Abstract
There are only a few smelters processing copper concentrates directly into blister copper. Despite the many advantages of this process, a serious challenge of this technology is the need to process the resulting flash smelting slag. It contains 12–15% copper and 2.5–4% lead. In this form, it cannot be considered as waste material and, therefore, a high-temperature reduction process is carried out. This decopperization process is energy- and time-consuming. The use of mineralurgical and hydrometallurgical processes, selective enrichment of the appropriate slag fractions in copper and lead, followed by its hydrometallurgical processing and recovery of Cu and Pb could be an interesting supplement to the methods used so far. The article presents results of research on the possibility of separation of useful components from copper slag using the original method of sieve analysis, gravitational enrichment and magnetic separation. Preliminary results of tests were made on a laboratory scale. Then, selective leaching of copper and lead from flash smelting slag was carried out, obtaining very promising results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In KGHM Polska Miedź (Poland) direct-to-blister copper flash smelting furnace, the input materials are selected primarily from the point of view of optimizing the content of the main impurities, i.e. lead and arsenic. The dried concentrate is fed through a concentrate burner to a flash furnace, and exothermic oxidation reactions of charge components, i.e. coal and copper and other metal sulfides, take place in the reaction shaft. In addition, there are endothermic dissociation reactions of calcium and magnesium carbonates and sulfates contained in dust, as well as melting processes of gangue. The article [1] presents in detail the entire process of direct-to-blister concentrate smelting in a flash furnace and the modernization of the process carried out over the last few years. The quality of copper produced in a flash smelting furnace is determined by lead content, which should be lower than 0.3%. The concentration of lead in copper depends on the state of thermodynamic equilibrium of the reaction:
The equilibrium concentration of lead in copper is a function of the cuprous oxide content in the slag and the Pb content in the concentrate, and consequently the PbO content in the slag. To obtain the required concentration of lead in copper, it is necessary to significantly oxidize the concentrate components in the furnace and transfer lead to the slag. This procedure transports significant amount of copper to the slag. On the basis of information from work [2], it can be concluded that copper in slag is found mainly in the form of cuprous oxide, and lead in the form of oxide and silicates of various stoichiometry. The degree of oxidation of flash smelting process products, determined by the concentration of Cu2O in the slag and the oxygen content in blister copper, depends mainly on the content of lead in the concentrate. The degree of oxidation of the concentrate components is used to obtain a Pb concentration in the copper lower than 0.3% by mass. Literature data [3] states that the content of the main components of slag can vary within the following limits: 10–15% Cu, 2.5–4% Pb, 4–6% Fe, 32–36% SiO2, 12–17% CaO, 8–11% MgO, 9–12% Al2O3, 2.5–4% K2O.
Due to the high copper content in the slag, the decopperization process is carried out. This process involves the reduction of cuprous oxide and other oxides, mainly lead and iron, in a liquid state in an electric arc furnace in the presence of coke covering the surface of liquid slag and a technological additive, which is calcium carbonate. Articles [4, 5] describe in detail the slag processing procedure.
The papers [6, 7] stated that as a result of the decopperization process of flash smelting slag in an electric furnace, the following is obtained:
-
Cu–Pb–Fe alloy (69–80% Cu), which is subjected to a converting process;
-
waste slag, containing on average 0.5% Cu;
-
dusts containing on average 35% Pb and 20% Zn;
-
gases that after oxidation of CO in the afterburning chamber and dedusting are directed to the chimney.
In this work, it was decided to analyze the properties of flash smelting slag in terms of the possibility of enriching it in copper with mineralurgical methods to a level that allows processing of obtained fractions differently from high-temperature processing in an electric furnace and converter. In the next step, research was carried out that allowed the estimation of the effectiveness of the process of selective extraction of Cu and Pb from slag using hydrometallurgical processes.
2 Materials and methods
2.1 Properties of flash smelting slag
The chemical composition of flash smelting slag was analyzed by atomic absorption spectrometry (AAS). Table 1 presents the contents of the main slag components and the density of the corresponding oxides from the work [8].
Due to the necessity to remove the maximum amount of lead from the metal alloy in the furnace, the melting of copper concentrates in the furnace maintains a highly oxidizing atmosphere. Hence, the components of the obtained slag occur in oxidized forms. Assuming no reaction between the oxides of elements forming the slag, it was decided to analyze the possibility of separation of slag components by gravitational methods. Three chemical compounds with a density of not more than 2.63 g/cm3 (Na2O—2.27 g/cm3, K2O—2.32 g/cm3, SiO2—2.63 g/cm3) can be noticed in the slag, which could theoretically be separated from the entire slag mass using separation in heavy liquids using bromoform (2.89 g/cm3). These compounds give together 37.08% of the initial mass of slag. In total, iron compounds found in the analyzed slag account for less than 10% of the slag mass.
The tested slag was in a crushed form (Fig. 1). Therefore, it was subjected to the grain composition test. The particle size distribution of the flash smelting slag was measured using sieve analysis. After the screening, the shares of individual slag fractions were determined, and then they were analyzed for copper and lead. The results of the above tests are presented in Table 2.
The sieve analysis in connection with the analysis on the Cu and Pb content in individual grain fractions clearly indicates the presence of the fraction (1.00 ÷ 0.63 mm) in which there is a significantly increased, compared to the entire slag mass, Cu content and reduced Pb content. The content of these elements is 46.2% and 1.95%, respectively. Although the participation of this fraction is relatively low, however, the amount of copper carried out with this grain fraction is almost 5.8% of the copper contained in all slag fractions.
Further tests that were carried out on the slag sample were EDS analysis (Fig. 2), confirming the occurrence of elements analyzed in the AAS test and identification of the slag phase composition was performed by XRD (Fig. 3).
It can be seen that the flash smelting slag consists of complex silicates of various elements, which are accompanied by copper, iron, lead, and magnesium oxides. The phase diagram indicates the presence of two compounds whose density is lower than 2.47 g/cm3. It is leucite (2.47 g/cm3) and hydroxyapofyllite (2.37 g/cm3). Lead appears only in the oxidized form as PbO (9.25 g/cm3). Copper occurs as Cu2O (6.1 g/cm3) and in the form of a copper and iron oxide compound with CuFeO2 stoichiometry (5.41 g/cm3). It should be assumed that the slag separation based on differences in specific gravity of forming components may occur to a limited range when heavy liquids are used. The method of melting copper concentrates in a flash furnace (temperature above 1200 °C, oxidizing atmosphere, presence of SiO2) leads to the formation of aluminum, calcium, iron, magnesium and lead silicates. In the case of Pb, silicates with different stoichiometry may appear (nPbO·SiO2, n = 1, 2, 4). The thermodynamic rules indicate (Fig. 4) that there is the highest probability of Pb4SiO6 formation, which meant that even a small amount of unreacted silica would bind the PbO appearing in the reaction shaft in a ratio of 1 mol SiO2 to 4 mol PbO. Due to the amorphous form, these silicates were not identifiable in the XRD method.
The tested slag was characterized by specific fragmentation and occurrence of various grain fractions. Therefore, EDS and electron microprobe (EMP) analysis of three slag grain fractions were performed: fine fraction (0–0.16 mm), intermediate fraction (0.16–0.32 mm) and coarse fraction (0.32–1 mm). Figure 5 presents scanning microscope images of these fractions, and Fig. 6 shows EDS from the field of observation of the tested slags. Table 3 presents the elemental analyzes of the characteristic grains appearing in the images from individual grain fractions. Both EDS and EMP results indicate differences in the composition of the analyzed grain fractions. In the fine fraction, mainly calcium, magnesium, potassium, iron, and aluminum silicates can be expected. The medium grain size material contains a lot of lead silicates, and the coarse fraction consists mainly of copper compounds.
2.2 Slag separation in heavy liquids
One of the commonly used and described in the literature [9] methods of separation of materials with different specific gravity is their placement in a liquid with such a density that some of the grains sink and some remain on the surface of the liquid. Grain material with a specific gravity close to that of the liquid remains suspended in it. In this work, an attempt was made to separate flash smelting slag using a heavy liquid in the form of bromoform. This substance is classified as homogeneous organic liquid and has a relatively high specific weight of 2.89 g/cm3. The purpose of the test was to check the possibility of separation of slag into fractions differing in specific gravity, and thus also in chemical composition. 1 dm3 bromoform and 20 g flash smelting slag were used in the test. After mixing the two phases, two segregation products were obtained: a phase suspended in bromoform and embedded grains. The shares of the obtained products in relation to the initial slag mass were: suspended phase—21.07%, sunken grains—78.93%. The content of Pb, Cu, and Fe in the separated phases was as follows:
Suspended phase | Sunken material |
|---|---|
5.16% Pb | 2.62% Pb |
19.31% Cu | 11.10% Cu |
9.58% Fe | 7.51% Fe |
Analyzing the obtained results, it is difficult to draw unequivocal conclusions. The suspended phase has a certain degree of enrichment in lead, copper, and iron relative to the starting material. About 31.7% of the copper contained in slag before separation was in this phase. By segregating slag in heavy liquids, large amounts of embedded material are obtained, in which the content of the studied metals is slightly lower compared to the initial slag and, as a consequence, there are no prospects for economically justified further processing of the materials thus obtained.
2.3 Magnetic separation of crushed flash smelting slag
During magnetic separation, the phenomenon is used where the grain placed in the magnetic field interacts with it, as a result of which it is pulled in or pushed out of the field. The way grains interact with a magnetic field is called magnetic susceptibility. Magnetic separation of the tested material is possible when the components forming it differ with a magnetic susceptibility sign or, in the case of the same signs, have different susceptibility values. Information from papers [10, 11] on the behavior of various substances in a magnetic field allows us to state that in the case of magnetic separation process certain chemical compounds may accumulate in the phases obtained during the process. Flash smelting slag was subjected to magnetic separation operation using two different devices. The first of these was the belt separator (Fig. 7). The diagram of the device is presented on the basis of the work [12]. The slag feed was poured onto a moving belt that circulated the magnetized drum, and three separation products were obtained: magnetic, non-magnetic, and intermediate fraction. The shares of these separation products were as follows: magnetic fraction—67.7%, non-magnetic—7.6%, intermediate—24.7%. The content of Cu, Pb, and Fe in the obtained phases is shown in Table 4.
The second device used for magnetic separation of flash smelting slag was a plate separator. It was made up of a vertically placed steel plate and windings of electromagnets matched to it, generating a magnetic field at the bottom of the plate. As a result of passing a certain weight of slag through the plate separator, two product fractions were obtained—magnetic and non-magnetic. The mass shares of these fractions were 19.5 and 80.5%, respectively. Information on the Cu, Pb, and Fe content in the obtained fractions is given in Table 5.
The results obtained during magnetic separation indicate that it is possible to receive the fraction significantly enriched in copper from the total slag stream (non-magnetic fraction from the belt separator). The share of this fraction in the total slag mass was not very high (7.6%) but the coefficient of copper enrichment of this material in relation to the initial slag was 3.87. In the non-magnetic fraction accumulated over 29.4% of copper contained in flash smelting slag before separation. In the other fractions obtained from both the belt and plate separators, no significant differences in copper and lead content were observed. Magnetic fractions are of course enriched in iron, and non-magnetic ones are characterized by a lower content of Fe in relation to the initial material, but this does not entail visible changes in the content of Cu and Pb in these fractions. Earlier analyzes (XRD) indicate that iron in slag occurs in a bound form with copper, silicon, magnesium, aluminum, calcium and, as a consequence, it is not possible to effectively separate the tested slag into fractions in which copper and lead will accumulate separately.
2.4 Hydrometallurgical processing of flash smelting slag
Despite the fact that copper is the most valuable slag component, and once it is removed, the lead contained in this slag can be easily neutralized, it was decided to perform both Cu and Pb leaching tests. Hydrometallurgical processing of flash smelting slag was carried out in two series of experiments. In the first series, selective Cu and Pb extraction tests were carried out in citric acid solutions using diametrically different leaching parameters. The second experimental series consisted of two-stage slag leaching. In the first stage, the material was leached with a sulfuric acid solution for maximum extraction of copper into the solution. In the second stage, tests were planned and conducted to remove lead from slag into the solution. Lead extraction tests were carried out with the use of acetic acid solution with the addition of urea, NaOH solutions, and citric acid solutions. For all the tests set of devices was used, which consisted of a mechanical stirrer and water bath (Fig. 8). The rotational speed of the stirrer was set at 250 rpm and the temperature required in the leaching process was controlled by the water bath settings. At the end of the process, the slurry was filtered, sediment washed with distilled water, dried and weighed. The content of Cu and Pb in this material was determined by the AAS method.
2.4.1 Leaching of flash smelting slag in citric acid solutions
Studies by Sonmez and Kumar [13] have shown that leaching of lead in the form of PbO is effective in citric acid solution. They performed the experiments using analytically pure lead (II) oxide and citric acid monohydrate. The authors in their work showed that lead can be converted into the crystalline salt Pb(C6H6O7)·H2O, which in turn can form a precursor for the extraction of lead from waste lead materials. The experiments were conducted so as to optimize the molar ratio of reagents, temperature, duration of the process and l/s ratio. The fact of the effective action of citric acid was also used to conduct research in this paper. The process of lead and copper leaching in a solution of citric acid monohydrate can be represented by the following reactions:
Two experiments were planned and carried out, radically differing in the conditions of the process. One test was performed in conditions very similar to those presented by the authors of the work [13] allowed for effective leaching of lead. The parameters used were: C6H8O7·H2O concentration—1 mol/dm3, temperature—25 °C, leaching time—15 min, l/s ratio—10. The second leaching test was carried out with a significant intensification of leaching parameters, namely C6H8O7·H2O concentration—5 mol/dm3, temperature—70 °C, time—30 min and l/s ratio—20. Below are Cu and Pb contents in the samples after leaching and the calculated leaching efficiency of these metals in accordance with the given relationship:
where A: Cu, Pb. mA,0: “A” mass in slag before leaching, mA,0 = %A,0·ms,0. %A,0: “A” percentage in the slag before leaching. ms,0: slag mass before leaching. mA,f: “A” mass in slag after leaching, mCu,k = %A,f·ms,f. %A,f: “A” percentage in the slag after leaching. ms,f: slag mass after leaching.
In the first test (1 mol/dm3 of C6H8O7·H2O, 25 °C, 15 min, l/s = 10), a material containing 0.99% Pb and 11.6% Cu was obtained after leaching. Based on the results obtained, the following leaching efficiency was calculated according to Eq. (4):
ηCu = 10.95%
ηPb = 69.11%
In the second test (5 mol/dm3 of C6H8O7·H2O, 70 °C, 30 min, l/s = 20), a material containing 3.02% Pb and 0.48% Cu was obtained. Leaching efficiency was as follows:
ηCu = 93.24%
ηPb = 15.84%
It can be seen that differentiation of leaching parameters with citric acid solutions transpose into selective copper and lead extraction into the solution phase. After leaching tests, two different materials were obtained in terms of Cu and Pb content. In the first one, a significant decrease in lead content was observed with a substantially unchanged level of copper content and the second in which the Pb content remains practically unchanged and the Cu content decreases from the initial level of 12.44–0.48%. Both in the first and second tests, there is a high leaching efficiency of one element with a fairly low index for the second element. It seems that it would be possible to process flash smelting slag and extract Cu and Pb using citric acid solutions. In specific process parameters, lead could be selectively removed from the slag, and copper would be transferred from the slag to solution under other leaching conditions.
2.4.2 Two-stage hydrometallurgical processing of flash smelting slag
In this part of the research, it was decided to conduct tests of selective leaching of copper and lead in two stages using various leaching reagents. Copper leaching was carried out using sulfuric acid solution, and for lead leaching, the use of acetic acid with the addition of urea, citric acid solution, and sodium hydroxide solutions was analyzed.
-
(A)
Leaching of copper in sulfuric acid solution
Leaching was carried out in sulfuric acid solution at a concentration of 180 g/dm3, for a period of 120 min., at a temperature of 60 °C and a liquid to solid ratio of 10. Every 15 min, 30% hydrogen peroxide as an oxidant was added to the solution in an amount of 1% of the total volume of the solution. Metallic copper passed into solution according to the reaction:
$${\text{Cu }} + {\text{ H}}_{2} {\text{SO}}_{4} + 0.5{\text{O}}_{2} = {\text{ CuSO}}_{4} + {\text{ H}}_{2} {\text{O}}{.}$$(5)The following reactions also occur in the sulfuric acid solution:
$${\text{Na}}_{2} {\text{O }} + {\text{ H}}_{2} {\text{SO}}_{4} = {\text{ Na}}_{2} {\text{SO}}_{4} + {\text{ H}}_{2} {\text{O,}}$$(6)$${\text{K}}_{2} {\text{O }} + {\text{ H}}_{2} {\text{SO}}_{4} = {\text{ K}}_{2} {\text{SO}}_{4} + {\text{ H}}_{2} {\text{O,}}$$(7)$${\text{CaO}} + {\text{ H}}_{2} {\text{SO}}_{4} = {\text{ CaSO}}_{4} + {\text{ H}}_{2} {\text{O,}}$$(8)$${\text{MgO }} + {\text{ H}}_{2} {\text{SO}}_{4} = {\text{ MgSO}}_{4} + {\text{ H}}_{2} {\text{O,}}$$(9)$${\text{Al}}_{2} {\text{O}}_{3} + \, 3{\text{H}}_{2} {\text{SO}}_{4} = {\text{ Al}}_{2} \left( {{\text{SO}}_{4} } \right)_{3} + \, 3{\text{H}}_{2} {\text{O,}}$$(10)$${\text{Fe}}_{2} {\text{O}}_{3} + {\text{ H}}_{2} {\text{SO}}_{4} = {\text{ Fe}}_{2} \left( {{\text{SO}}_{4} } \right)_{3} + {\text{ H}}_{2} {\text{O,}}$$(11)$${\text{FeO}} + {\text{ H}}_{2} {\text{SO}}_{4} = {\text{ FeSO}}_{4} + {\text{ H}}_{2} {\text{O,}}$$(12)$${\text{ZnO }} + {\text{ H}}_{2} {\text{SO}}_{4} = {\text{ ZnSO}}_{4} + {\text{ H}}_{2} {\text{O,}}$$(13)$${\text{CuO }} + {\text{ H}}_{2} {\text{SO}}_{4} = {\text{ CuSO}}_{4} + {\text{ H}}_{2} {\text{O,}}$$(14)$${\text{PbO }} + {\text{ H}}_{2} {\text{SO}}_{4} = {\text{ PbSO}}_{4} + {\text{ H}}_{2} {\text{O,}}$$(15)$$n{\text{PbO}} \cdot {\text{SiO}}_{2} + n{\text{H}}_{2} {\text{SO}}_{4} = n{\text{PbSO}}_{4} + n{\text{H}}_{2} {\text{O }} + {\text{SiO}}_{2} \, (n = \, 1, \, 2, \, 4).$$(16)Among the products of the above-mentioned reactions, only PbSO4 and CaSO4 are hardly soluble or completely insoluble in water. It can be assumed that the remaining sulfates will be in the liquid phase after the leaching process.
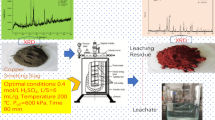
At the end of the process, the solution was filtered and the solid phase dried. The resulting precipitate was leached again in a fresh portion of the solution to check that the total amount of copper was leached. No visible color change of the solution to bluish color was observed, which allowed to state that all the copper in the earlier leaching stage passed into the solution. A change in the color of the material after leaching was observed (Fig. 9). The starting material was brown, and after treatment in sulfuric acid solution a gray material was obtained.
To accurately determine copper and lead content in leaching slag, AAS analysis was performed. The analysis showed that 0.77% copper and 4.44% lead remained in the slag. Based on Eq. (4), copper leaching efficiency was also calculated, which amounted to 96%.
Slag after leaching with sulfuric acid was subjected to microstructure observation (Fig. 10) and chemical composition test by SEM–EDS (Fig. 11) and phase analysis by XRD (Fig. 12). Based on the presented EDS image, it can be seen that the peak height derived from copper is very small, which confirms that almost the total amount of copper was leached from the slag. Also, by means of X-ray analysis, copper was not identified in any phase. In the case of lead, phase analysis showed that Pb exists in the form of PbSO4. It can be assumed that the use of sulfuric acid solution for slag leaching leads to the breakdown of lead silicates and obtaining lead sulfate as a result of the reaction (16).
-
(B)
Lead removal from the material after H2SO4 leaching
The second leaching stage was to transfer to solution lead accumulated in the material obtained after leaching with sulfuric acid solution. Three types of reagents were used—two acid and one alkaline. Therefore, acetic acid with the addition of urea and citric acid solution was used for acid leaching, and sodium hydroxide solutions were used for alkaline leaching. Each of the leaching tests used optimized parameters obtained for processing the same flash smelting slag only without pre-treatment in sulfuric acid. In the case of acetic acid with the addition of urea, leaching parameters from [14] were used, in the case of the citric acid solution the data (temperature, citric acid concentration, process time, l/s ratio) from [15] were used, and the alkaline leaching was based on the parameters obtained in [16].
-
(B) - 1
Leaching in acetic acid solution with the addition of urea
The slag after leaching with sulfuric acid was subjected to acid leaching in a solution of acetic acid with the addition of urea. The process was carried out in the following conditions: 5 mol/dm3 CH3COOH solution in the presence of 150 g/dm3 urea, 30 min, process temperature—70 °C, l/s = 20. The use of acetic acid with the addition of urea increases the dissolution rate of compounds lead in this solution. The degree of acetic acid dissociation is relatively low. However, information from work [17] indicates that the addition of urea significantly increases this coefficient:
$${\text{CH}}_{4} {\text{N}}_{2} {\text{O }} + {\text{ CH}}_{3} {\text{COOH }} = {\text{ CH}}_{5} {\text{N}}_{2} {\text{O}}^{ + } + {\text{ CH}}_{3} {\text{COO}}^{ - } .$$(17)In the presence of a large amount of CH3COO− ions, they react with PbSO4 and form a soluble compound (CH3COO)2Pb:
$${\text{PbSO}}_{4} + 2{\text{CH}}_{3} {\text{COO}}^{ - } = \, \left( {{\text{CH}}_{3} {\text{COO}}} \right)_{2} {\text{Pb }} + {\text{ SO}}_{4}^{2 - } .$$(18)Chemical composition analysis by AAS showed that the lead content in slag after leaching in CH3COOH solution with the addition of urea decreased from 4.44 to 0.39%. The lead leaching efficiency determined on the basis of relationship (4) was 88%.
-
(B) - 2
Leaching in citric acid solution
Flash smelting slag after copper removal was subjected to leaching process under the following conditions: 1 mol/dm3 citric acid solution (C6H8O7·H2O), 25 °C, 15 min, l/s = 10. Chemical composition analysis revealed that the lead content in the slag after leaching fell to 3.61% and the lead leaching efficiency was 26.88%. The obtained result indicates that citric acid acts primarily on lead (II) oxide in flash smelting slag and on lead oxide associated with SiO2, while it reacts slightly with lead (II) sulfate, which was formed as a result of acid leaching with sulfuric acid. Literature information [18] states that when both C6H5Na3O7·2H2O and C6H8O7·H2O reactants were used together, it was possible to achieve effective leaching of lead:
$$3{\text{PbSO}}_{4} + 2\left( {{\text{Na}}_{3} {\text{C}}_{6} {\text{H}}_{5} {\text{O}}_{7} \cdot 2{\text{H}}_{2} {\text{O}}} \right) = \left[ {3{\text{Pb}} \cdot 2\left( {{\text{C}}_{6} {\text{H}}_{5} {\text{O}}_{7} } \right)} \right] \cdot 3{\text{H}}_{2} {\text{O}} + 3{\text{Na}}_{2} {\text{SO}}_{4} + {\text{H}}_{2} {\text{O}}{.}$$(19) -
(B) - 3
Alkaline leaching
In the next step, the slag after leaching with sulfuric acid was subjected to alkaline leaching in a NaOH solution. During leaching, lead in the form of PbSO4 is converted into a soluble Na2PbO2 compound according to the following reaction:
$${\text{PbSO}}_{4} + 4{\text{NaOH}} \to {\text{Na}}_{2} {\text{PbO}}_{2} + {\text{Na}}_{2} {\text{SO}}_{4} + 2{\text{H}}_{2} {\text{O}}{.}$$(20)Two alkaline leaching tests were performed, differing in NaOH concentration, temperature and l/s ratio. The test time was identical in both attempts.
-
1.
4 mol/dm3 NaOH, 80 °C, 120 min. and l/s = 50.
Alkaline leaching carried out for these parameters allowed reducing the lead content in the slag from 4.44 to 0.99% and achieving a lead leaching efficiency of 77.4%.
-
2.
2 mol/dm3 NaOH, 40 °C, 120 min. and l/s = 30.
Under these conditions, a decrease in the lead concentration in the slag to 0.84% was noted. Lead leaching efficiency was 79.3%.
-
1.
-
(B) - 1
3 Results and discussion
3.1 Results of experimental research
The analysis of the chemical composition of flash smelting slag from direct-to-blister process indicates that it is characterized by high contents of copper, lead, and zinc. From the point of view of economics and ecology, this material cannot be treated as waste and therefore recovery of the above-mentioned metals is carried out. The tests of slag processing carried out in the first part of this paper showed that it is possible to separate fractions from the whole mass of obtained slag in which the content of interesting metals is increased and, as a consequence, they can be easily and cheaply processed in the direction of extraction of these metals. Such material is the grain fraction 0.63 ÷ 1.00 mm obtained during sieve analysis of flash smelting slag. Although its share in the resulting slag is only 1.56%, it contains 46.2% Cu and 1.95% Pb and can, for example, be used as a rich input material for a flash furnace. The use of slag enrichment methods in heavy liquids seems to be problematic. Despite the fact that the material in which copper enrichment was over 55% (suspended phase) was obtained, it is difficult at this point to show the ways of its use in the existing system of devices. Magnetic separation tests performed on crushed slag samples indicated another fraction that could be used in the production process. In the magnetic belt separator, a non-magnetic fraction was obtained in which the copper content was over 48%. The non-magnetic fraction was 7.6% of the total slag mass and, like the 0.63 ÷ 1.00 mm grain fraction, it could be recycled for melting in a flash furnace.
Hydrometallurgical studies of flash smelting slag processing have shown that selective copper and lead extraction from this slag is possible. Leaching with a low concentration of citric acid solution, carried out for a short time at a low temperature, leads to an effective leaching of lead from the slag with a low copper leaching efficiency. Under conditions of high concentration of citric acid and higher process temperature, copper passes into the solution, and most of the lead is not leached.
The use of a sulfuric acid solution with the addition of an oxidant allowed the removal of copper from the slag with a yield of 96%. The material after this processing step contained 0.77% Cu and 4.44% Pb. Tests carried out at a later stage to identify the reagent that can be used to remove lead from the material obtained in the first stage indicated that high efficiency of transferring lead to the solution is obtained when using acetic acid solution with the addition of urea and NaOH solutions. The residue after leaching with acetic acid and urea contained 0.39% Pb, and the process yield was 88%. The best result obtained for NaOH solutions was 0.84% Pb in the residue, and leaching efficiency was equal to 79.3%. The extraction of copper from aqueous solutions can take place in the process of electrolysis using insoluble anodes. Lead recovery can take place through precipitation or also in the electrolysis process of obtained solutions.
3.2 Potential benefits of flash smelting slag processing with the use of mineralurgical and hydrometallurgical methods
The annual production of copper in the direct-to-blister technology at the Głogów II smelter is around 220,000 tonnes. The specificity of the process is associated with the need to copper remove from approximately 500,000 tons of slag with Cu content of 12–16%. In the works [3, 19] one can find information that about 185,000 MWh of electricity is used annually in an electric furnace for slag processing. According to the available data [20,21,22], about 16,000 tons of carbon in the form of coke and electrode mass are used annually for the reduction process. As a result of the reduction of metal oxides, CO2 is produced in an amount of almost 60,000 tons. The analysis of the permissible levels of pollutants in the plant given in the work [20] allowed for the estimation of the reduction of emissions to the atmosphere of arsenic, cadmium, and lead after removing the electric furnace and converters for processing Cu–Pb–Fe alloy from the process line. Based on the maximum hourly emission and the annual emission time from the electric furnace and converters, it was found that the share of these aggregates in the total emission of the entire technological process of cathode copper production is over 41% for arsenic, over 59% for cadmium and over 84% for lead. Therefore, it can be concluded that if the flash smelting slag was not processed in electric furnace and converters, it would reduce the annual emissions to the atmosphere in the amount of 120.6 kg As, 9.6 kg Cd and over 1587 kg Pb.
The processing of slag from direct-to-blister technology by mineralurgical and hydrometallurgical methods requires its grinding. It can be assumed that this process will be the most energy-consuming of all activities aimed at extracting the most valuable components from the slag. According to available literature sources [23,24,25], crushing and grinding of slag of similar composition and form involves energy inputs in the amount of 40–66 kWh/ton. Assuming the most unfavorable variant of consumption and grinding 500,000 tons of slag, the annual energy consumption for this processing stage is 33,000 MWh. Specified amounts of energy would be needed to drive the conveying equipment, leach mixers, pumps, filter presses, and to maintain the temperature of the leaching solutions at the required level. Since the flash smelting process is a highly exothermic process, surplus thermal energy could be successfully used in the process of heating and maintaining the required temperature of the leaching solution. Assuming that, if all other auxiliary processes would consume another 33,000 MWh/year, the total energy consumption for mineralurgical and hydrometallurgical processing would be approximately 66,000 MWh.
Therefore, by balancing the two methods of handling slag, the existing one and the one consisting of mineralurgical and hydrometallurgical processing, it can be concluded that replacing the current technology would reduce energy consumption by almost 120,000 MWh/year. Carbon consumption in the reduction process would drop by 16,000 tons, and as a consequence, almost 60,000 tons of CO2 would not be emitted into the atmosphere. In addition, about 120 kg of arsenic, almost 10 kg of cadmium and over 1500 kg of lead would not be emitted into the atmosphere each year.
Although the above analysis includes only some elements of the technological process of slag processing in two alternative variants, the authors believe that they are the most important from the ecological and economic point of view in the two compared processes. Thus, after the presented analysis, one can risk a theorem that grinding slag, separation of fractions enriched in copper or lead, and then hydrometallurgical processing of the remaining part will be much less negative project affecting the natural environment due to lower CO2 and heavy metal emissions. Much lower energy consumption will result in lower processing costs and may constitute an economic justification for the need to change the current technology.
4 Conclusions
During direct-to-blister smelting of copper concentrates, slag is formed in which the copper content reaches up to 15%. In addition, it may contain up to 4% Pb. The recovery of copper and, by the way, other valuable elements contained in the slag takes place in an electric furnace. The slag flows from the direct-to-blister furnace directly into an electric furnace where it is settled for about 10 h under a 0.25 m blanket of metallurgical coke. The above process is long and energy-consuming. The research carried out in this work indicates that it would be possible to carry out Cu and Pb recovery in a different way, using mechanical slag processing: grinding, sieving, segregation based on differences in specific weight, magnetic separation. This way it is possible to get certain amounts of materials in which there is an increased content of interesting elements.
The application of hydrometallurgical processes and the selective transfer of copper and lead to the solution are possible using citric acid solutions and conducting the process in diametrically different process conditions. A two-stage slag processing method is also possible. In the first stage, copper is removed from slag with a sulfuric acid solution. In the second stage, the lead is extracted into the solution using acetic acid with the addition of urea or with NaOH solutions.
The use of mineralurgical and hydrometallurgical methods seems to be much more environmentally friendly compared to the currently used high-temperature slag processing process in an electric furnace and converter. The analysis carried out indicate that energy consumption, emission of pollutants into the environment and, as a consequence, the cost of slag processing in properly selected operations of mechanical slag processing, its leaching and recovery of valuable components would be much lower than the values obtained in currently used technologies.
References
Gostyński Z, Haze D. Flash smelting furnace of the KGHM Glogow copper plant - technological and process challenges as a driving force of its continuous modernization. In: Warmer AEM, Newman CJ, Vahed A, George DG, Mackey PJ, Warczok A, editors, Copper/cobre 2007, vol. III, Book 2: the carlos diaz symposium of pyrometallurgy. 2007. pp. 233–243.
Taskinen P, Kojo I. Fluxing options in the direct-to-blister copper smelting. In: Proceedings of molten 2009 international conference. 2009. pp. 1139–1151.
Piestrzyński A, Zaleska-Kuśmierczyk M (2007) Monograph of KGHM Polska Miedź SA, 2 Eds., Wrocław: KGHM Cuprum Lubin.
Kucharski M, Sak T, Madej P, Wędrychowicz M. A study on the copper recovery from the slag of the outokumpu direct-to-copper process. Metall Mater Trans B. 2014;45:590–602.
Czernecki J, Śmieszek Z, Miczkowski Z, et al. The slag cleaning technologies for one-stage flash smelting of KGHM Polska Miedz concentrates. In: Kongoli F, Reddy RG, editors. Sohn international symposium, vol. 8, International symposium on sulfide smelting. 2006. pp. 181–197.
Śmieszek Z, Czernecki J, Sak T, Madej P. Metallurgy of non-ferrous metals in Poland. J Chem Technol Metall. 2017;52:221–34.
Byszyński L, Garycki L, Gostyński Z, Stodulski T, Urbanowski J. Present and future modernization of metallurgical production lines of the Glogow copper smelter. In: Proceedings of copper 2010, vol. 2, Pyrometallurgy I. 2010. pp. 631–647.
Mineralogy Database. http://webmineral.com. Accessed 12 Dec 2019.
Gupta A, Yan DS. Mineral processing design and operation: an introduction. Amsterdam: Elsevier; 2006.
Svoboda J, Fujita T. Recent developments in magnetic methods of material separation. Miner Eng. 2003;16:785–92.
Oberteuffer J. Magnetic separation: a review of principles, devices, and applications. IEEE Trans Magn. 1974;10:223–38.
Drzymała J. Podstawy mineralurgii (basics of mineralurgy). 2nd ed. Wrocław: Oficyna Wydawnicza Politechniki Wrocławskiej; 2009.
Sonmez MS, Kumar RV. Leaching of waste battery paste components. Part 1: lead citrate synthesis from PbO and PbO2. Hydrometallurgy. 2009;95:53–60.
Gargul K, Boryczko B, Bukowska A. Hydrometallurgical recovery of lead from direct-to-blister copper flash smelting slag. Arch Civ Mech Eng. 2017;17:905–11.
Gargul K, Boryczko B, Bukowska A, Jarosz P, Małecki S. Leaching of lead and copper from flash smelting slag by citric acid. Arch Civ Mech Eng. 2019;19:648–56.
Gargul K, Bukowska A. Leaching of direct-to-blister copper flash smelting slag with sodium hydroxide solutions. Przem Chem. 2017;96:2464–6.
Buzatu T, Ghica VG. Solubilization kinetics of lead hydroxide obtained from sulfated-oxide waste from lead-acid battery in acetic acid in the presence of urea. Rev Chim. 2013;64:170–3.
Sonmez MS, Kumar RV. Leaching of waste battery paste components Part 2: leaching and desulphurisation of PbSO4 by citric acid and sodium citrate solution. Hydrometallurgy. 2009;95:82–6.
The National Centre for Research and Development. https://www.ncbr.gov.pl/fileadmin/user_upload/import/tt_content/files/zalacznik_nr_1_do_regulaminu_zakres_merytoryczny.pdf. Accessed 12 Sept 2020.
Decision of the Marshal of the Lower Silesian Voivodeship no PZ 83.13/2017 of July 12, 2017, sign: DOWS-IV.7222.0.2017.LS. https://bip.dolnyslask.pl/urzad/Article/get/id,110836.html. Accessed 15 Sept 2020
Siwiec G, Sozańska M, Blacha L, Smalcerz A. Behaviour of iron during reduction of slag obtained from copper flash smelting. Metalurgija. 2015;54:113–5.
Kucharski M, Rogóż K. Method for decoppering waste slag, especially after flash furnace process of obtaining copper. Patent nr. 213767. 2009.
Coursol P, Mackey PJ, Díaz CM. Energy consumption in copper sulphide smelting. In: Proceedings of copper 2010, vol. 2, Pyrometallurgy I (Clausthal-Zellerfeld: GDMB, 2010), pp. 649–668.
Li Y, Guan J. Life cycle assessment of recycling copper process from copper-slag. In: 2009 International conference on energy and environment technology, ICEET. pp 198–201.
Mateuszuk S. Selected issues of materials milling in vertical roller-mills. Sci Works Inst Ceram Build Mater. 2012;9:113–24.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declared that they have no conflict of interest.
Ethical approval
Research was done according to ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gargul, K., Boryczko, B., Bukowska, A. et al. Behavior of copper and lead during mineralurgical and hydrometallurgical processing of flash smelting slag. Archiv.Civ.Mech.Eng 21, 26 (2021). https://doi.org/10.1007/s43452-021-00184-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s43452-021-00184-9