, C. L. R., Dalboni, M. A., Oliveira, A. P. L., Cogo, J. C., & Zamuner, S. R. (2020). Photobiomodulation reduces cell death and cytokine production in C2C12 cells exposed to Bothrops venoms. Lasers in Medical Science. https://doi.org/10.1007/s10103-019-02884-4
Barbosa, A. M., Villaverde, A. B., Guimarães-Sousa, L., Soares, A. M., Zamunér, S. F., Cogo, J. C., & Zamunér, S. R. (2010). Low-level laser therapy decreases local effects induced by myotoxins isolated from Bothrops jararacussu snake venom. Journal of Venomous Animals and Toxins Including Tropical Diseases. https://doi.org/10.1590/S1678-91992010000300014
Amorim, F. G., Costa, T. R., Baiwir, D., De Pauw, E., Quinton, L., & Sampaio, S. V. (2018). Proteopeptidomic, functional and immunoreactivity characterization of Bothrops moojeni snake venom: Influence of snake gender on venom composition. Toxins (Basel). https://doi.org/10.3390/toxins10050177
Assis, L., Moretti, A. I., Abrahão, T. B., de Souza, H. P., Hamblin, M. R., & Parizotto, N. A. (2013). Low-level laser therapy (808 nm) contributes to muscle regeneration and prevents fibrosis in rat tibialis anterior muscle after cryolesion. Lasers in Medical Science. https://doi.org/10.1007/s10103-012-1183-3
Brunelli, R. M., Rodrigues, N. C., Ribeiro, D. A., Fernandes, K., Magri, A., Assis, L., Parizotto, N. A., Cliquet, A., Jr., Renno, A. C., & Abreu, D. C. (2014). The effects of 780-nm low-level laser therapy on muscle healing process after cryolesion. Lasers in Medical Science. https://doi.org/10.1007/s10103-013-1277-6
Relaix, F., & Zammit, P. S. (2012). Satellite cells are essential for skeletal muscle regeneration: The cell on the edge returns centre stage. Development. https://doi.org/10.1242/dev.069088
Kuang, S., & Rudnicki, M. A. (2008). The emerging biology of satellite cells and their therapeutic potential. Trends in Molecular Medicine. https://doi.org/10.1016/j.molmed.2007.12.004
Tedesco, F. S., Dellavalle, A., Diaz-Manera, J., Messina, G., & Cossu, G. (2010). Repairing skeletal muscle: Regenerative potential of skeletal muscle stem cells. The Journal of Clinical Investigation. https://doi.org/10.1172/JCI40373
Sambasivan, R., & Tajbakhsh, S. (2015). Adult skeletal muscle stem cells. Results and Problems in Cell Differentiation. https://doi.org/10.1007/978-3-662-44608-9_9
Trajano, L. S., Stumbo, A. C., Silva, C. L., Mencalha, A. L., & Fonseca, A. S. (2016). Low-level infrared laser modulates muscle repair and chromosome stabilization genes in myoblasts. Lasers in Medical Science. https://doi.org/10.1007/s10103-016-1956-1
Lepper, C., Conway, S. J., & Fan, C. M. (2009). Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature. https://doi.org/10.1038/nature08209
Ehrhardt, J., & Morgan, J. (2005). Regenerative capacity of skeletal muscle. Current Opinion in Neurology. https://doi.org/10.1097/01.wco.0000177382.62156.82
Shefer, G., Oron, U., Irintchev, A., Wernig, A., & Halevy, O. (2001). Skeletal muscle cell activation by low-energy laser irradiation: A role for the MAPK/ERK pathway. Journal of Cellular Physiology. https://doi.org/10.1002/1097-4652(2001)9999:9999%3c::AID-JCP1053%3e3.0.CO;2-9
Shefer, G., Partridge, T. A., Heslop, L., Gross, J. G., Oron, U., & Halevy, O. (2002). Low-energy laser irradiation promotes the survival and cells cycle entry of skeletal muscle satellite cells. Journal of Cell Science, 115(7), 1461–1469 PMID: 11896194.
Füchtbauer, E. M., & Westphal, H. (1992). MyoD and myogenin are coexpressed in regenerating skeletal muscle of the mouse. Developmental Dynamics. https://doi.org/10.1002/aja.1001930106
Marsh, D. R., Criswell, D. S., Carson, J. A., & Booth, F. W. (1997). Myogenic regulatory factors during regeneration of skeletal muscle in young, adult, and old rats. Journal of Applied Physiology. https://doi.org/10.1152/jappl.1997.83.4.1270
Mesquita-Ferrari, R. A., Alves, A. N., Cardoso, V. O., Artilheiro, P. P., Bussadori, S. K., Rocha, L. A., Nunes, F. D., & Fernandes, K. P. S. (2015). Low-level laser irradiation modulates cell viability and creatine kinase activity in C2C12 muscle cells during the differentiation process. Lasers in Medical Science. https://doi.org/10.1007/s10103-015-1715-8
Silva, L. M. G., Silva, C. A. A., Silva, A., Vieira, R. P., Mesquita-Ferrari, R. A., Cogo, J. C., & Zamunér, S. R. (2016). Photobiomodulation protects and promotes differentiation of C2C12 myoblast cells exposed to snake venom. PLoS ONE. https://doi.org/10.1371/journal.pone.0152890
Blais, A., Tsikitis, M., Acosta-Alvear, D., Sharan, R., Kluger, Y., & Dynlacht, B. D. (2005). An initial blueprint for myogenic differentiation. Genes and Development. https://doi.org/10.1101/gad.1281105
Alves, A. N., Ribeiro, B. G., Fernandes, K. P. S., Souza, N. H. C., Rocha, L. A., Nunes, F. D., Bussadori, S. K., & Mesquita-Ferrari, R. A. (2016). Comparative effects of low-level laser therapy pre- and post-injury on mRNA expression of MyoD, myogenin, and IL-6 during the skeletal muscle repair. Lasers in Medical Science. https://doi.org/10.1007/s10103-016-1908-9
Huang, Y.-Y., Sharma, S. K., Carroll, J., & Hamblin, M. R. (2011). Biphasic dose response in low level light therapy—An update. Dose Response. https://doi.org/10.2203/dose-response.11-009.Hamblin
Khacho, M., & Slack, R. S. (2017). Mitochondrial activity in the regulation of stem cell self-renewal and differentiation. Current Opinion in Cell Biology. https://doi.org/10.1016/j.ceb.2017.11.003
Xu, X., Duan, S., Yi, F., Ocampo, A., Liu, G.-H., & Belmonte, J. C. I. (2013). Mitochondrial regulation in pluripotent stem cells. Cell Metabolism. https://doi.org/10.1016/j.cmet.2013.06.005
Karu, T. I. (2008). Mitochondrial signaling in mammalian cells activated by red and near-IR radiation. Photochemistry and Photobiology. https://doi.org/10.1111/j.1751-1097.2008.00394.x
Koch, S., Tugues, S., Li, X., Gualandi, L., & Claesson-Welsh, L. (2011). Signal transduction by vascular endothelial growth receptors. Biochem. https://doi.org/10.1042/BJ20110301
Germani, A., Di Carlo, A., Mangoni, A., Straino, S., Giacinti, C., Turrini, P., Biglioli, P., & Capogrossi, M. C. (2003). Vascular endothelial growth factor modulates skeletal myoblast function. American Journal of Pathology. https://doi.org/10.1016/S0002-9440(10)63499-2
Dourado, D. M., Fávero, S., Matias, R., Carvalho, P. T. C., & da Cruz-Höfling, M. A. (2011). Low-level laser therapy promotes vascular endothelial growth factor receptor-1 expression in endothelial and nonendothelial cells of mice gastrocnemius exposed to snake venom. Photochemistry and Photobiology. https://doi.org/10.1111/j.1751-1097.2010.00878.x
Acknowledgements
This work was supported by Grants from São Paulo Research Foundation (FAPESP) (Proc. 05/53625-1) (http://www.fapesp.br/) and National Council for Scientific and Technological Development (CNPq, Grants #488792/2011 and #486142/2012-4) (http://www.cnpq.br/). MACH is an IA research fellow from CNPq (Grant #305099/2011-6); WFV was a Master Sci. student granted with a scholarship from Coordination of Improvement of Higher Education Personnel (CAPES) (http://www.capes.gov.br/) at the Department of Semiconductors, Instruments and Photonics (FEEC-UNICAMP), and Department of Biochemistry and Tissue Biology (IB-UNICAMP). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We would like to thank Professor Vitor Baranauskas (Brazilian Academy of Sciences, in memoriam) by his contribution to science in the last years and for all support given for the realization of this study. Professor Baranauskas passed way in October, 2014.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
Ethics approval
All experiments were done in accordance to ethical guidelines of the Brazilian National Council for Animal Experimentation Control (CONCEA) and the Brazilian College of Animal Experimentation (COBEA), and were approved by the University of Campinas (UNICAMP) institutional Committee for Ethics in Animal Use (CEUA/UNICAMP, protocol no. 2950-1). Good laboratory practices were followed according to the international standards for animal experimentation, such as the National Institutes of Health (NIH) guides for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978), and comply with the ARRIVE guidelines.
Additional information
Vitor Baranauskas: Deceased.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vieira, W.F., Kenzo-Kagawa, B., Alvares, L.E. et al. Exploring the ability of low-level laser irradiation to reduce myonecrosis and increase Myogenin transcription after Bothrops jararacussu envenomation. Photochem Photobiol Sci 20, 571–583 (2021). https://doi.org/10.1007/s43630-021-00041-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s43630-021-00041-x