Abstract
Salt-affected soils urgently need to be remediated to achieve the goals of carbon neutrality and food security. Limited reviews are available on biochar performance in remediating salt-affected soils in the context of carbon neutrality and climate change mitigation. This work summarized the two pathways to achieve carbon neutrality during remediating salt-affected soils using biochars, i.e., biochar production from sustainable feedstock using thermal technologies, application for promoting plant productivity and mitigating greenhouse gas (GHG) emission. Converting biomass wastes into biochars can reduce GHG emission and promote carbon dioxide removal (CDR), and collection of halophyte biomass as biochar feedstocks, development of biochar poly-generation production systems with carbon neutrality or negativity could be promising strategies. Biochar can effectively improve plant growth in salt-affected soils, showing that the grand mean of plant productivity response was 29.3%, via improving physicochemical characteristics, shifting microbial communities, and enhancing plant halotolerance. Moreover, biochar can mitigate GHG emission via inducing negative priming effect, improving soil properties, changing microbial communities associated with carbon and nitrogen cycle, direct adsorption of GHG. However, biochar also may pose negative effects on plant growth because of stress of toxic compounds and free radicals, and deterioration of soil properties. The promoted GHG emission is mainly ascribed to positive priming effect, and provision of labile carbon and inorganic nitrogen fractions as microbial substrates. Finally, this review pointed out the gaps in the current studies and the future perspectives. Particularly, the development of “carbon neutral” or “carbon negative” biochar production system, balancing the relationship of biochar effectiveness and functionality with its environmental risks and costs, and designing biochar-based GHG adsorbents would be important directions for remediating salt-affected soils to achieve carbon neutrality and abate climate change.
Graphical Abstract

Highlights
-
Sustainable biochar production could be a promising strategy to achieve carbon neutrality.
-
Biochar can significantly improve plant productivity in salt-affected soils by 29.3%.
-
Biochar could reduce GHG emission and facilitate CO2 removal in salt-affected soils.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The global climate change including temperature anomalies and extreme rainfall patterns has brought severe challenges to the stability and health of natural ecosystems, as well as to human survival and development (Hoegh-Guldberg et al. 2019). Carbon neutrality, achieving the “net zero emission” of carbon dioxide (CO2) by offsetting increasing amount of CO2 released into atmosphere, has become one of the leading global environmental goals of the twenty-first century (Wang et al. 2021; Wu et al. 2022). Effective strategies for climate change mitigation require both reduction of greenhouse gas (GHG) emission and enhancement of CO2 removal (CDR) from atmosphere (Lehmann et al. 2021). Soil is the largest terrestrial organic carbon pool (~ 1500–2400 Pg carbon), which profoundly determines the achievement of carbon neutrality (Rumpel et al. 2020). Therefore, enhancing carbon sink function in soil ecosystems through reducing GHG release and increasing vegetation carbon sequestration has been recommended as a win–win strategy for mitigating climate change and sustaining food productivity (Wang et al. 2021). However, about 29% of the global land area is facing degradation such as desertification, erosion, pollution, acidification, sodification, and salinization (Jia et al. 2019), leading to the irreversible loss of soil ability to sequester carbon (Ferreira et al. 2022). The Intergovernmental Panel on Climate Change (IPCC) estimated that the loss of soil organic carbon (SOC) from soil degradation and land use change contributed to an emission of 0.9 Gt carbon per year on average between 2002 and 2011 (Jia et al. 2019). Salinization and sodification, the typical types of soil degradation resulted from the combined action of natural and human factors (Daliakopoulos et al. 2016; Hassani et al. 2021), have generated a large number of salt-affected soils worldwide (FAO 2015). As shown in Fig. 1, soil salinization is also driven by climate change, including rising earth surface temperature and sea-level, and changing in precipitation rates, which in turn would further contribute to global climate change (Eswar et al. 2021). Salt-affected soils occupy approximately 10.3 × 108 ha in the world (FAO 2015). Approximately 3.69 × 107 ha soil, accounting for 4.88% of total available land in China, has been subjected to salinization and sodification (Li et al. 2014). The excessive concentrations of sodium (Na+) in salt-affected soils lead to a large amount of SOC decomposition and CO2 emission due to lack of physical protection of aggregates (Setia et al. 2011). For example, the SOC content in surface coastal salt-affected soils is 6 g kg−1, only accounting for 42% of those in agricultural soils (Lin et al. 2015). In addition, an extreme example is the coastal blue carbon ecosystem that emits 0.15–1.02 Pg CO2 annually due to soil degradation or altered use patterns, equivalent to 3–19% of those from deforestation globally (Pendleton et al. 2012). Furthermore, the loss of SOC can cause deterioration of other soil physicochemical properties such as bulk density, water holding capacity, pH, cation exchange capacity (CEC), and nutrient availability, thus leading to low primary productivity and biodiversity of salt-affected soils (Haj-Amor et al. 2022). Low primary productivity results in the low input and accumulation of SOC in salt-affected soils, which not only intensifies the loss of soil fertility, but also decreases the potential of soil carbon sequestration (Zheng et al. 2018b). In addition, salt-affected soil is an important reserve land resource for implementing land-based climate change mitigation strategies because they can serve as potential lands for biochar and bioenergy feedstock production (Kumar et al. 2022). Therefore, it is imperative to develop sustainable strategies to reclaim salt-affected soils to expand arable land availability and enhance carbon sinks to contribute the goal of carbon neutrality.
Formation process of salt-affected soils and traditional approaches for remediating salt-affected soils. The light blue arrows show the primary salinization/sodification processes, including: (A) salt accumulation from rock weathering; (B) salt accumulation due to excessive water evaporation in arid and semiarid regions; (C) salt accumulation due to sea breeze; (D) salt accumulation from wet deposition of oceanic salts. The orange arrows show the secondary salinization/sodification by human activities, including (a) irrigation with saline water; (b) groundwater recession due to poor land and water management; (c) seawater incursion due to rising sea levels or over-exploitation of underground waters. Traditional approaches for reclaiming salt-affected soils include physical methods such as (i) leaching and (ii) draining off salt; chemical methods such as (iii) application of gypsum and (iv) application of compost, and biological methods such as (v) phytoremediation, (vi) microbial remediation, (vii) plant-microbial remediation
Although traditional techniques including physical, chemical, and biological methods, which are summarized in Fig. 1, could improve salt-affected soil quality and mitigate salt stress for plants to a certain extent (Arif et al. 2020; Qin et al. 2016), the new viewpoints should be considered during remediation to achieve the goals of carbon neutrality or carbon negative and climate change mitigation. Biochar, a technology coupled with carbon sequestration and soil improvement, provides an option (Lehmann & Joseph 2015; Zheng et al. 2018a). Biochar is a solid and highly aromatized carbon rich material pyrolyzed from various biomass at a relatively low-temperature (≤ 700 °C) under oxygen-limited conditions (Lehmann and Joseph 2015). So far, biochar has been widely used for multiple purposes, including carbon sequestration (Song et al. 2022), soil improvement and remediation (El-Naggar et al. 2019), and bio-energy resource utilization (Yang et al. 2021a), because of its excellent characteristics such as high carbon stability, rich pore structure, large specific surface area, and abundant surface functional groups (Al-Wabel et al. 2018; Xiao et al. 2018). In particular, the aromatic structure of biochar is the primary reason for its strong persistence, as a result, biochar can be used as a carbon negative tool in promoting carbon sequestration and reducing GHG emission in soils (Wang et al. 2015). A meta-analysis based on two documented databases (128 observations from 24 studies for biochar persistence and 116 observations from 21 studies for biochar effect on soil organic matter (SOM) mineralization) showed that the size of recalcitrant carbon pools in biochars was about 97%, and only reduced native SOM mineralization by 3.70%, contributing to long-term soil carbon sequestration (Wang et al. 2015). In addition, Liu et al. (2019) reported that biochar could significantly decrease soil GHG emission intensity by 29.0% through a meta-analysis based on 81 observations from 28 studies. These studies and analyses show the promising potentials of biochar in long-term carbon sequestration and soil GHG emission reduction, contributing to soil carbon sink functions. Recently, Lehmann et al. (2021) proposed that biochar systems, including the biomass production sub-system, thermal conversion sub-system, and soil sub-system, can be used as promising strategies to mitigate climate change by reducing GHG emission and facilitating CDR (Fig. 2). So far, increasing studies have demonstrated the positive effects regarding plant growth following biochar application in salt-affected soils (Cui et al. 2022; Zheng et al. 2018a). This promotion effect on plant growth is an important way for biochar technology to facilitate CDR in soil sub-systems (Lehmann et al. 2021). However, not all biochars may bring positive effects on physicochemical characteristics and plant growth in salt-affected soils (Kazemi et al. 2019; Zhang et al. 2019b). Additionally, biochar also could mitigate or increase GHG emission from salt-affected soils (Zhang et al. 2018). Obviously, the uncertainties on plant growth and GHG emission still exist following biochar application into salt-affected soils. To better understand the performance of biochar in improving salt-affected soils in terms of GHG emission reduction and CDR enhancement, a comprehensive review to gauge research progress and identify the knowledge gaps for highlighting future research directions is a prerequisite.
Biochar system on climate change mitigation. Biochar system can be divided into biomass sub-system (green gear), thermal conversion sub-system (yellow gear), and soil sub-system (blue gear) (Lehmann et al. 2021). The plus “ + ” indicates the processes that promote GHG emission, including collection and transportation of biomass feedstock or biochar and emissions associated with the thermochemical conversion such as the emissions about electricity, heat, and labor force. The minus “−” indicates the processes that reduce GHG emission, including replacing unreasonable biomass management, replacing fossil fuels with bioenergy (syngas or bio-oil), promoting carbon capture and storage (CCS), and enhancing plant growth and reducing GHG emission in soils. Reproduced and modified from Lehmann et al. (2021) after a permission of the publisher
Many reviews have been published on production and characterization of biochars (Khosravi et al. 2022; Xu et al. 2021), and their applications as fertilizers (El-Naggar et al. 2019), adsorbents (Ambika et al. 2022), and amendments for soil quality improvement and pollutant remediation (El-Naggar et al. 2019). To our best knowledge, only three reviews simply summarized biochar application in salt-affected soils (Ali et al. 2017; Amini et al. 2015; Saifullah et al. 2018). However, several gaps still exist in these reviews: (1) the understanding of biochar amelioration in salt-affected soils from the perspective of carbon neutrality and climate change mitigation is absent; (2) the carbon-negative production mode in salt-affected soil improvement using biochar has not been fully discussed yet; (3) the potential mechanisms of biochars on plant growth in salt-affected soils, especially the negative response mechanisms, still need to be critically assessed to avoid potential environmental risks; and (4) the effects and mechanisms of biochar on GHG emission in salt-affected soils have not been reviewed. Therefore, the aims of this review are to: (1) summarize the pathways to achieve carbon neutrality during remediating salt-affected soils using biochars; (2) explore the carbon-negative production mode in salt-affected soil improvement using biochar; (3) clarify the effects and potential mechanisms of biochars on plant growth in salt-affected soils; and (4) elucidate the effects and mechanisms of biochars on GHG emission from salt-affected soils. Finally, this review points out the gaps in the current studies and the prospects that need to be addressed.
2 Biochar production and classification
In recent years, the mass production of waste biomass and adoption of inappropriate recycling technologies (e.g., landfilling, direct burning) significantly contribute to GHG emission (Yang et al. 2021a). As shown in Fig. 2, converting biomass wastes into biochars followed by soil applications can reduce soil GHG emission and promote CDR, which is conducive to abate climate change (Lehmann et al. 2021; Yang et al. 2021a). Due to its persistence, the carbon mineralization and non-CO2 emissions from biochar in soils are one to two orders of magnitude lower than those of unpyrolysed biomass (Zhang et al. 2019a), and the GHG mitigation potential of biochar largely depends on the sustainability of biomass feedstock (Lehmann et al. 2021). In addition, bio-oil (also called pyroligneous acids, wood vinegar, liquid smoke) and syngas produced during biomass thermal conversion can be used as an alternative to fossil fuels, combining with carbon capture and storage (CCS) technology to reduce GHG release (Tisserant and Cherubini 2019).
2.1 Biochar production
2.1.1 Feedstock
Parent feedstock is a critical factor for controlling biochar physiochemical characteristics and functionalities (Xiao et al. 2018). Compared with original biomass (scale of week to years), biochar itself has lower carbon mineralization and non-CO2 emissions due to its high persistence of centennial to millennial time scales, which is discussed in the subsequent soil sub-systems (Fig. 2).
Selection of parent feedstock would significantly affect the potential of biochar to mitigate climate change, depending on the sustainability of biomass and the allocation of land for biochar production (Fig. 2, Additional file 1: Fig. S1). Sustainably available biomass refers to the biomass that can be collected as biochar feedstock within a certain period and then can regrow without influencing the current productivity of the ecosystem (Lehmann et al. 2021; Woolf et al. 2010). In early period, Woolf et al. (2010) evaluated that the sustainably available biomass of biochar such as crop residues, manures, crops, timber and forest residues, and green waste could decrease 1.8 Pg CO2 equivalent (CO2e) per year and 130 Pg CO2e over the course of one century. Recently, Lehmann et al. (2021) assessed the carbon sequestration potential of planting annual and perennial biomass and converting it into biochars on all the abandoned farmlands which have not been converted into cities, forests, or pastures using a biochar global response assessment model (BGRAM) algorithm. They found that the emission reduction potential would reach 3.40–6.30 Pg CO2e year−1, with the CDR potential accounting for 44.0–49.0%. Similarly, most salt-affected soils are not suitable for crop production, and the production of biochar feedstock (mainly referring to halophyte biomass waste) using these soils can relieve the direct competition with food production for high quality land. Halophytes, accounting for 1% of the global flora, grow in a wide range of salt-affected soils from arid regions to coastal wetlands (Debez et al. 2010), and the average dry biomass yield on salt-affected soils was 3.1 t ha−1 year−1 (Wicke et al. 2011). Therefore, converting the halophyte biomass wastes (e.g., Suaeda salsa, alfalfa, Phragmites australis) (Xiao et al. 2022), or halophytic invasive plants (e.g., Spartina alterniflora, Sorghum halepense) (Cui et al. 2021), or even algae in coastal wetlands (Zhao et al. 2021) to biochars and returning them as amendments to salt-affected soils or other marginal lands may be a win–win strategy to strengthen rational use of waste resources and enhance soil carbon sequestration capacity (Al-Marzooqi and Yousef 2017; Dong et al. 2022; Xiao et al. 2022). For example, Al-Marzooqi and Yousef (2017) used Salicornia bigelovii to produce biochar at 550 °C and applied it to remediate a sandy salt-affected soil in Abu Dhabi. They observed that the biochar application significantly increased SOC contents by 1.0–2.6% (Al-Marzooqi and Yousef 2017). More information regarding expansion of biochar feedstock types is provided in Additional file 1: Text S1. Notably, the selected halophyte biomass should first be targeted according to a specific soil (El-Naggar et al. 2019). Considering the further application of halophyte-derived biochars for remediating salt-affected soils or other marginal lands (e.g., acidic soil and contaminated soil), the contents of nutrients, salts, and contaminants in these biomasses and corresponding biochars need to be carefully tested by proximate, ultimate, and thermogravimetric analyses to identify the best options for a particular application. Additional file 1: Table S1 summarizes the properties of typical halophytes and their derived biochars, as well as the targeted soils that the biochars were added to. The selection of halophyte biomass should also follow the principle of accessibility (Wang et al. 2023). However, one potential concern is that halophytes are important components of salt-affected soil ecosystems, and the unreasonable harvest may disrupt ecosystem stability and decrease biodiversity (Xie et al. 2018). Hence, it is recommended to collect halophyte biomass in salt-affected soils which produce abundant biomass waste and the biomass can be regenerated quickly, such as lands undergoing ecological restoration project using halophytes (Wang et al. 2023). Also, future studies should specify the collection frequency and the maximum single harvest amount of halophyte biomass based on the growth rate of the specific halophyte species, the size and density of the local halophyte population, and soil conditions to ensure that the biomass harvesting is sustainable.
2.1.2 Thermal conversion
In the thermal conversion sub-system of biochar systems (Fig. 2), the processes associated with thermochemical conversion of biomass will result in GHG emissions (Rajabi Hamedani et al. 2019; Yang et al. 2021a), while the bioenergy can be used as alternatives to conventional fuels and combined with CCS technology to promote CDR (Chen et al. 2022; Lund et al. 2022; Woolf et al. 2021).
Biomass can be converted into char, bio-oil, and syngas by different thermal technologies, including slow pyrolysis, fast pyrolysis, flash pyrolysis, microwave pyrolysis, gasification, and hydrothermal carbonization (HTC) (Additional file 1: Fig. S1), which has been well-reviewed previously (Khosravi et al. 2022; Liu et al. 2017b). Throughout a life cycle of biochar systems, the processes associated with thermochemical conversion of biomass, including biomass transportation, pre-drying treatment, and consumption of building materials, energy, labor, and tap water, would result in a large amount of CO2 emission (Additional file 1: Table S2). For example, according to an analysis carried out by Yang et al. (2021a), the carbon emission caused by collecting 1 t of crop residues in the field was equivalent to 76.5 kg CO2e, and converting 1 t of crop residue into biochar by slow pyrolysis released 392 kg CO2e, the largest contributor to the whole biochar system. Although bioenergy generated in the heat conversion process can be recycled as a substitute for fossil fuel, and biochar has strong carbon sequestration potential when applied to soils (Lehmann et al. 2021; Yang et al. 2021a), how to minimize the additional carbon footprint generated in the heat conversion process of biomass to enhance the negative carbon potential of an entire biochar system needs to be investigated. HTC is attractive because it can directly transfer wet biomass containing high moisture content (> 30%) (e.g., sewage sludge, pig manure, and algae) into char product without pre-drying treatment (Khosravi et al. 2022). This is the greatest advantage for HTC among all thermal technologies because it can largely decrease extra energy input and cost (Khosravi et al. 2022), thus potentially reducing CO2 emissions from thermal conversion process of wet biomass (Medick et al. 2018). Medick et al. (2018) reported that the emission reduction potential of hydrochar could reach 7.1 × 104 t CO2e year−1 if compost derived from green waste is converted to hydrochars in Berlin, Germany. Comparing the emission reduction data of different thermal technologies used for biochar and hydrochar production will provide insights into their potentials for reducing carbon emission and mitigating climate change, enabling reasonable selection of appropriate thermal technologies. However, so far, little information is available on comparison of the emission reduction potentials of different thermal technologies used for biochar and hydrochar production. In addition, current biochar thermal technologies also need to be further improved (e.g., development of solar heating and autothermal system, application of CCS technologies in thermal conversion process) to develop “carbon neutral” or “carbon negative” systems for biochar production.
So far, limited studies have reported that typical halophyte biomass (e.g., Spartina alterniflora, S. alterniflora powder, and Suaeda salsa) could produce biochar, bio-oil, and syngas with a yield of 9.80–94.3%, 1.67–76.4%, and 13.2–45.2%, respectively (Additional file 1: Table S3). Both bio-oil and syngas with calorific values of 19.7–24.7 MJ kg−1 and 30.0–79.0 MJ kg−1 (Additional file 1: Table S3) can be directly used as biofuel or industrial raw materials (Wang et al. 2023). However, large scale utilization of bio-oil or syngas as biofuels may show great challenges such as low energy density and instability (Duarah et al. 2022), which need to be upgraded or pre-treated (e.g., emulsification, steam reforming, catalytic cracking, and zeolite cracking) to meet the quality and specification requirements for their intended applications (Ahamed et al. 2021). In addition, bioenergy can be combined with CCS technologies to capture the extra CO2 produced during bioenergy combustion by carbon sequestration in underground, referred to as bioenergy with carbon capture and storage (BECCS) (Tanzer et al. 2021). BECCS is one of CDR techniques endorsed by the IPCC (Tanzer et al. 2021). A typical case is the BECCS pilot project of Drax power station in the UK, which can capture up to 1 t of CO2 per day from flue gas (Affan and Maaroof 2016). Similarly, bioenergy from halophyte-derived biomass may also be combined with CCS to enhance the negative carbon potential of biochar systems, unfortunately, which has not been reported yet. Hence, future studies are needed to optimize the process of converting halophyte biomass into biochar and bioenergy to develop efficient BECCS technologies. In addition, the pilot projects should be initiated to test the feasibility and scalability of combining halophyte-derived bioenergy with CCS technologies in different regions or specific application scenarios. Recently, Yang et al. (2021b) first evaluated the contribution of biomass intermediate pyrolysis poly-generation (BIPP) to carbon emission reduction in China. BIPP, a multi-product production mode including a pyrolytic reaction system and a heat recovery system, not only can produce biochar with comparatively high stability for soil improvement and carbon sequestration, but also can provide considerable heat and opportunities for generation of electricity via production of syngas and bio-oil (Chen et al. 2016). They found that the BIPP system can be profitable without governmental subsidies, and can cumulatively reduce 8.62 Gt CO2e GHG emission by 2050, contributing to 13–31% of the global GHG emission reduction goal for BECCS. While there are no engineering case studies for deploying BIPP in salt-affected soils, several laboratory-scale researchers have preliminarily demonstrated the feasibility of this multi-product production mode (Iaccarino et al. 2021; Irfan et al. 2016; Makkawi et al. 2021). For example, Makkawi et al. (2021) used Salicornia bigelovii seed and the seedless-plant as feedstock to produce biofuel and biochar through pyrolysis in an auger reactor at 550 °C. They showed that the pyrolysis of seeds had a high bio-oil yield (80%), and the biochar had a high potential for soil carbon sequestration. In the future, with the further technological development and policy support, this multi-product production mode is expected to be more widely applied in salt-affected soils, while providing additional bioenergy.
2.2 Biochar standards and classification
The standards and classification of biochars, regulating feedstock selection and thermal conversion process (Camps-Arbestain et al. 2015; Meyer et al. 2017), are basics for developing negative biochar technology to reduce global GHG emission (Wang et al. 2021). Several voluntary biochar standards or guidelines have been proposed by different biochar organizations, including International Biochar Initiative (IBI) (Camps-Arbestain et al. 2015), European Biochar Community (EBC) (Schmidt et al. 2016), and Biochar Quality Mandate (BQM) (Shackley et al. 2014). Meanwhile, several countries including China, Germany, Austria, Switzerland, and Italy, also fitted biochar into the existing national legislation of fertilizers, soil amendments and composts (Meyer et al. 2017). Figure 3 and Additional file 1: Table S4 document the biochar different standards from voluntary organizations and different national policies in detail. These different biochar standards include quality requirements for biochar, environmental thresholds for organic pollutants and heavy metals, and other requirements of feedstock type, transportation distance, and production technology (see more details in Additional file 1: Text S2). Based on IBI guidelines (IB1 2015), the pyrolytic product containing organic C (Corg) less than 10% (w%) or the molar H/Corg ratio higher than 0.7 cannot be classified as a biochar. According to the EBC requirements (Schmidt et al. 2016), the Corg content of a biochar must be higher than 50% (w%), or the molar H/Corg and O/Corg ratio must be lower than 0.7 and 0.4, respectively. A product with a Corg content lower than 50% or H/Corg ratio lower than 0.7 can be classified as a pyrogenic carbonaceous material (PCM), but cannot be classified as a biochar (Schmidt et al. 2016). The BQM defined that stable Corg content of a biochar should at least be 10% (w%), and the H/Corg ratio must be lower than 0.7 (Shackley et al. 2014).
Standards and classification systems of biochars. Voluntary biochar standards or guidelines have been proposed by several biochar organizations, including International Biochar Initiative (IBI), European Biochar Community (EBC), and Biochar Quality Mandate (BQM). Several countries including China, Germany, Austria, Switzerland, and Italy, attempted to fit biochar production and application into their national legislations for fertilizers, composts, and soil amendments. A classification system based on IBI and EBC classified biochar into five categories according to its potential benefits in soils, which was proposed by Camps-Arbestain et al. (2015). sBC+100, representing the C storage value of biochar, is calculated by the estimated fraction of biochar organic carbon (Corg) that has remained stable in soil for more than 100 years. Liming value of biochar is expressed as CaCO3 equivalent (CaCO3-eq)
Based on the standards proposed by IBC and EBC, Camps-Arbestain et al. (2015) classified biochars according to the potential benefits of their application in soils into five categories, including (i) carbon storage, (ii) fertilizer value, (iii) liming potential, (iv) particle‐size classes, and (v) use in potting mixes and soilless agriculture (Fig. 3). Although the current standards and guidelines do not stipulate the application rules of biochar in specific types of soils, biochar applied in salt-affected soils still needs to comply with the requirements for feedstock, production methods, and quality control established by IBI, EBC, BQM, or national laws and regulations. In addition, the requirements of biochar quality and stability should address the specific challenges associated with poor soil structure, low carbon content, high salt content, and low nutrient availability in diffident salt-affected soils. Saline soils contain excess salts to endow their electrical conductivity (EC) values greater than 4 dS m−1, which generally cause osmotic stress to plants and microorganisms (Kotula et al. 2020). Therefore, it is recommended to select biochar with high nutrient value and low liming value (CaCO3-eq < 1%) to minimize additional salt input during improving soil primary productivity. For saline-sodic soil or sodic soil with exchangeable sodium percentage (ESP) higher than 15 or sodium adsorption ratio (SAR) greater than 13, high Na+ and low salt levels may cause serious clay dispersion, degradation of aggregate structure, and loss of macroporosity (Rengasamy and Olsson 1991). Hence, when applying biochar to these two types of soils, the selection of biochar with high carbon storage value (sBC+100 ≥ 600 g kg−1) and large particle size (50% biochar fraction > 16 mm) should be emphasized (Fig. 3).
It is worth noting that the current biochar standards and guidelines face much limitations and challenges. The complexity and diversity of biochar feedstocks, characteristics, and applications pose great challenges in establishing standardized classification. Variability in production methods adds another layer of complexity for establishing the standards. Overall, the selection and application of biochar need to be optimized based on the specific problems for a given salt-affected soil. In addition, universal adoption of a single set of standards is lacking, leading to inconsistencies and hindrances in international trade and cooperation, which needs to be addressed in future.
3 Effect of biochar amendments on plant growth in salt-affected soils
Promoting plant growth and restoring primary productivity are the primary goals of remediating salt-affected soils (Mukhopadhyay et al. 2021). Biochar promoting plant growth for CDR is an effective strategy for climate change mitigation (Lehmann et al. 2021). Biochar enhancing plant photosynthesis and soil primary productivity would remove additional CO2 from the atmosphere (Horton et al. 2021), and greater crop yields may provide an incentive for adoption of the technology (Guo et al. 2022). However, although great advances have been made in understanding biochar positive effects on plant growth (Additional file 1: Table S5), there are still many uncertainties (e.g., negative and no effect) in salt-affected soils (Additional file 1: Table S6).
3.1 Positive effects of biochar amendments on plant growth in salt-affected soils
3.1.1 Positive response of plant growth
Increasing studies have demonstrated that biochar application into salt-affected soils can effectively improve plant growth (Fig. 4, Additional file 1: Table S5). This study was based on a meta-analysis of 254 comparisons of biochar on salt-affected soils published in 35 articles (Additional file 1: Text S3, Tables S7, S8, and S9). The grand mean of plant productivity response (PPR, the change of plant yield in the biochar-amended soils compared with the un-amended soils) was estimated to be 29.3%, in any case of biochar properties or soil conditions (Fig. 4). The results of heterogeneity test, showing significant Qb values existed in most of the cases except the groups of BC-HTT and soil-pH (Additional file 1: Table S10), suggested that most of the examined biochar properties and soil conditions significantly influenced the PPR. The results of the publication bias test (Additional file 1: Table S11) showed that all the groups passed the publication bias test, indicating that the publication biases in the literature had little effect on the reliability and robustness of the output results. Notably, the PPR resulting from biochar addition in salt-affected soils is higher than estimates from previous studies including all soil types (16.0–25.3%) (Bai et al. 2022; Dai et al. 2020). Thus, biochar amendments could show better performance in promoting plant growth in salt-affected soils more than the all-soil types. For example, Liu et al. (2013) found that biochar averagely increased crop productivity by 11.0% using a meta-analysis based on 880 paired comparisons from 103 published studies. Dai et al. (2020) reported that biochar significantly improved PPR by 16.0% regardless of biochar and soil properties using a meta-analysis based on 1254 paired comparisons from 153 published studies. The differences in the grand plant response to biochar addition in these meta-analyses may be attributed to the size of dataset based on different amounts of published studies regarding the corresponding topics, on which limited studies are available for the salt-affected soils (Additional file 1: Table S7).
Effect of biochar on plant productivity categorized by biochar (BC) properties (a) and salt-affected soil properties (b). Symbols indicate the grand mean change (%) of plant productivity relative to control with 95% confidence intervals. The y-axis shows the groups categorized by biochar and soil properties correspondingly. The numbers after the group names indicate the amount of pairwise comparison. The red dotted line shows the grand mean change (%) of plant productivity responding to biochar application, regardless of the biochar properties and soil conditions
A better promoting effect on plant productivity in BC-application rate groups was recorded in the case of biochar application rate less than 5% (w%) or 40 t/ha (15.0%, Fig. 3a), emphasizing that the balance of application rate, plant productivity, and economy should be considered to ensure the sustainable development of biochar technology. Compared with the biochar prepared above 350 °C (23.7–25.1%), the biochar prepared below 350 °C (28.6%) showed a better promoting effect (Fig. 4a), which may be due to the higher nutrient availability and retention (Al-Wabel et al. 2018). It is noted that the best positive effects of PPR were not observed in biochar groups with lower pH (≤ 7, 20.6%) and higher CEC (> 30 cmol kg−1, 33.9%), but in the biochar with medium pH (8.0–10.0, 41.5%) and low CEC (≤ 10 cmol kg−1, 49.9%) (Fig. 4a). Biochar with higher pH is accompanied by higher ash content (Al-Wabel et al. 2018), and the direct input of multivalent cations (e.g., Ca2+ and Mg2+) may be conducive to the formation of soil aggregates though electrostatic adsorption and cation exchange, thus improving soil structure and promoting plant growth (Zheng et al. 2018b). Biochar prepared at a high temperature is associated with lower CEC due to its fewer oxygen-containing functional groups (Al-Wabel et al. 2018), but this type of biochar tends to have higher pore volume and surface area, which will improve soil aeration and permeability (Ge et al. 2023). Similarly, the best positive effects of PPR were observed in the biochar groups with medium TC (30.0–50.0%, 42.7%) and TN (10.0–15.0 g kg−1, 39.7%) (Fig. 4a). This was mainly ascribed to the higher nutrient availability of these biochars produced at low or moderate heating temperatures, although the total nutrient contents were not the highest (Zheng et al. 2018a, 2018b). Considering soil properties, the higher increase in PPR was found in the groups of soil-pH (> 9 (42.4%), soil-CEC > 10 (27.3%), soil-SOM ≤ 20 (52.6%), soil-TN ≤ 1.0 (44.6%), and soil-AP of 50–150 (53.6%) (Fig. 4b). Furthermore, the results of regression analysis (Additional file 1: Text S3) demonstrated that biochar characteristics including pH (R2 = 0.0242, P < 0.05) and TN (R2 = 0.0183, P < 0.05) (Additional file 1: Fig. S2a, b), and soil properties including pH (R2 = 0.0326, P < 0.05), SOM (R2 = 0.0112, P < 0.05), and TN (R2 = 0.1720, P < 0.05) (Additional file 1: Fig. S2c–e) significantly influenced the results of PPR, while biochar TC and CEC (Additional file 1: Fig. S2f, g), and soil CEC had little effect (Additional file 1: Fig. S2h). According to these data, the promoting effect of biochar on plant growth should be the interactive results of different biochar properties and soil conditions. However, due to the limited available data, the effects of biochar characteristics such as EC, ESP, SAR, surface area, and porosity on plant growth cannot further be analyzed. In the future, more studies regarding different biochar properties on different plant growth in different salt-affected soils should be conducted.
3.1.2 Potential mechanisms underlying the improved plant growth in salt-affected soils following biochar amendments
Although the studies regarding plant productivities in salt-affected soils following biochar input are limited, several important potential mechanisms have been proposed. These mechanisms mainly include (Fig. 5a): (1) improving physicochemical properties of salt-affected soils, (2) shifting microbial community to beneficial taxa, and (3) regulating plant metabolism and enhancing salt-tolerance of plants.
Positive (a) or negative (b) effects of biochars on plant growth in salt-affected soils. The positive effects mainly include enhancing seed germination, improving root development, increasing biomass and crop yield, which are mainly attributed to (1) improving soil physical and chemical properties, (2) changing microbial community associated with nutrient cycling, and (3) enhancing salt tolerance of plants. Biochar properties associated with promoting plant growth include developed porous structure, large surface area, abundant oxygen-containing functional groups and multivalent cations (e.g., Ca2+ and Mg2+), strong stability and high carbon content, and abundant nutrients (e.g., nitrogen, phosphorus, and potassium). The negative effects mainly include inhibiting seed germination, damaging plant morphology, decreasing root development, and reducing biomass and crop yield, which are mainly attributed to (1) stress of inherent toxic compounds released from biochars to plants, (2) damages of plant roots by persistent free radicals (PFRs) on biochar surfaces, and (3) deterioration of soil physicochemical properties following biochar application. Biochar properties associated with inhibiting plant growth include high ash content and salinity, harmful compounds, and surface PFRs
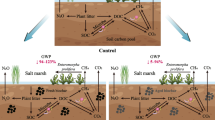
A few studies have proved that biochar application could enhance plant growth through improving the physicochemical characteristics of salt-affected soils (Luo et al. 2017, 2016b; Zheng et al. 2018a). Biochar with multivalent cations and developed pore structure could facilitate formation of biochar-organic-mineral complexes with native SOM and minerals through electrostatic adsorption and cation exchange, and thus reduce the damage of soil structure caused by Na+ (Jing et al. 2022; Zheng et al. 2018b). These interactions could enhance the formation and stability of soil aggregates, resulting in the decreased bulk density and increased soil porosity (Kim et al. 2016). Accordingly, the presence of oxygen-containing functional groups (e.g., carboxyl and hydroxyl) and multivalent cations (e.g., Ca2+ and Mg2+) on biochar surfaces is the key factor determining the aggregate structure of salt-affected soils (Zheng et al. 2018b). Biochar prepared from low-temperature (< 500 °C) pyrolysis of manure contains high amounts of oxygen-containing functional groups and multivalent cations, which would easily form biochar-organic matter-mineral complexes (Han et al. 2020). In addition, pore structure, particle size, and shape of biochar can influence the water hydraulic conditions (e.g., saturated hydraulic conductivity, water field capacity) of salt-affected soils, thus contributing to the positive response of plant growth (Xiao et al. 2020). Compared with the biochar with larger particle size (2–16 mm), the fine powdered biochar (< 2 mm) is easily able to block the pores of sandy soil and reduce its water infiltration capacity (Esmaeelnejad et al. 2017; Liu et al. 2017c). Clay coastal salt-affected soils are tightly compacted, and biochar can easily enhance the connectivity and quantity of soil pores, thus significantly increasing water infiltration capacity (Omondi et al. 2016). Biochar also may improve chemical characteristics of salt-affected soils, such as decreasing pH and EC (Singh et al. 2019), increasing CEC (Kim et al. 2016; Zheng et al. 2018a). This was further confirmed by the significant negative correlations between the PPR and pH values of biochars or the amended salt-affected soils (Additional file 1: Fig. S2a, c). Increasing studies also evidenced that biochar could mitigate salt-stress by decreasing exchange sodium percentage (ESP) or sodium adsorption ratio (SAR) (Zhu et al. 2020) through the exchange of Ca2+ and Mg2+ with Na+, and/or adsorption of Na+ on biochars. In addition, the improvement of soil structure by biochar increased soil aeration and water permeability, thus promoting the leaching of Na+ to the subsoil (Luo et al. 2019). The pore structure, surface area, and multivalent cations of biochar are the key factors determining its ability to alleviate salt stress. For example, Cui et al. (2022) observed that a wheat straw derived biochar significantly decreased the total soluble salt by 11.7–42.2% in the coastal salt-affected soils located in Yancheng, China, and thus increased the wheat and corn yields. Biochar can significantly increase nutrient availability of salt-affected soils due to its nutrient supply (Zhang et al. 2021a; Zheng et al. 2013) and enhance soil nutrient retention by electrostatic attraction or cation bridges (Zhang et al. 2021a), confirmed by the positively correlation between TN contents of biochars and PPR (Additional file 1: Fig. S2b). On the one hand, biochar rich in nutrients such as nitrogen, phosphorus, potassium, and calcium can directly provide essential nutrients for plant growth in salt-affected soils (Luo et al. 2017; Xu et al. 2017; Zheng et al. 2018a). On the other hand, biochar with abundant surface oxygen-containing functional groups and pore structure can also hold these nutrients in salt-affected soils through electrostatic adsorption and/or cation bridging and exchange (Luo et al. 2016a). These results showed that the positive influences of biochar on plant growth are combined outcome of soil properties and biochar characteristics, i.e., one biochar cannot solve all the problems of a soil. Therefore, biochar should be modified according to the specific problems of the target salt-affected soils, and the characteristics of biochars such as pH, CEC, and nutrient content particularly nitrogen and phosphorus should be carefully designed and adjusted. Acid modification endows alkaline biochar with the desired properties such as acidic and highly porous by directly introducing H+, and removing alkaline minerals concentrated in biochar (Wang et al. 2022b; Zhang et al. 2022). Particularly, H3PO4 and HNO3 are highly recommended to pretreat biochar before applying it to salt-affected soils (Wang et al. 2022b).
In recent years, a growing body of studies have revealed that biochar could enhance plant growth through increasing microbial abundance, diversity, and activity in salt-affected soils (Azadi and Raiesi 2021; Yao et al. 2021). Biochar may improve the activity of soil enzymes such as urease, invertase, phosphatase, β-glucosidase, and leucine aminopeptidase, which benefit to plant physiological activity via promoting soil microbial abundance and metabolic activity (Qin et al. 2016; Song et al. 2020). For example, Zheng et al. (2018a) concluded that a peanut shell biochar significantly increased the richness and diversity of bacteria in the rhizosphere soils grown with halophyte Sesbania (Sesbania cannabina) and Seashore mallow (Kosteletzkya virginica), and the biochar also strengthened the bacterial taxon with carbon sequestration (e.g., Alphaproteobacteria, Cytophagia) and phosphorus solubilization (e.g., Pseudomonas, Bacillus). The abundant pore structure of biochar can provide habitats for soil microorganisms to be protected from predators (Zhu et al. 2017). Also, biochar can directly provide substrates for microorganisms such as carbon and nitrogen, thus increasing microbial activity and abundance (Zheng et al. 2018a). The pore structure, unstable carbon content, surface functional groups and hydrophilicity of biochar are the key factors that regulate the structure and function of microbial community in salt-affected soils (Liu et al. 2017a). However, it is still difficult to distinguish the direct and indirect effects of biochars on soil microbial community associated with plant growth. Also, the relationships between biochar properties and salt tolerance of halophytes are unclear. Notably, a small number of studies reported that biochar may negatively affect microorganisms in salt-affected soils (Nguyen et al. 2020; Xu et al. 2018). Harmful substances (e.g., PAHs and heavy metals) in biochars are important reasons for the negative response of microorganism (Godlewska et al. 2021). However, the response of specific microorganisms in salt-affected soils to certain substances in biochar requires further investigations. Recently, increasing evidence has proved that “core microbiome” in rhizosphere, the smallest subset of the natural microbiome in soil that can stably, efficiently and continuously maintain the function of the natural microbiome (Toju et al. 2018), is essential for promoting plant adaptation to drought and salt stress (Qin et al. 2016). Although a few studies have made great efforts in the identification, isolation, and assembling of salt-resistant rhizosphere core microbiome (Rath et al. 2019; Zhang et al. 2020), technical challenges such as functional species recruitment and multiple core microbiomes combination still need to be overcome in future. Meanwhile, how to effectively regulate the structure and function of microbial community to achieve efficient salt-affected soil carbon sequestration is also an important research direction in the future. The emerging molecular biology techniques such as metagenomic analysis, single cell culturing, and microfluidic technology should be used to evaluate the succession and contribution of core microbial community to the enhanced plant growth following biochar application in salt-affected soils.
Regulating plant metabolism and enhancing halotolerance of plants in salt-affected soils amended with biochars is another important reason for the promoted plant growth (Fig. 5a). Several studies reported that biochar amendments may enhance plant growth in salt-affected soils by enhancing salt tolerance through improving Na+ exclusion and K+ uptake (He et al. 2020), reducing stress of endogenous hormones (Hafez et al. 2021), increasing growth hormones secretion, and alleviating oxidative stress of cellular membranes by Na+ stress (Parveen et al. 2021; Torabian et al. 2018). However, the specific mechanisms underlying these biochar effects remain unclear, and the relationship between biochar properties and plant salt tolerance needs to be further studied. Moreover, a recent study reported that a low rate (0.5%, w%) of graphene application into a simulated salt-affected soil alleviates salinity and alkalinity stress in alfalfa (Medicago sativa L.) through enhancing photosynthesis and antioxidative defense system by regulating gene expression (Chen et al. 2021b). Biochar, particularly nano-biochar, containing graphene-like structure (e.g., graphene sheets and wrinkled edges) (Liu et al. 2018), could also improve plant halotolerance in salt-affected soils, which needs further research to distinguish the relationships between biochar characteristics and physiological process related to plant halotolerance. Recently, the development of nanotechnology has led to the increasing application of many nanomaterials (e.g., TiO2, ZnO, and CeO2) in salt-affected soils to enhance salt tolerance of plants (Gomez et al. 2021; Liu et al. 2021b; Wang et al. 2022a). It is reasonable to speculate that the surface modification of biochar using nanomaterials (e.g., TiO2, ZnO, and CeO2) is an effective strategy to improve the halotolerance of plants in salt-affected soils (Liu et al. 2020a). Synthesizing biochar-based nanocomposites can combine the advantages of biochars and these nanomaterials, which may be a promising strategy to develop functional biochars for enhancing plant halotolerance, carbon sequestration efficiency of plant photosynthesis, and rhizosphere carbon deposition.
3.2 Negative effects of biochar amendments on plant growth in salt-affected soils
3.2.1 Negative response of plant growth
Unfortunately, several studies also reported the negative effects of biochars on plant growth in salt-affected soils (Fig. 5b, Additional file 1: Table S6), such as decreasing seed germination, inhibiting root development (Bu et al. 2020), and decreasing plant biomass and even crop yield (Xiao et al. 2020). For example, Luo et al. (2017) reported that a biochar-based compost applied at 1.5–5% significantly increased the total biomass, root length, surface area and tips of Sesbania (Sesbania cannabina) by 71.4–129%, 127–165%, 143–176%, and 200–265%, respectively, while the high rate of 10% application decreased the shoot biomass, length, surface area and tips of Sesbania by 9.60%, 23.7%, 67.1%, 58.6%, and 37.0%. These negative effects highlight the uncertainties and potential environmental risks of biochars used in remediating salt-affected soils. As an important component of the global carbon cycle, plants absorb CO2 from the atmosphere and convert it into organic matter through photosynthesis (Tkemaladze and Makhashvili 2016). Meanwhile, plant growth is crucial for maintaining ecological services such as soil retention, hydrological cycling, and climate regulation (Gouda et al. 2018). In the event of limited plant growth, the ability of plant to absorb CO2 and the input of plant-derived organic carbon into the soil would decrease, resulting in the reduced soil carbon sequestration (Dusenge et al. 2019). Therefore, biochar application may weaken CDR potential associated with plant growth, but the impact on climate change still needs to be further evaluated. Thus, future studies are warranted to examine the negative effects of more types of both biochars and plants in different salt-affected soils, especially to evaluate the negative effects of biochar on CDR associated with plant growth.
3.2.2 Potential mechanisms underlying the inhibited plant growth in salt-affected soils
Although limited studies are available (Additional file 1: Table S6), several potential mechanisms underlying the negative effects of biochars on plant growth in salt-affected soils are proposed or can be extrapolated from the findings regarding the non-salt-affected soils (Fig. 5b). These potential mechanisms mainly include: (1) stress of inherent toxic compounds to plants released from biochars (Benavente et al. 2018; Intani et al. 2018); (2) damages of plant roots by biochar-derived nanoparticles or persistent free radicals (PFRs) (Zhang et al. 2019c; Zheng et al. 2019), and (3) deterioration of soil physicochemical properties following biochar application (Kazemi et al. 2019).
Biochar degradation can also affect plant growth in salt-affected soils. On the one hand, during the degradation of biochar in soils, the release of harmful substances (e.g., heavy metals, polycyclic aromatic hydrocarbons, volatile organic compounds, and dioxins) from the biochar may result in toxicity to seeds, roots, and soil microbial communities (Godlewska et al. 2021). On the other hand, biochar applied to salt-affected soils may be broken into fine particles or even nano-sized biochar with weathering, tillage, and biological activities, thus impairing plant root development and growth (Liu et al. 2018; Ma et al. 2023). Liu et al. (2022) found that hot pepper stalk-derived nano-biochar decreased cucumber seed germination and inhibited root development and seedling growth due to oxidative stress and root injury. Moreover, studies have shown that PFRs in biochars posed adverse effects on organisms including plants (Liao et al. 2014), soil animals (Lieke et al. 2018), and microorganisms via inducing oxidative stress and destabilizing the cellular membranes (Baltrėnaitė-Gedienė et al. 2022). However, environmental behaviors and ecological effects of biochar-derived nanoparticles and PFRs in salt-affected soils are unknown, which could be much complicated due to the special soil conditions such as high pH and anions (e.g., Cl−, CO32−, and HCO3−) (Odinga et al. 2020). Therefore, strategies should be considered to quench PFRs in biochars before their application into salt-affected soils via different pretreatments such as preparing biochar at relatively high heating temperatures (e.g., > 500 °C) and composting with other amendments (e.g., sludge, livestock, and green manure). However, PFRs in biochars also have positive effects in degrading organic and inorganic contaminants (e.g., diethyl phthalate, 2-chlorobiphenyl) in soils, as they generate reactive oxygen species (ROS) (Odinga et al. 2020). Consequently, optimizing pyrolytic conditions and pretreating biochars (e.g., modification, composting) to avoid the environmental risk should be conducted before the large-scale application in salt-affected soils, but the balance between the functionality and risk of PFRs in biochar should be carefully considered, particularly for the salt-affected soils contaminated with organic pollutants.
The negative impacts of biochars on plant growth in salt-affected soils are also related to the unexpected deterioration of soil physicochemical characteristics (Fig. 5b, Additional file 1: Fig. S2). Biochar with high contents of alkaline components (e.g., carbonates, alkaline earth metals) may inevitably increase soil pH and EC (Wang et al. 2019), thus showing salt-stress to plant growth. This was directly confirmed by the negative correlation between pH values of biochar and the PPR (Additional file 1: Fig. S2a, c). Thus, for mitigating soil salinity and alkalinity, the biochars with high ash contents derived from sewage sludge and manure would not be suitable for ameliorating salt-affected soils (Al-Wabel et al. 2018). However, biochars with high contents of Ca2+ and Mg2+ often have higher CEC and are more likely to exchange with excess Na+ in soil colloids (Farhangi-Abriz and Torabian 2018). Hence, balancing biochar salinity and its contents of Ca2+ or Mg2+ for achieving simultaneous salt reduction and soil CEC promotion still needs to be further verified and practical application (Chen et al. 2022). In addition, the increase in pH and salinity caused by biochars is also related to the experimental conditions at lab-scale. At present, most studies adopted pot experiments grown with or without plants in greenhouse, and the soil moisture contents were generally maintained at 60–80% of WHC (Luo et al. 2017; Sun et al. 2016). Due to the absence of water leaching in the whole cultivation periods, even though biochar removes Na+ via cation exchange and adsorption, Na+ are still kept in soils and cannot be leached from the experimental soils. In order to alleviate the negative effects of biochar salinity on salt-affected soils, the combination of biochar and modern irrigation technologies such as drip irrigation, surface or subsurface drainage may be an important direction. In addition, preparation of high water retaining biochars with high porosity and surface hydrophilic functional groups (e.g., carboxyl and hydroxyl) may be an important supplement to the combined salt reduction technology of biochar and engineering irrigation (Gray et al. 2014). Alternatively, hydrochars, nutrient-rich carbonaceous materials with low ash contents of 27.2–37.5% and acidic pHs of 3.3–6.2 (Benedetti et al. 2022; Saha et al. 2019), could be combined application with biochars to overcome the limitation of biochar alkalinity in remedaiting salt-affected soils. Moreover, the combined application of biochars with other soil amendments such as pyroligneous acids (Yuan et al. 2022), manure composts (Liu et al. 2021a), and microbial agents (Cui et al. 2021), could be an effective strategy to overcome the above-mentioned negative effects on plant growth and thus enhance CDR because of their potential synergistic effects.
4 Effects of biochars on GHG emission in salt-affected soils
Effective climate change mitigation requires reductions of GHG emissions to achieve the net zero emissions to meet the Paris Agreement goal (Schleussner et al. 2016). Reducing GHG emission from soils is one of the most effective strategies for mitigating global climate change (Wang et al. 2021). Biochar can mitigate climate change in the soil sub-systems of biochar system by reducing SOC mineralization and mitigating non-CO2 GHG emission (Fig. 2, Additional file 1: Table S12).
4.1 Biochar effects on CO2 emission from salt-affected soils
4.1.1 Responses of CO2 emission from salt-affected soils amended with biochars
Generally, a large amount of CO2 emission from salt-affected soils is caused by the lack of physical protection of SOC due to dispersion and poor aggregation of soil aggregates (Setia et al. 2011). In a meta-analysis of 163 studies, Chagas et al. (2022) found that biochar significantly increased soil total carbon and organic carbon by 64.3% and 84.3%, respectively, regardless of soil types. However, the studies regarding CO2 emission from salt-affected soils following biochar application are just tentative (Additional file 1: Table S12). Most of these limited studies reported that biochars can effectively decrease CO2 emissions from salt-affected soils (Akanji et al. 2021; Zhao et al. 2020). However, a small number of studies also reported that biochar application stimulated CO2 emission from salt-affected soils (Sial et al. 2019; Zhang et al. 2016). These inconsistent results are mainly related with biochar physicochemical properties, soil characteristics, experimental conditions, plant types, and biochar application rate. However, the relationships between these factors are still not well recognized and linked, which need further research.
4.1.2 Potential mechanisms underlying changes of CO2 emission from salt-affected soils amended with biochars
The potential mechanisms underlying the variation of CO2 release in salt-affected soils amended with biochar can be speculated from these limited studies (Additional file 1: Table S12) in parallel with those in the non-salt-affected soils (Shakoor et al. 2021a; Zhang et al. 2019a). These mechanisms mainly include the following aspects (Fig. 6a). (1) Negative priming effect: the high pH and salt content of salt-affected soils generally lead to the disintegration of soil aggregates and thus decomposition of SOM without physical protection (Feng et al. 2021a), evidenced by the positive correlation between effect size of CO2 emissions and biochar pH or EC values in salt-affected soils (Fig. 7a, b). Biochar application may induce negative priming effect on native SOC mineralization through promoting physical protection of soil aggregates weakened by excess Na+ in salt-affected soils (Bhaduri et al. 2016; Zheng et al. 2018b), enhancing the adsorption and encapsulation of organic carbon (OC) molecules by biochars (Feng et al. 2021b; Zheng et al. 2018b), and shifting the microbial communities to low carbon turnover microorganism groups (Ni et al. 2021; Zheng et al. 2018a). (2) Enhanced formation of soil inorganic carbon (SIC) fractions: SIC is one of the important carbon sources and sinks in terrestrial and marine ecosystems (Ferdush and Paul 2021; Srivastava et al. 2017). The alkaline environment caused by biochar or the original alkalinity of salt-affected soil (e.g., CO32− and HCO3−) is prone to formation of stable carbonate (e.g., CaCO3, MgCO3) from CO2 via precipitation (Luo et al. 2016b; Zhang et al. 2016). Hence, enhancing SIC accumulation by adding biochars (Luo et al. 2016b), particularly animal waste or algae derived biochars, may be one of the effective strategies to capture CO2 in alkaline salt-affected soils in arid or semi-arid regions. (3) Accumulation of microbial necromass carbon: increasing studies evidenced that microbial necromass carbon plays more important roles (e.g., 32.6–61.8%) in SOC accumulation than plant litter components due to its stability and “entombing effect”, known as the novel concept of “microbial carbon pump” (Liang et al. 2019). A previous study reported that a maize straw-derived biochar increased the contribution of microbial necromass carbon to SOC by enhancing the biomass of living microorganisms in a maize field trial in north-eastern China (Zhang et al. 2021b). However, the effect of biochar on the accumulation and transformation of microbial necromass carbon in salt-affected soils is unknown. (4) Adsorption of CO2 by biochars: biochar has great potential to capture CO2 (10.0–82.0 mg g−1) via van der Waals force, pore-filling, and precipitation due to its developed pore structure, large specific surface area, alkaline matter like N-containing functional groups (Creamer and Gao 2016; Dissanayake et al. 2020). It is worth noting that no direct evidence on the adsorption process and mechanism of GHG by biochar in soil is available so far due to the complexity of soil systems. However, the good correlation between biochar surface area and the effect size of CO2 emissions implied that CO2 adsorption by biochar might also occur in salt-affected soils (Fig. 7c).
The potential mechanisms underlying the decreased (light green) and increased (saffron yellow) GHG emission from salt-affected soils amended with biochars. Reduced CO2 emission is mainly ascribed to: (1) negative priming effect, (2) enhanced formation of SIC, (3) accumulation of microbial necromass carbon, (4) adsorption of CO2. Promoted CO2 emission is mainly attributed to: (1) positive priming effect, (2) mineralization of labile carbon fractions released from biochars, (3) co-metabolism of labile biochar C fractions and native SOM. Decreased CH4 emission is mainly ascribed to: (1) improvement of soil aeration, (2) adsorption of CH4, and (3) changes in methanotrophs and methanogens community. Promoted CH4 emission is mainly ascribed to: (1) provision of exogenous substrate for methanogens and (2) reduction of Na+ bioavailability. Reduced N2O emission is mainly ascribed to: (1) enhanced soil aeration, (2) elevated soil pH, (3) adsorption of nitrification and denitrification substrate, enzyme, and N2O, and (4) shifted microbial community associated with nitrogen biogeochemical cycle. Promoted N2O emission is mainly attributed to: (1) provision of exogenous substrate (e.g., NH4+, NO3−) from biochars for nitrifiers and denitrifiers, (2) increased soil anaerobic microzones by biochar and soil moisture
Biochars generally result in short-term positive priming effects on SOC mineralization and increased CO2 release in salt-affected soils, which may be ascribed to the following reasons (Fig. 6a). (1) Positive priming effect: the rich porous structures of biochars provide excellent microhabitats for soil microorganisms, thus promoting their growth and activity and directly enhancing native SOC mineralization (Chen et al. 2021a; Luo et al. 2020). (2) Mineralization of labile carbon fractions released from biochars: the dissolved organic carbon (DOC) in biochars released into soils can be directly mineralized into CO2 (Han et al. 2020; Virk et al. 2021). This is consistent with the positive correlation between biochar OC contents and the effect size of CO2 release in salt-affected soils (Fig. 7d). (3) Co-metabolism: the labile carbon fractions in biochars could provide growth substrate for soil microorganisms, and simultaneously stimulate these microorganisms to secrete non-specific enzymes to mineralize the native SOC (Jiang et al. 2019; Singh and Cowie 2014) (Fig. 7d). In addition, extensive studies demonstrated that biochars can reduce rhizosphere priming effect, an important cause responsible for SOC pool loss (Zhou et al. 2020a), by adsorbing root exudates and enhancing rhizosphere carbon deposition (Keith et al. 2015; Pei et al. 2020). However, the rhizosphere priming effect of biochar on salt-affected soils remains unknown, which needs to be further examined using advanced technologies such as nanoscale secondary ion mass spectrometry (NanoSIMS) and biological chip technology to distinguish the mineralization intensity of rhizodeposition and native soil carbon and quantify the contribution of rhizosphere deposition in soil carbon storage. The GHG emission from soil will alter the vegetation and ecosystem stability, thus exacerbating climate change (Rumpel et al. 2020). However, it is noted that the promotion effect of GHG release from salt-affected soils amended with biochar has not been quantitatively evaluated due to the limited studies, and the negative impact on GHG changes still needs further examination.
4.2 Biochar effects on CH4 emission from the salt-affected soils
4.2.1 Response of CH4 emission from salt-affected soils amended with biochars
CH4 is the second critical GHG after CO2 which shows 25 times higher global-warming potential than CO2 and accounts for 20% of the anthropogenic warming effect (Tan et al. 2021). In a meta-analysis of 61 studies, Ji et al. (2018) found that biochar significantly decreased CH4 release rates by 12% for paddy soil and 72% for upland soil, and decreased CH4 uptake rate by 84% for upland soil. These results indicated the inconsistent effects of biochar on CH4 release from different soil ecosystems. High salinity and sulfate in salt-affected soils can reduce CH4 emissions due to inhibition of methanogen activities (Zhao et al. 2020). So far, a few studies showed inconsistent effects of biochar on CH4 emissions from salt-affected soils (Additional file 1: Table S12), including increased (Lin et al. 2015), decreased (Zhang et al. 2016), and no effects (Maucieri et al. 2017). These inconsistent results reflect the uncertainties of biochars on regulating CH4 emission from salt-affected soils, which should be further examined using more different biochars and different salt-affected soils, for example, coastal wetland soils with alternate wetting and drying and salinity evolution, and inland salt-affected soils with prolonged drought.
4.2.2 Potential mechanisms underlying changes of CH4 emission from salt-affected soils amended with biochars
Several potential mechanisms responsible for CH4 emission can be deduced from the published studies related with biochars, regardless of the salt-affected soil and non-salt-affected soils (Shakoor et al. 2021a; Zhang et al. 2019a). These potential mechanisms include (Fig. 6b): (1) changes in methanotrophs and methanogens community; (2) improvement of soil aeration, (3) adsorption of CH4. The relatively high C/N ratios of biochars (116–269) could limit the utilization of SOM by methanogens (e.g., Desulfobacca and Clostridium), and thus inhibit their abundances and activities for CH4 production (Nguyen et al. 2020). In addition, the increased soil pH and EC following biochar addition may increase abundance and activities of methanotrophs (e.g., Gamma-proteobacteria and Alpha-proteobacteria) and thus reduce CH4 emissions, consistent with the negative correlation between biochar pH values and the effect size of CH4 release in salt-affected soils (Fig. 7e). Biochar can improve soil porosity due to the high surface area, and thus improve salt-affected soil aeration and increase oxygen (O2) diffusion, thus would stimulate CH4 oxidation and/or suppress CH4 production by inhibiting methanogens (Sial et al. 2019; Zhang et al. 2016). Notably, although the improvement of soil aeration conditions may reduce CH4 release, CO2 release could increase due to the oxidization of CH4. Hence, future studies should design engineered biochar by regulating its SA, pore structure and surface basic functional groups to achieve GHG storage through reducing CH4 release and promoting CO2 adsorption. Moreover, biochars with rich pore structure and large surface area may favor CH4 adsorption on them and/or soil particles (Chiu and Huang 2020; La et al. 2019), supported by the negative correlation between biochar surface area and the effect size of CH4 release (Fig. 7f).
The potential mechanisms underlying the promoted CH4 emissions from salt-affected soils following biochar application were not well studied, and limited studies were mainly explained by the input of labile carbon fractions released from biochars and reducing Na+ bioavailability (Fig. 6b). The increase of OC contents in biochar maintained a trend consistent with the increase of the effect of CH4 release (Fig. 7g), indicating that the labile carbon components of biochars can be used as substrates for methanogens in anoxic environments, thus promoting CH4 production (Zhang et al. 2016). In addition, studies showed that low salinity conditions are favorable for methanogenic activity (Lu et al. 2019), because appropriate salinity conditions may provide necessary Na+ for amino acid transport and internal pH regulation of methanogens (Theint et al. 2016). Hence, biochar applied to salt-affected soils with high ESP or SAR, which generally inhibits the activity of methanogens, especially in sodic soils, may increase CH4 release by reducing Na+ bioavailability via biochar adsorption. Moreover, the effect of biochar on the activity of methanogens and ultimately CH4 production and emission under different types and salinity conditions remains unclear, which needs to be confirmed in future study.
4.3 Biochar effects on N2O emission from salt-affected soils
4.3.1 Response of N2O emission from salt-affected soils amended with biochars
N2O has a global warming potential 298 times as high as that of CO2, and the main source of global anthropogenic N2O emissions is owing to the extensive utilization of nitrogen fertilizers (Shi et al. 2017). Increasing salinity in soils may promote N2O emission because high soil salinity can directly inhibit the transformation of N2O to N2 in denitrification (Zhou et al. 2017). In a meta-analysis of 186 studies, Shakoor et al. (2021b) found that biochar can decrease global agricultural soil N2O emission by 19.7%. However, only several studies reported that biochar amendments effectively reduced N2O emission from salt-affected soils (Additional file 1: Table S12). For example, Liu et al. (2020b) demonstrated that a rice straw-derived biochar applied in a alkaline sandy loamy soil at 2.25, 6.75, and 11.25 t ha−1 decreased the cumulative N2O emission by 28.6–38.4% during ten consecutive crop growing seasons. The complex environmental conditions of salt-affected soils (such as pH, salinity, and drought), greatly altering mineralization, ammoniation, nitrification and denitrification (Zhou et al. 2017), may further complicate N2O emission from salt-affected soils amended with biochars, which should be carefully considered in the context of serious disturbance soil biogeochemical nitrogen cycle caused by intensified human activities (e.g., excessive application of chemical nitrogen fertilizers, fossil fuel burning).
4.3.2 Potential mechanisms of biochar on N2O emission from salt-affected soils
The potential mechanisms underlying the mitigated N2O emissions from salt-affected soils mainly include: (1) enhancing soil aeration; (2) promoting the reduction of N2O; (3) adsorption of nitrification and denitrification substrate, enzyme, and N2O; and (4) shifting microbial community associated with nitrification and denitrification (Fig. 6c). (1) Enhancing soil aeration: biochar may enhance salt-affected soil aeration and promote O2 diffusion, thereby reducing the anaerobic microzones of the soil in which denitrification takes place (Romero et al. 2021). (2) Promoting the reduction of N2O: biochar may elevate pH and electron-transfer ability of salt-affected soils due to its inherent alkalinity and abundant functional groups on biochar surfaces, which could enhance N2O reductase (the enzyme reducing N2O to N2) activity (Zhang et al. 2021c), thus promoting reduction of N2O to N2 (Dong et al. 2020; Wang et al. 2012). This view can be supported by the opposite trend of biochar pH values and the effect size of N2O emission (Fig. 7h). (3) Adsorption of nitrification and denitrification substrate, enzyme, and N2O: the results of correlation analysis showed negative correlation between biochar SA and the effect size of N2O emissions (Fig. 7i). The adsorption of substrate by biochars, or the increased adsorption of NO3− and NH4+ by biochar-amended soils (Duan et al. 2020; Zheng et al. 2013), may decrease the substrate availability to nitrifying and denitrifying bacteria (Lin et al. 2015). Moreover, biochar can directly adsorb N2O (5 × 104–1.3 × 105 μg g−1) (Cornelissen et al. 2013). Particularly, the adsorption and desorption of N2O by biochar in actual salt-affected soils for long-term scale is still difficult to be evaluated. (4) Shifted microbial community associated with nitrification and denitrification: biochar may inhibit the abundance and activity of denitrifying bacteria, and promote the expression of the N2O reductase genes (NosZ) of denitrifiers, thus promoting a complete reduction of NO3− to N2 instead of N2O (Xiao et al. 2019; Zhang et al. 2016). Besides, biochar may directly supply inorganic nitrogen and labile carbon fractions as substrates for microorganism growth, thereby triggering nitrification and denitrification for N2O production (Zhou et al. 2020b). This can be directly confirmed by the positive correlation between biochar TN contents and the effect size of N2O release in salt-affected soils (Fig. 7j). In addition, due to the excellent water retention ability of biochars (Adhikari et al. 2022), the anaerobic microzones formed by biochar and soil particles conducive to denitrification in soils may be hotspots for N2O production (Lyu et al. 2022), which should be verified in future.
5 Concluding remarks and perspectives
From the perspective of mitigating climate change and achieving carbon neutrality, this review comprehensively summarized the production and classification systems of biochars, and clarified the potential positive and negative effects and underlying mechanisms of biochars on plant growth and GHG emission in salt-affected soils. Sustainable biochar production is facilitated by utilization of salt-affected soils for biomass production, expansion of feedstock types (e.g., halophytes and marine algae), and development of biochar poly-generation production systems with carbon neutrality or negativity. Biochar can effectively improve plant growth (the grand mean of PPR was estimated to be 29.3%) and mitigate GHG emission in salt-affected soils, while negative effects of biochars on plant growth and GHG emission should not be ignored. This review concluded that biochar is one of the promising candidates to achieve carbon neutrality during remediating salt-affected soils by reducing GHG emission and facilitating CDR. As summarized in Fig. 8, the current knowledge gaps and research areas are as follows:
-
(1)
Expanding accessibility of biomass feedstock is a prerequisite strategy for maintaining sustainable biochar production, which requires evaluation of the economic feasibility and carbon emission potentials of feedstock transportation and storage, as well as the sustainability of the biomass feedstock supplying. Moreover, in order to advance the usability of biochars prepared from extended feedstocks (e.g., marine algae wastes and invasive plants) for salt-affected soils, it is necessary to conduct further studies to establish the relationships of biochar structure-properties-application. In addition, upgrading thermal technologies for biochar production to meet the “carbon neutral” or “carbon negative” requirements should be further considered. Particularly, replacing the heat supply for the thermal reactor using clean or low-carbon energy such as solar energy, hydrogen energy, and autothermal pathway to replace fossil fuels or electricity is strongly recommended. Finally, unified standards and certifications for biochars at global and regional levels are still lacking, and it is imperative to establish unified standards and classification systems to facilitate biochar commercialization and marketization.
-
(2)
Effectively enhancing the positive effects of biochars on promoting plant growth is an effective way for biochar to mitigate climate change through CDR. Development of functional biochars such as biochar-based nanocomposites, biochar loaded with nutrients and acidic agents (e.g., H3PO4 and HNO3) to enhance plant halotolerance and biochar water holding capacity or nutrient supply capacity may provide new options for remediating salt-affected soils. Moreover, the coupling application of biochar amendments with other salt-affected soil improvement technologies (e.g., modern irrigation techniques, phytoremediation, microbial remediation, and plant-microbial remediation) is an important research and application direction in future. However, it should be noted that a balanced approach (e.g., effectiveness and stability, desired functions and risks, effectiveness, and cost) to designing the functional biochar should be taken to ensure both the practicability and the effectiveness of this technology. In addition, biochar modification may increase the application cost and carbon emissions, which need to further evaluate the carbon emission potential using life cycle analysis. Finally, the succession process, diversity, and stability of plant communities in salt-affected soils amended with biochars need further to be explored, particularly the halophyte and invasive plant communities.
-
(3)
Biochar has shown a great potential as a GHG adsorbent, but its adsorption capacity and mechanism in a real complex soil environment still need comprehensive studies. Additionally, more advanced technologies such as NanoSIMS, 13C-NMR spectrometry, biological chip technology, and proteomic analysis should be used to reveal the potential mechanisms underlying biochar effects on soil carbon cycling in salt-affected soils, particularly rhizosphere priming effect, SIC and microbial necromass carbon transformation and accumulation under specific salt-affected soil conditions such as dry–wet cycle or long-term drought. Finally, the development of GHG accounting protocols for biochar applications based on data from biochar or target soils in order to guide GHG reduction of biochar at regional, national, or global scale is encouraged.
-
(4)
The inherent toxic substances also pose major challenges for biochar application in improving salt-affected soils. Current assessment of environmental risks of biochars mainly focuses on only a narrow range of toxic substances including heavy metals, polycyclic aromatic hydrocarbons, volatile organic compounds, and dioxins, while the emerging contaminants in biochars such as surface PFRs and perfluorochemicals (PFCs) are relatively rarely considered. Thus, future studies should consider more compounds formed in biochars with potential toxicity in salt-affected soil ecosystems. Furthermore, prior to biochar application, appropriate methods, such as water washing or composting should be used to reduce the contents of harmful substances in biochars. Moreover, a systematic database including the hazardous substance contents of biochars and the conditions of salt-affected soils needs to be developed based on current biochar standards to guide precise and reasonable biochar application patterns. Finally, the effects of pollutants remaining in biochars on emission reduction potential in salt-affected soils still needs to be answered in future.
-
(5)
For the practical application of biochars, one critical question is how to reduce the production and application costs. To better promote the commercialization and marketization of biochars, appropriate measures should be taken to enhance the participation from different industries. Developing poly-generation instruments for biochars and by-products like biochar-gas-oil poly-generation system and selling biochar by-products such as bio-oil or syngas may be incentive for biochar manufacturers to gain additional profits. In addition, although the carbon value of biochar is still being explored, carbon sequestration value of biochar is undoubtedly attractive in the context of carbon neutrality. The combined carbon emission reduction potential of soil improvement, carbon sequestration, GHG reduction, and CDR provided by biochar should be comprehensively evaluated, and incorporated into the global, regional, or national carbon emission trading systems, and relevant enterprises or countries can obtain corresponding carbon credits from biochar application in salt-affected soils to offset carbon emissions. It is worth noting that if the carbon sequestration and emission reduction value of biochar is to be included in the global carbon trading system, more extensive emission reduction data for different application strategies will be needed, as well as the assessment of biochar production and its stability.
Research directions of biochar application in remediating salt-affected soils for abating climate change. Biochar application in salt-affected soils can provide alternative strategies for remediating salt-affected soils, and thus can be conducive to carbon neutrality and climate change mitigation. Five key research fields are listed in the middle of the figure, including biochar production, plant growth promotion, GHG emission reduction, environmental risk assessment and mitigation, and biochar commercialization and marketization
Availability of data and materials
The datasets generated and analyzed during the current study are available from the corresponding authors on reasonable request.
References
Adhikari S, Timms W, Mahmud MAP (2022) Optimising water holding capacity and hydrophobicity of biochar for soil amendment—a review. Sci Total Environ 851:158043. https://doi.org/10.1016/j.scitotenv.2022.158043
Affan FB, Maaroof A (2016) A critical evaluation of the UK Drax Power Station. J Ecosys Ecograph 6:206. https://doi.org/10.4172/2157-7625.1000206
Ahamed TS, Anto S, Mathimani T, Brindhadevi K, Pugazhendhi A (2021) Upgrading of bio-oil from thermochemical conversion of various biomass—mechanism, challenges and opportunities. Fuel 287:119329. https://doi.org/10.1016/j.fuel.2020.119329
Akanji MA, Usman ARA, Al-Wabel MI (2021) Influence of acidified biochar on CO2–C efflux and micronutrient availability in an alkaline sandy soil. Sustainability 13(9):5196. https://doi.org/10.3390/su13095196
Al-Wabel MI, Hussain Q, Usman ARA, Ahmad M, Abduljabbar A, Sallam AS, Ok YS (2018) Impact of biochar properties on soil conditions and agricultural sustainability: a review. Land Degrad Dev 29(7):2124–2161. https://doi.org/10.1002/ldr.2829
Al-Marzooqi F, Yousef LF (2017) Biological response of a sandy soil treated with biochar derived from a halophyte (Salicornia bigelovii). Appl Soil Ecol 114:9–15. https://doi.org/10.1016/j.apsoil.2017.02.012
Ali S, Rizwan M, Qayyum MF, Ok YS, Ibrahim M, Riaz M, Arif MS, Hafeez F, Al-Wabel MI, Shahzad AN (2017) Biochar soil amendment on alleviation of drought and salt stress in plants: a critical review. Environ Sci Pollut Res Int 24(14):12700–12712. https://doi.org/10.1007/s11356-017-8904-x
Ambika S, Kumar M, Pisharody L, Malhotra M, Kumar G, Sreedharan V, Singh L, Nidheesh PV, Bhatnagar A (2022) Modified biochar as a green adsorbent for removal of hexavalent chromium from various environmental matrices: mechanisms, methods, and prospects. Chem Eng J 439:135716. https://doi.org/10.1016/j.cej.2022.135716
Amini S, Ghadiri H, Chen C, Marschner P (2015) Salt-affected soils, reclamation, carbon dynamics, and biochar: a review. J Soil Sediment 16(3):939–953. https://doi.org/10.1007/s11368-015-1293-1
Arif I, Batool M, Schenk PM (2020) Plant microbiome engineering: expected benefits for improved crop growth and resilience. Trends Biotechnol 38(12):1385–1396. https://doi.org/10.1016/j.tibtech.2020.04.015
Azadi N, Raiesi F (2021) Sugarcane bagasse biochar modulates metal and salinity stresses on microbial functions and enzyme activities in saline co-contaminated soils. Appl Soil Ecol 167:104043. https://doi.org/10.1016/j.apsoil.2021.104043
Bai S, Omidvar N, Gallart M, Kamper W, Tahmasbian I, Farrar MB, Singh K, Zhou G, Muqadass B, Xu C, Koech R, Li Y, Nguyen TTN, van Zwieten L (2022) Combined effects of biochar and fertilizer applications on yield: a review and meta-analysis. Sci Total Environ 808:152073. https://doi.org/10.1016/j.scitotenv.2021.152073
Baltrėnaitė-Gedienė E, Lomnicki S, Guo C (2022) Impact of biochar, fertilizers and cultivation type on environmentally persistent free radicals in agricultural soil. Environ Technol Inno 28:102755. https://doi.org/10.1016/j.eti.2022.102755
Benavente I, Gasco G, Plaza C, Paz-Ferreiro J, Mendez A (2018) Choice of pyrolysis parameters for urban wastes affects soil enzymes and plant germination in a Mediterranean soil. Sci Total Environ 634:1308–1314. https://doi.org/10.1016/j.scitotenv.2018.04.120
Benedetti V, Pecchi M, Baratieri M (2022) Combustion kinetics of hydrochar from cow-manure digestate via thermogravimetric analysis and peak deconvolution. Bioresour Technol 353:127142. https://doi.org/10.1016/j.biortech.2022.127142
Bhaduri D, Saha A, Desai D, Meena HN (2016) Restoration of carbon and microbial activity in salt-induced soil by application of peanut shell biochar during short-term incubation study. Chemosphere 148:86–98. https://doi.org/10.1016/j.chemosphere.2015.12.130
Bu X, Xue J, Wu Y, Ma W (2020) Effect of biochar on seed germination and seedling growth of Robinia pseudoacacia L. in karst calcareous soils. Commun Soil Sci Plan 51(3):352–363. https://doi.org/10.1080/00103624.2019.1709484
Camps-Arbestain M, Amonette JE, Singh B, Wang T, Schmidt HP (2015) Biochar for environmental management: science, technology and implementation. In: Lehmann J, Joseph S (eds) A biochar classification system and associated test methodsm. Routledge, New York, pp 165–193
Chagas JKM, Figueiredo CC, Ramos MLG (2022) Biochar increases soil carbon pools: evidence from a global meta-analysis. J Environ Manage 305:114403. https://doi.org/10.1016/j.jenvman.2021.114403
Chen G, Fang Y, Van Zwieten L, Xuan Y, Tavakkoli E, Wang X, Zhang R (2021a) Priming, stabilization and temperature sensitivity of native SOC is controlled by microbial responses and physicochemical properties of biochar. Soil Biol Biochem 154:108139. https://doi.org/10.1016/j.soilbio.2021.108139
Chen S, Liu J, Zhang Q, Teng F, McLellan BC (2022) A critical review on deployment planning and risk analysis of carbon capture, utilization, and storage (CCUS) toward carbon neutrality. Renew Sust Energ Rev 167:112537. https://doi.org/10.1016/j.rser.2022.112537
Chen Y, Yang H, Wang X, Chen W, Chen H (2016) Biomass pyrolytic polygeneration system: adaptability for different feedstocks. Energy Fuel 30(1):414–422. https://doi.org/10.1021/acs.energyfuels.5b02332
Chen Z, Niu J, Guo Z, Sui X, Xu N, Kareem HA, Hassan MU, Yan M, Zhang Q, Cui J, Kang J, Wang Z, Mi F, Karagić Đ, Wang Q (2021b) Graphene enhances photosynthesis and the antioxidative defense system and alleviates salinity and alkalinity stresses in alfalfa (Medicago sativa L.) by regulating gene expression. Environ Sci-Nano 8(9):2731–2748. https://doi.org/10.1039/D1EN00257K
Chiu C, Huang Z (2020) Microbial methane oxidation and gas adsorption capacities of biochar-modified soils. INT J Geosynth Groun 6:24. https://doi.org/10.1007/s40891-020-00202-5
Cornelissen G, Rutherford DW, Arp HP, Dorsch P, Kelly CN, Rostad CE (2013) Sorption of pure N2O to biochars and other organic and inorganic materials under anhydrous conditions. Environ Sci Technol 47(14):7704–7712. https://doi.org/10.1021/es400676q
Creamer AE, Gao B (2016) Carbon-based adsorbents for postcombustion CO2 capture: a critical review. Environ Sci Technol 50(14):7276–7289. https://doi.org/10.1021/acs.est.6b00627
Cui L, Liu Y, Yan J, Hina K, Hussain Q, Qiu T, Zhu J (2022) Revitalizing coastal saline-alkali soil with biochar application for improved crop growth. Ecol Eng 179:106594. https://doi.org/10.1016/j.ecoleng.2022.106594
Cui Q, Xia J, Yang H, Liu J, Shao P (2021) Biochar and effective microorganisms promote Sesbania cannabina growth and soil quality in the coastal saline-alkali soil of the Yellow River Delta, China. Sci Total Environ 756:143801. https://doi.org/10.1016/j.scitotenv.2020.143801
Dai Y, Zheng H, Jiang Z, Xing B (2020) Combined effects of biochar properties and soil conditions on plant growth: a meta-analysis. Sci Total Environ 713:136635. https://doi.org/10.1016/j.scitotenv.2020.136635
Daliakopoulos IN, Tsanis IK, Koutroulis A, Kourgialas NN, Varouchakis AE, Karatzas GP, Ritsema CJ (2016) The threat of soil salinity: a European scale review. Sci Total Environ 573:727–739. https://doi.org/10.1016/j.scitotenv.2016.08.177
Debez A, Huchzermeyer B, Abdelly C, Koyro HW (2010) Sabkha Ecosystems. In: Öztürk M et al (eds) Current challenges and future opportunities for a sustainable utilization of halophytes., Sabkha ecosystems, tasks for vegetation science, pp. 59–77
Dissanayake PD, You S, Igalavithana AD, Xia Y, Bhatnagar A, Gupta S, Kua H, Kim S, Kwon JH, Tsang DCW, Ok YS (2020) Biochar-based adsorbents for carbon dioxide capture: a critical review. Renew Sust Energ Rev 119:109582. https://doi.org/10.1016/j.rser.2019.109582
Dong W, Walkiewicz A, Bieganowski A, Oenema O, Nosalewicz M, He C, Zhang Y, Hu C (2020) Biochar promotes the reduction of N2O to N2 and concurrently suppresses the production of N2O in calcareous soil. Geoderma 362:114091. https://doi.org/10.1016/j.geoderma.2019.114091
Dong X, Wang J, Liu X, Singh BP, Sun H (2022) Characterization of halophyte biochar and its effects on water and salt contents in saline soil. Environ Sci Pollut Res 29:11831–11842. https://doi.org/10.1007/s11356-021-16526-2
Duan X, Cao Y, Liu T, Li L, Wang B, Wang X (2020) Nutrient stability and sorption of sewage sludge biochar prepared from co-pyrolysis of sewage sludge and stalks/mineral materials. Env Pollut Bioavail 32(1):12–18. https://doi.org/10.1080/26395940.2019.1710259
Duarah P, Haldar D, Patel AK, Dong CD, Singhania RR, Purkait MK (2022) A review on global perspectives of sustainable development in bioenergy generation. Bioresour Technol 348:126791. https://doi.org/10.1016/j.biortech.2022.126791
Dusenge ME, Duarte AG, Way DA (2019) Plant carbon metabolism and climate change: elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol 221(1):32–49. https://doi.org/10.1111/nph.15283
El-Naggar A, Lee SS, Rinklebe J, Farooq M, Song H, Sarmah AK, Zimmerman AR, Ahmad M, Shaheen SM, Ok YS (2019) Biochar application to low fertility soils: a review of current status, and future prospects. Geoderma 337:536–554. https://doi.org/10.1016/j.geoderma.2018.09.034
Esmaeelnejad L, Shorafa M, Gorji M, Hosseini SM (2017) Impacts of woody biochar particle size on porosity and hydraulic conductivity of biochar-soil mixtures: an incubation study. Commun Soil Sci Plan 48(14):1710–1718. https://doi.org/10.1080/00103624.2017.1383414
Eswar D, Karuppusamy R, Chellamuthu S (2021) Drivers of soil salinity and their correlation with climate change. Curr Opin Env Sust 50:310–318. https://doi.org/10.1016/j.cosust.2020.10.015
FAO, Itps (2015) The Status of the world’s soil resources (main report). FAO, Rome
Farhangi-Abriz S, Torabian S (2018) Effect of biochar on growth and ion contents of bean plant under saline condition. Environ Sci Pollut R 25(12):11556–11564. https://doi.org/10.1007/s11356-018-1446-z
Feng H, Wang S, Gao Z, Pan H, Zhuge Y, Ren X, Hu S, Li C (2021a) Aggregate stability and organic carbon stock under different land uses integrally regulated by binding agents and chemical properties in saline-sodic soils. Land Degrad Dev 32(15):4151–4161. https://doi.org/10.1002/ldr.4019
Feng Z, Fan Z, Song H, Li K, Lu H, Liu Y, Cheng F (2021b) Biochar induced changes of soil dissolved organic matter: the release and adsorption of dissolved organic matter by biochar and soil. Sci Total Environ 783:147091. https://doi.org/10.1016/j.scitotenv.2021.147091
Ferdush J, Paul V (2021) A review on the possible factors influencing soil inorganic carbon under elevated CO2. CATENA 204:105434. https://doi.org/10.1016/j.catena.2021.105434
Ferreira CSS, Seifollahi-Aghmiuni S, Destouni G, Ghajarnia N, Kalantari Z (2022) Soil degradation in the European Mediterranean region: processes, status and consequences. Sci Total Environ 805:150106. https://doi.org/10.1016/j.scitotenv.2021.150106
Ge S, Wang S, Mai W, Zhang K, Tanveer M, Wang L, Tian C (2023) Characteristics and acidic soil amelioration effects of biochar derived from a typical halophyte Salicornia europaea L. (common glasswort). Environ Sci Pollut Res 30:66113–66124. https://doi.org/10.1007/s11356-023-27182-z
Godlewska P, Ok YS, Oleszczuk P (2021) The dark side of black gold: ecotoxicological aspects of biochar and biochar-amended soils. J Hazard Mater 403:123833. https://doi.org/10.1016/j.jhazmat.2020.123833
Gomez A, Narayan M, Zhao L, Jia X, Bernal RA, Lopez-Moreno ML, Peralta-Videa JR (2021) Effects of nano-enabled agricultural strategies on food quality: Current knowledge and future research needs. J Hazard Mater 401: 123385. https://doi.org/10.1016/j.jhazmat.2020.123385
Gouda S, Kerry RG, Das G, Paramithiotis S, Shin HS, Patra JK (2018) Revitalization of plant growth promoting rhizobacteria for sustainable development in agriculture. Microbiol Res 206:131–140. https://doi.org/10.1016/j.micres.2017.08.016
Gray M, Johnson MG, Dragila MI, Kleber M (2014) Water uptake in biochars: the roles of porosity and hydrophobicity. Biomass Bioenerg 61:196–205. https://doi.org/10.1016/j.biombioe.2013.12.010
Guo Z, Chen X, Zhang Y (2022) Impact of environmental regulation perception on farmers’ agricultural green production technology adoption: a new perspective of social capital. Technol Soc 71:102085. https://doi.org/10.1016/j.techsoc.2022.102085
Hafez EM, Osman HS, El-Razek UAA, Elbagory M, Omara AE, Eid MA, Gowayed SM (2021) Foliar-applied potassium silicate coupled with plant growth-promoting rhizobacteria improves growth, physiology, nutrient uptake and productivity of faba bean (Vicia faba L.) irrigated with saline water in salt-affected soil. Plants 10(5):894. https://doi.org/10.3390/plants10050894
Haj-Amor Z, Araya T, Kim DG, Bouri S, Lee J, Ghiloufi W, Yang Y, Kang H, Jhariya MK, Banerjee A, Lal R (2022) Soil salinity and its associated effects on soil microorganisms, greenhouse gas emissions, crop yield, biodiversity and desertification: a review. Sci Total Environ 843:156946. https://doi.org/10.1016/j.scitotenv.2022.156946
Han L, Sun K, Yang Y, Xia X, Li F, Yang Z, Xing B (2020) Biochar’s stability and effect on the content, composition and turnover of soil organic carbon. Geoderma 364:114184. https://doi.org/10.1016/j.geoderma.2020.114184
Hassani A, Azapagic A, Shokri N (2021) Global predictions of primary soil salinization under changing climate in the 21st century. Nat Commun 12(1):6663. https://doi.org/10.1038/s41467-021-26907-3
He K, He G, Wang C, Zhang H, Xu Y, Wang S, Kong Y, Zhou G, Hu R (2020) Biochar amendment ameliorates soil properties and promotes Miscanthus growth in a coastal saline-alkali soil. Appl Soil Ecol 155:103674. https://doi.org/10.1016/j.apsoil.2020.103674
Hoegh-Guldberg O, Jacob D, Taylor M, Guillen BT, Bindi M, Brown S, Camilloni IA, Diedhiou A, Djalante R, Ebi K, Engelbrecht F, Guiot J, Hijioka Y, Mehrotra S, Hope CW, Payne AJ, Portner HO, Seneviratne SI, Thomas A, Warren R, Zhou G (2019) The human imperative of stabilizing global climate change at 1.5°C. Science 365(6459):eaaw6974. https://doi.org/10.1126/science.aaw6974
Horton P, Long SP, Smith P, Banwart SA, Beerling DJ (2021) Technologies to deliver food and climate security through agriculture. Nature Plants 7(3):250–255. https://doi.org/10.1038/s41477-021-00877-2
Iaccarino A, Gautam R, Sarathy SM (2021) Bio-oil and biochar production from halophyte biomass: effects of pre-treatment and temperature on Salicornia bigelovii pyrolysis. Sustain Energ Fuels 5(8):2234–2248. https://doi.org/10.1039/D0SE01664K
IBI (2015) Standardized product definition and product testing guidelines for biochar that is used in soil: version number 2.1. http://www.biocharinternational.org/sites/default/files/IBI_Biochar_Standards_V2.1_Final.pdf
Intani K, Latif S, Islam M, Müller J (2018) Phytotoxicity of corncob biochar before and after heat treatment and washing. Sustainability 11(1):30. https://doi.org/10.3390/su11010030
Irfan M, Chen Q, Yue Y, Pang R, Lin Q, Zhao X, Chen H (2016) Co-production of biochar, bio-oil and syngas from halophyte grass (Achnatherum splendens L.) under three different pyrolysis temperatures. Bioresour Technol 211:457–463. https://doi.org/10.1016/j.biortech.2016.03.077
Jia G, Shevliakova E, Artaxo P, De Noblet-Ducoudré N, Houghton R, House J, Kitajima K, Lennard C, Popp A, Sirin A, Sukumar R, Verchot L (2019). Land–climate interactions. In: Shukla PR, Skea J, Calvo Buendia E, Masson-Delmotte V, Pörtner HO, Roberts DC, Zhai P, Slade R, Connors S, van Diemen R, Ferrat M, Haughey E, Luz S, Neogi S, Pathak M, Petzold J, Portugal Pereira J, Vyas P, Huntley E, Kissick K, Belkacemi M, Malley J (eds) Climate change and land: an IPCC special report on climate change, desertification, land degradation, sustainable land management, food security, and greenhouse gas fluxes in terrestrial ecosystems. https://www.ipcc.ch/srccl/chapter/chapter-2/
Ji C, Jin Y, Li C, Chen J, Kong D, Yu K, Liu S, Zou J (2018) Variation in soil methane release or uptake responses to biochar amendment: a separate meta-analysis. Ecosystems 21(8):1692–1705. https://doi.org/10.1007/s10021-018-0248-y
Jiang X, Tan X, Cheng J, Haddix ML, Cotrufo MF (2019) Interactions between aged biochar, fresh low molecular weight carbon and soil organic carbon after 3.5 years soil-biochar incubations. Geoderma 333:99–107. https://doi.org/10.1016/j.geoderma.2018.07.016
Jing F, Sun Y, Liu Y, Wan Z, Chen J, Tsang DCW (2022) Interactions between biochar and clay minerals in changing biochar carbon stability. Sci Total Environ 809:151124. https://doi.org/10.1016/j.scitotenv.2021.151124
Kazemi R, Ronaghi A, Yasrebi J, Ghasemi-Fasaei R, Zarei M (2019) Effect of shrimp waste–derived biochar and arbuscular mycorrhizal fungus on yield, antioxidant enzymes, and chemical composition of corn under salinity stress. J Soil Sci Plant Nut 19(4):758–770. https://doi.org/10.1007/s42729-019-00075-2
Keith A, Singh B, Dijkstra FA (2015) Biochar reduces the rhizosphere priming effect on soil organic carbon. Soil Biol Biochem 88:372–379. https://doi.org/10.1016/j.soilbio.2015.06.007
Khosravi A, Zheng H, Liu Q, Hashemi M, Tang Y, Xing B (2022) Production and characterization of hydrochars and their application in soil improvement and environmental remediation. Chem Eng J 430:133142. https://doi.org/10.1016/j.cej.2021.133142
Kim HS, Kim KR, Yang JE, Ok YS, Owens G, Nehls T, Wessolek G, Kim KH (2016) Effect of biochar on reclaimed tidal land soil properties and maize (Zea mays L.) response. Chemosphere 142:153–159. https://doi.org/10.1016/j.chemosphere.2015.06.041
Kotula L, Garcia Caparros P, Zorb C, Colmer TD, Flowers TJ (2020) Improving crop salt tolerance using transgenic approaches: an update and physiological analysis. Plant Cell Environ 43(12):2932–2956. https://doi.org/10.1111/pce.13865
Kumar R, Singh A, Datta A, Yadav RP, Dinesh D, Verma K (2022) Plans and policies for soil organic carbon management in agriculture. In: Meena RS, Rao CS, Kumar A (eds) Carbon sequestration in degraded lands: current prospects, practices, and future strategies. Springer Nature Singapore, Singapore, pp 221–255
La H, Hettiaratchi JPA, Achari G (2019) The influence of biochar and compost mixtures, water content, and gas flow rate, on the continuous adsorption of methane in a fixed bed column. J Environ Manage 233:175–183. https://doi.org/10.1016/j.jenvman.2018.12.015
Lehmann J, Cowie A, Masiello CA, Kammann C, Woolf D, Amonette JE, Cayuela ML, Camps-Arbestain M, Whitman T (2021) Biochar in climate change mitigation. Nat Geosci 14(12):883–892. https://doi.org/10.1038/s41561-021-00852-8
Lehmann J, Joseph S (2015) Biochar for environmental management: science, technology and implementation. Routledge, New York
Li J, Pu L, Han M, Zhu M, Zhang R, Xiang Y (2014) Soil salinization research in China: Advances and prospects. J Geogr Sci 24(5):943–960. https://doi.org/10.1007/s11442-014-1130-2
Liang C, Amelung W, Lehmann J, Kastner M (2019) Quantitative assessment of microbial necromass contribution to soil organic matter. Glob Change Biol 25(11):3578–3590. https://doi.org/10.1111/gcb.14781
Liao S, Pan B, Li H, Zhang D, Xing B (2014) Detecting free radicals in biochars and determining their ability to inhibit the germination and growth of corn, wheat and rice seedlings. Environ Sci Technol 48(15):8581–8587. https://doi.org/10.1021/es404250a
Lieke T, Zhang X, Steinberg CEW, Pan B (2018) Overlooked risks of biochars: persistent free radicals trigger neurotoxicity in Caenorhabditis elegans. Environ Sci Technol 52(14):7981–7987. https://doi.org/10.1021/acs.est.8b01338
Lin X, Xie Z, Zheng J, Liu Q, Bei Q, Zhu J (2015) Effects of biochar application on greenhouse gas emissions, carbon sequestration and crop growth in coastal saline soil. Eur J Soil Sci 66(2):329–338. https://doi.org/10.1111/ejss.12225
Liu D, Ding Z, Ali EF, Kheir AMS, Eissa MA, Ibrahim OHM (2021a) Biochar and compost enhance soil quality and growth of roselle (Hibiscus sabdariffa L.) under saline conditions. Sci Rep 11(1):8739. https://doi.org/10.1038/s41598-021-88293-6
Liu G, Pan M, Song J, Guo M, Xu L, Xin Y (2022) Investigating the effects of biochar colloids and nanoparticles on cucumber early seedlings. Sci Total Environ 804:150233. https://doi.org/10.1016/j.scitotenv.2021.150233
Liu G, Zheng H, Jiang Z, Zhao J, Wang Z, Pan B, Xing B (2018) Formation and physicochemical characteristics of nano biochar: insight into chemical and colloidal stability. Environ Sci Technol 52(18):10369–10379. https://doi.org/10.1021/acs.est.8b01481
Liu J, Jiang J, Meng Y, Aihemaiti A, Xu Y, Xiang H, Gao Y, Chen X (2020a) Preparation, environmental application and prospect of biochar-supported metal nanoparticles: a review. J Hazard Mater 388:122026. https://doi.org/10.1016/j.jhazmat.2020.122026
Liu J, Li G, Chen L, Gu J, Wu H, Li Z (2021b) Cerium oxide nanoparticles improve cotton salt tolerance by enabling better ability to maintain cytosolic K+/Na+ ratio. J Nanobiotechnol 19(1):153. https://doi.org/10.1186/s12951-021-00892-7
Liu S, Meng J, Jiang L, Yang X, Lan Y, Cheng X, Chen W (2017a) Rice husk biochar impacts soil phosphorous availability, phosphatase activities and bacterial community characteristics in three different soil types. Appl Soil Ecol 116:12–22. https://doi.org/10.1016/j.apsoil.2017.03.020
Liu W, Li W, Jiang H, Yu H (2017b) Fates of chemical elements in biomass during its pyrolysis. Chem Rev 117(9):6367–6398. https://doi.org/10.1021/acs.chemrev.6b00647
Liu X, Mao P, Li L, Ma J (2019) Impact of biochar application on yield-scaled greenhouse gas intensity: a meta-analysis. Sci Total Environ 656:969–976. https://doi.org/10.1016/j.scitotenv.2018.11.396
Liu X, Zhang A, Ji C, Joseph S, Bian R, Li L, Pan G, Paz-Ferreiro J (2013) Biochar’s effect on crop productivity and the dependence on experimental conditions–a meta-analysis of literature data. Plant Soil 373:583–594. https://doi.org/10.1007/s11104-013-1806-x
Liu Y, Bi Y, Xie Y, Zhao X, He D, Wang S, Wang C, Guo T, Xing G (2020b) Successive straw biochar amendments reduce nitrous oxide emissions but do not improve the net ecosystem economic benefit in an alkaline sandy loam under a wheat–maize cropping system. Land Degrad Dev 31(7):868–883. https://doi.org/10.1002/ldr.3495
Liu Z, Dugan B, Masiello CA, Gonnermann HM (2017c) Biochar particle size, shape, and porosity act together to influence soil water properties. PLoS ONE 12(6):e0179079. https://doi.org/10.1371/journal.pone.0179079
Lu Q, Yu Z, Yu S, Liang Z, Li H, Sun L, Wang S (2019) Organic matter rather than salinity as a predominant feature changes performance and microbiome in methanogenic sludge digesters. J Hazard Mater 377:349–356. https://doi.org/10.1016/j.jhazmat.2019.05.075
Lund H, Skov IR, Thellufsen JZ, Sorknæs P, Korberg AD, Chang M, Mathiesen BV, Kany MS (2022) The role of sustainable bioenergy in a fully decarbonised society. Renew Energ 196:195–203. https://doi.org/10.1016/j.renene.2022.06.026
Luo R, Kuzyakov Y, Liu D, Fan J, Luo J, Lindsey S, He J, Ding W (2020) Nutrient addition reduces carbon sequestration in a Tibetan grassland soil: disentangling microbial and physical controls. Soil Biol Biochem 144:107764. https://doi.org/10.1016/j.soilbio.2020.107764
Luo X, Chen L, Zheng H, Chang J, Wang H, Wang Z, Xing B (2016a) Biochar addition reduced net N mineralization of a coastal wetland soil in the Yellow River Delta, China. Geoderma 282:120–128. https://doi.org/10.1016/j.geoderma.2016.07.015
Luo X, Liu G, Xia Y, Chen L, Jiang Z, Zheng H, Wang Z (2017) Use of biochar-compost to improve properties and productivity of the degraded coastal soil in the Yellow River Delta, China. J Soil Sediment 17(3):780–789. https://doi.org/10.1007/s11368-016-1361-1
Luo X, Wang L, Liu G, Wang X, Wang Z, Zheng H (2016b) Effects of biochar on carbon mineralization of coastal wetland soils in the Yellow River Delta, China. Ecol Eng 94:329–336. https://doi.org/10.1016/j.ecoleng.2016.06.004
Luo X, Wang Z, Meki K, Wang X, Liu B, Zheng H, You X, Li F (2019) Effect of co-application of wood vinegar and biochar on seed germination and seedling growth. J Soil Sediment 19(12):3934–3944. https://doi.org/10.1007/s11368-019-02365-9
Lyu H, Zhang H, Chu M, Zhang C, Tang J, Chang S, Mašek O, Ok YS (2022) Biochar affects greenhouse gas emissions in various environments: A critical review. Land Degrad Dev 33:3327–3342. https://doi.org/10.1002/ldr.4405
Ma P, Qi Z, Wu X, Ji R, Chen W (2023) Biochar nanoparticles-mediated transport of organic contaminants in porous media: dependency on contaminant properties and effects of biochar aging. Carbon Res 2:4. https://doi.org/10.1007/s44246-023-00036-6
Makkawi Y, El Sayed Y, Lyra DA, Pour FH, Khan M, Badrelzaman M (2021) Assessment of the pyrolysis products from halophyte Salicornia bigelovii cultivated in a desert environment. Fuel 290:119518. https://doi.org/10.1016/j.fuel.2020.119518
Maucieri C, Zhang Y, McDaniel MD, Borin M, Adams MA (2017) Short-term effects of biochar and salinity on soil greenhouse gas emissions from a semi-arid Australian soil after re-wetting. Geoderma 307:267–276. https://doi.org/10.1016/j.geoderma.2017.07.028
Medick J, Teichmann I, Kemfert C (2018) Hydrothermal carbonization (HTC) of green waste: mitigation potentials, costs, and policy implications of HTC coal in the metropolitan region of Berlin, Germany. Energ Policy 123:503–513. https://doi.org/10.1016/j.enpol.2018.08.033
Meyer S, Genesio L, Vogel I, Schmidt HP, Soja G, Someus E, Shackley S, Verheijen FGA, Glaser B (2017) Biochar standardization and legislation harmonization. J Environ Eng Landsc 25(2):175–191. https://doi.org/10.3846/16486897.2016.1254640
Mukhopadhyay R, Sarkar B, Jat HS, Sharma PC, Bolan NS (2021) Soil salinity under climate change: challenges for sustainable agriculture and food security. J Environ Manage 280:111736. https://doi.org/10.1016/j.jenvman.2020.111736
Nguyen BT, Trinh NN, Bach QV (2020) Methane emissions and associated microbial activities from paddy salt-affected soil as influenced by biochar and cow manure addition. Appl Soil Ecol 152:103531. https://doi.org/10.1016/j.apsoil.2020.103531
Ni H, Jing X, Xiao X, Zhang N, Wang X, Sui Y, Sun B, Liang Y (2021) Microbial metabolism and necromass mediated fertilization effect on soil organic carbon after long-term community incubation in different climates. ISME J 15(9):2561–2573. https://doi.org/10.1038/s41396-021-00950-w
Odinga ES, Waigi MG, Gudda FO, Wang J, Yang B, Hu X, Li S, Gao Y (2020) Occurrence, formation, environmental fate and risks of environmentally persistent free radicals in biochars. Environ Int 134:105172. https://doi.org/10.1016/j.envint.2019.105172
Omondi MO, Xia X, Nahayo A, Liu X, Korai PK, Pan G (2016) Quantification of biochar effects on soil hydrological properties using meta-analysis of literature data. Geoderma 274:28–34. https://doi.org/10.1016/j.geoderma.2016.03.029
Parveen A, Ahmar S, Kamran M, Malik Z, Ali A, Riaz M, Abbasi GH, Khan M, Sohail AB, Rizwan M, Afzal S, Ali S (2021) Abscisic acid signaling reduced transpiration flow, regulated Na+ ion homeostasis and antioxidant enzyme activities to induce salinity tolerance in wheat (Triticum aestivum L.) seedlings. Environ Technol Inno 24:101808. https://doi.org/10.1016/j.eti.2021.101808
Pei J, Dijkstra FA, Li J, Fang C, Su J, Zhao J, Nie M, Wu J (2020) Biochar-induced reductions in the rhizosphere priming effect are weaker under elevated CO2. Soil Biol Biochem 142:107700. https://doi.org/10.1016/j.soilbio.2019.107700
Pendleton L, Donato DC, Murray BC, Crooks S, Jenkins WA, Sifleet S, Craft C, Fourqurean JW, Kauffman JB, Marba N, Megonigal P, Pidgeon E, Herr D, Gordon D, Baldera A (2012) Estimating global “blue carbon” emissions from conversion and degradation of vegetated coastal ecosystems. PLoS ONE 7(9):e43542. https://doi.org/10.1371/journal.pone.0043542
Qin Y, Druzhinina IS, Pan X, Yuan Z (2016) Microbially mediated plant salt tolerance and microbiome-based solutions for saline agriculture. Biotechnol Adv 34(7):1245–1259. https://doi.org/10.1016/j.biotechadv.2016.08.005
Rajabi Hamedani S, Kuppens T, Malina R, Bocci E, Colantoni A, Villarini M (2019) Life cycle assessment and environmental valuation of biochar production: two case studies in Belgium. Energies 12(11):2166. https://doi.org/10.3390/en12112166
Rath KM, Maheshwari A, Rousk J (2019) Linking microbial community structure to trait distributions and functions using salinity as an environmental filter. Mbio 10(4):e01607-e1619. https://doi.org/10.1128/mBio.01607-19
Rengasamy P, Olsson K (1991) Sodicity and soil structure. Soil Res 29(6):935–952. https://doi.org/10.1071/SR9910935
Romero CM, Li C, Owens J, Ribeiro GO, McAllister TA, Okine E, Hao X (2021) Nutrient cycling and greenhouse gas emissions from soil amended with biochar-manure mixtures. Pedosphere 31(2):289–302. https://doi.org/10.1016/S1002-0160(20)60071-6
Rumpel C, Amiraslani F, Chenu C, Garcia Cardenas M, Kaonga M, Koutika LS, Ladha J, Madari B, Shirato Y, Smith P, Soudi B, Soussana JF, Whitehead D, Wollenberg E (2020) The 4p1000 initiative: opportunities, limitations and challenges for implementing soil organic carbon sequestration as a sustainable development strategy. Ambio 49(1):350–360. https://doi.org/10.1007/s13280-019-01165-2
Saha N, Saba A, Reza MT (2019) Effect of hydrothermal carbonization temperature on pH, dissociation constants, and acidic functional groups on hydrochar from cellulose and wood. J Anal Appl Pyrol 137:138–145. https://doi.org/10.1016/j.jaap.2018.11.018
Saifullah Dahlawi S, Naeem A, Rengel Z, Naidu R (2018) Biochar application for the remediation of salt-affected soils: challenges and opportunities. Sci Total Environ 625:320–335. https://doi.org/10.1016/j.scitotenv.2017.12.257
Schleussner CF, Rogelj J, Schaeffer M, Lissner T, Licker R, Fischer EM, Knutti R, Levermann A, Frieler K, Hare W (2016) Science and policy characteristics of the Paris Agreement temperature goal. Nat Clim Change 6(9):827–835. https://doi.org/10.1016/j.scitotenv.2017.12.257
Schmidt HP, Bucheli T, Kammann C, Glaser B, Abiven S, Leifeld J (2016) European biochar certificate-guidelines for a sustainable production of biochar. Eur Biochar Found. https://doi.org/10.13140/RG.2.1.4658.7043
Setia R, Marschner P, Baldock J, Chittleborough D, Verma V (2011) Relationships between carbon dioxide emission and soil properties in salt-affected landscapes. Soil Biol Biochem 43(3):667–674. https://doi.org/10.1016/j.soilbio.2010.12.004
Shackley S, Esteinou RI, Hopkins D, Hammond J (2014) Biochar quality mandate (BQM) version 1.0. British Biochar Foundation
Shakoor A, Arif MS, Shahzad SM, Farooq TH, Ashraf F, Altaf MM, Ahmed W, Tufail MA, Ashraf M (2021a) Does biochar accelerate the mitigation of greenhouse gaseous emissions from agricultural soil?—a global meta-analysis. Environ Res 202:111789. https://doi.org/10.1016/j.envres.2021.111789
Shakoor A, Shahzad SM, Chatterjee N, Arif MS, Farooq TH, Altaf MM, Tufail MA, Dar AA, Mehmood T (2021b) Nitrous oxide emission from agricultural soils: application of animal manure or biochar? A global meta-analysis. J Environ Manage 285:112170. https://doi.org/10.1016/j.jenvman.2021.112170
Shi X, Hu H, Zhu-Barker X, Hayden H, Wang J, Suter H, Chen D, He JZ (2017) Nitrifier-induced denitrification is an important source of soil nitrous oxide and can be inhibited by a nitrification inhibitor 3,4-dimethylpyrazole phosphate. Environ MIcrobiol 19(12):4851–4865. https://doi.org/10.1111/1462-2920.13872
Sial TA, Khan MN, Lan Z, Kumbhar F, Ying Z, Zhang J, Sun D, Li X (2019) Contrasting effects of banana peels waste and its biochar on greenhouse gas emissions and soil biochemical properties. Process Saf Environ 122:366–377. https://doi.org/10.1016/j.psep.2018.10.030
Singh BP, Cowie AL (2014) Long-term influence of biochar on native organic carbon mineralisation in a low-carbon clayey soil. Sci Rep 4:3687. https://doi.org/10.1038/srep03687
Singh R, Mavi MS, Choudhary OP (2019) Saline soils can be ameliorated by adding biochar generated from rice-residue waste. Clean: Soil, Air, Water 47(2):1700656. https://doi.org/10.1002/clen.201700656
Song B, Almatrafi E, Tan X, Luo S, Xiong W, Zhou C, Qin M, Liu Y, Cheng M, Zeng G, Gong J (2022) Biochar-based agricultural soil management: an application-dependent strategy for contributing to carbon neutrality. Renew Sust Energ Rev 164:112529. https://doi.org/10.1016/j.rser.2022.112529
Song X, Razavi BS, Ludwig B, Zamanian K, Zang H, Kuzyakov Y, Dippold MA, Gunina A (2020) Combined biochar and nitrogen application stimulates enzyme activity and root plasticity. Sci Total Environ 735:139393. https://doi.org/10.1016/j.scitotenv.2020.139393
Srivastava P, Singh R, Tripathi S, Singh P, Singh S, Singh H, Raghubanshi AS, Mishra PK (2017) Soil carbon dynamics under changing climate—a research transition from absolute to relative roles of inorganic nitrogen pools and associated microbial processes: a Review. Pedosphere 27(5):792–806. https://doi.org/10.1016/S1002-0160(17)60488-0
Sun J, He F, Shao H, Zhang Z, Xu G (2016) Effects of biochar application on Suaeda salsa growth and saline soil properties. Environ Earth Sci 75:630. https://doi.org/10.1007/s12665-016-5440-9
Tan L, Ge Z, Li S, Li Y, Xie L, Tang J (2021) Reclamation-induced tidal restriction increases dissolved carbon and greenhouse gases diffusive fluxes in salt marsh creeks. Sci Total Environ 773:145684. https://doi.org/10.1016/j.scitotenv.2021.145684
Tanzer SE, Blok K, Ramírez A (2021) Decarbonising Industry via BECCS: promising sectors, challenges, and techno-economic limits of negative emissions. Curr Sustain Renew Energy Rep 8:253–262. https://doi.org/10.1007/s40518-021-00195-3
Theint EE, Bellingrath-Kimura SD, Oo AZ, Motobayashi T (2016) Influence of gypsum amendment on methane emission from paddy soil affected by saline irrigation water. Front Environ Sci 3:79. https://doi.org/10.3389/fenvs.2015.00079
Tisserant A, Cherubini F (2019) Potentials, limitations, co-benefits, and trade-offs of biochar applications to soils for climate change mitigation. Land 8(12):179. https://doi.org/10.3390/land8120179
Tkemaladze GS, Makhashvili KA (2016) Climate changes and photosynthesis. Ann Agric Sci 14(2):119–126. https://doi.org/10.1016/j.aasci.2016.05.012
Toju H, Peay KG, Yamamichi M, Narisawa K, Hiruma K, Naito K, Fukuda S, Ushio M, Nakaoka S, Onoda Y, Yoshida K, Schlaeppi K, Bai Y, Sugiura R, Ichihashi Y, Minamisawa K, Kiers ET (2018) Core microbiomes for sustainable agroecosystems. Nat Plants 4(5):247–257. https://doi.org/10.1038/s41477-018-0139-4
Torabian S, Farhangi-Abriz S, Rathjen J (2018) Biochar and lignite affect H+-ATPase and H+-PPase activities in root tonoplast and nutrient contents of mung bean under salt stress. Plant Physiol Bioch 129:141–149. https://doi.org/10.1016/j.plaphy.2018.05.030
Virk AL, Kan ZR, Liu BY, Qi JY, He C, Liu QY, Zhao X, Zhang HL (2021) Impact of biochar water extract addition on soil organic carbon mineralization and C fractions in different tillage systems. Environ Technol Inno 21:101193. https://doi.org/10.1016/j.eti.2020.101193
Wang C, Yue L, Cheng B, Chen F, Zhao X, Wang Z, Xing B (2022a) Mechanisms of growth-promotion and Se-enrichment in Brassica chinensis L. by selenium nanomaterials: beneficial rhizosphere microorganisms, nutrient availability, and photosynthesis. Environ Sci-Nano 9(1):302–312. https://doi.org/10.1039/d1en00740h
Wang F, Harindintwali JD, Yuan Z, Wang M, Wang F, Li S, Yin Z, Huang L, Fu Y, Li L, Chang SX, Zhang L, Rinklebe J, Yuan Z, Zhu Q, Xiang L, Tsang DCW, Xu L, Jiang X, Liu J, Wei N, Kästner M, Zou Y, Ok YS, Shen J, Peng D, Zhang W, Barcelo D, Zhou Y, Bai Z, Li B, Zhang B, Wei K, Cao H, Tan Z, Zhao LB, He X, Zheng J, Bolan N, Liu X, Huang C, Dietmann S, Luo M, Sun N, Gong J, Gong Y, Brahushi F, Zhang T, Xiao C, Li X, Chen W, Jiao N, Lehmann J, Zhu Y, Jin H, Schaeffer A, Tiedje JM, Chen J (2021) Technologies and perspectives for achieving carbon neutrality. Innovation 2(4):100180. https://doi.org/10.1016/j.xinn.2021.100180
Wang J, Pan X, Liu Y, Zhang X, Xiong Z (2012) Effects of biochar amendment in two soils on greenhouse gas emissions and crop production. Plant Soil 360:287–298. https://doi.org/10.1007/s11104-012-1250-3
Wang J, Xiong Z, Kuzyakov Y (2015) Biochar stability in soil: meta-analysis of decomposition and priming effects. GCB Bioenergy 8(3):512–523. https://doi.org/10.1111/gcbb.12266
Wang J, Yuan G, Lu J, Wu J, Wei J (2019) Effects of biochar and peat on salt-affected soil extract solution and wheat seedling germination in the Yellow River Delta. Arid Land Res Manag 34(3):287–305. https://doi.org/10.1080/15324982.2019.1696423
Wang Y, Lin Q, Liu Z, Liu K, Wang X, Shang J (2023) Salt-affected marginal lands: a solution for biochar production. Biochar 5:21. https://doi.org/10.1007/s42773-023-00219-9
Wang Z, Pan X, Kuang S, Chen C, Wang X, Xu J, Li X, Li H, Zhuang Q, Zhang F, Wang X (2022b) Amelioration of coastal salt-affected soils with biochar, acid modified biochar and wood vinegar: enhanced nutrient availability and bacterial community modulation. Int J Environ Res Public Health 19(12):7282. https://doi.org/10.3390/ijerph19127282
Wicke B, Smeets E, Dornburg V, Vashev B, Gaiser T, Turkenburg W, Faaij A (2011) The global technical and economic potential of bioenergy from salt-affected soils. Energ Environ Sci 4(8):2669–2681. https://doi.org/10.1039/C1EE01029H
Woolf D, Amonette JE, Street-Perrott FA, Lehmann J, Joseph S (2010) Sustainable biochar to mitigate global climate change. Nat Commun 1:56. https://doi.org/10.1038/ncomms1053
Woolf D, Lehmann J, Ogle S, Kishimoto-Mo AW, McConkey B, Baldock J (2021) Greenhouse gas inventory model for biochar additions to soil. Environ Sci Technol 55(21):14795–14805. https://doi.org/10.1021/acs.est.1c02425
Wu F, Li F, Zhao X, Bolan NS, Fu P, Lam SS, Mašek O, Ong HC, Pan B, Qiu X, Rinklebe J, Tsang DCW, Van Zwieten L, Vithanage M, Wang S, Xing B, Zhang G, Wang H (2022) Meet the challenges in the “Carbon Age.” Carbon Res 1:1. https://doi.org/10.1007/s44246-022-00001-9
Xiao L, Yuan G, Feng L, Bi D, Wei J (2020) Soil properties and the growth of wheat (Triticum aestivum L.) and maize (Zea mays L.) in response to reed (phragmites communis) biochar use in a salt-affected soil in the Yellow River Delta. Agr Ecosyst Environ 303:107124. https://doi.org/10.1016/j.agee.2020.107124
Xiao X, Chen B, Chen Z, Zhu L, Schnoor JL (2018) Insight into multiple and multilevel structures of biochars and their potential environmental applications: a critical review. Environ Sci Technol 52(9):5027–5047. https://doi.org/10.1021/acs.est.7b06487
Xiao Z, Rasmann S, Yue L, Lian F, Zou H, Wang Z (2019) The effect of biochar amendment on N-cycling genes in soils: a meta-analysis. Sci Total Environ 696:133984. https://doi.org/10.1016/j.scitotenv.2019.133984
Xiao H, Lin Q, Li G, Zhao X, Li J, Li E (2022) Comparison of biochar properties from 5 kinds of halophyte produced by slow pyrolysis at 500 °C. Biochar 4:12. https://doi.org/10.1007/s42773-022-00141-6
Xie T, Cui B, Bai J, Li S, Zhang S (2018) Rethinking the role of edaphic condition in halophyte vegetation degradation on salt marshes due to coastal defense structure. Phys Chem Earth Parts ABC 103:81–90. https://doi.org/10.1016/j.pce.2016.12.001
Xu C, Tan X, Zhao J, Cao J, Ren M, Xiao Y, Lin A (2021) Optimization of biochar production based on environmental risk and remediation performance: take kitchen waste for example. J Hazard Mater 416:125785. https://doi.org/10.1016/j.jhazmat.2021.125785
Xu W, Wang G, Deng F, Zou X, Ruan H, Chen H, Aitkenhead M (2018) Responses of soil microbial biomass, diversity and metabolic activity to biochar applications in managed poplar plantations on reclaimed coastal saline soil. Soil Use Manage 34(4):597–605. https://doi.org/10.1111/sum.12460
Xu X, Zhao Y, Sima J, Zhao L, Masek O, Cao X (2017) Indispensable role of biochar-inherent mineral constituents in its environmental applications: a review. Bioresour Technol 241:887–899. https://doi.org/10.1016/j.biortech.2017.06.023
Yang Q, Mašek O, Zhao L, Nan H, Yu S, Yin J, Li Z, Cao X (2021a) Country-level potential of carbon sequestration and environmental benefits by utilizing crop residues for biochar implementation. Appl Energ 282:116275. https://doi.org/10.1016/j.apenergy.2020.116275
Yang Q, Zhou H, Bartocci P, Fantozzi F, Masek O, Agblevor FA, Wei Z, Yang H, Chen H, Lu X, Chen G, Zheng C, Nielsen CP, McElroy MB (2021b) Prospective contributions of biomass pyrolysis to China’s 2050 carbon reduction and renewable energy goals. Nat Commun 12(1):1698. https://doi.org/10.1038/s41467-021-21868-z
Yao T, Zhang W, Gulaqa A, Cui Y, Zhou Y, Weng W, Wang X, Liu Q, Jin F (2021) Effects of peanut shell biochar on soil nutrients, soil enzyme activity, and rice yield in heavily saline-sodic paddy field. J Soil Sci Plant Nut 21(1):655–664. https://doi.org/10.1007/s42729-020-00390-z
Yuan Y, Kong Q, Zheng Y, Zheng H, Liu Y, Cheng Y, Zhang X, Li Z, You X, Li Y (2022) Co-application of biochar and pyroligneous acid improved peanut production and nutritional quality in a coastal soil. Environ Technol Inno 28:102886. https://doi.org/10.1016/j.eti.2022.102886
Zhang C, Zeng G, Huang D, Lai C, Chen M, Cheng M, Tang W, Tang L, Dong H, Huang B, Tan X, Wang R (2019a) Biochar for environmental management: mitigating greenhouse gas emissions, contaminant treatment, and potential negative impacts. Chem Eng J 373:902–922. https://doi.org/10.1016/j.cej.2019.05.139
Zhang J, Zhou S, Sun H, Lu F, He P (2019b) Three-year rice grain yield responses to coastal mudflat soil properties amended with straw biochar. J Environ Manage 239:23–29. https://doi.org/10.1016/j.jenvman.2019.03.022
Zhang L, Song L, Wang B, Shao H, Zhang L, Qin X (2018) Co-effects of salinity and moisture on CO2 and N2O emissions of laboratory-incubated salt-affected soils from different vegetation types. Geoderma 332:109–120. https://doi.org/10.1016/j.geoderma.2018.06.025
Zhang P, Duan W, Peng H, Pan B, Xing B (2022) Functional biochar and its balanced design. ACS Environ Au 2(2):115–127. https://doi.org/10.1021/acsenvironau.1c00032
Zhang X, Bao D, Li M, Tang Q, Wu M, Zhou H, Liu L, Qu Y (2020) Bioremediation of petroleum hydrocarbons by alkali–salt-tolerant microbial consortia and their community profiles. J Chem Technol Biotechnol 96(3):809–817. https://doi.org/10.1002/jctb.6594
Zhang Y, Lin F, Wang X, Zou J, Liu S (2016) Annual accounting of net greenhouse gas balance response to biochar addition in a coastal saline bioenergy cropping system in China. Soil till Res 158:39–48. https://doi.org/10.1016/j.still.2015.11.006
Zhang Y, Wang J, Feng Y (2021a) The effects of biochar addition on soil physicochemical properties: a review. CATENA 202:105284. https://doi.org/10.1016/j.catena.2021.105284
Zhang Y, Xie H, Wang F, Sun C, Zhang X (2021b) Effects of biochar incorporation on soil viable and necromass carbon in the luvisol soil. Soil Use Manage 38(1):318–330. https://doi.org/10.1111/sum.12720
Zhang Y, Yang R, Si X, Duan X, Quan X (2019c) The adverse effect of biochar to aquatic algae—the role of free radicals. Environ Pollut 248:429–437. https://doi.org/10.1016/j.envpol.2019.02.055
Zhang Y, Zhang Z, Chen Y (2021c) Biochar mitigates N2O emission of microbial denitrification through modulating carbon metabolism and allocation of reducing power. Environ Sci Technol 55(12):8068–8078. https://doi.org/10.1021/acs.est.1c01976
Zhao M, Han G, Li J, Song W, Qu W, Eller F, Wang J, Jiang C (2020) Responses of soil CO2 and CH4 emissions to changing water table level in a coastal wetland. J Clean Prod 269:122316. https://doi.org/10.1016/j.jclepro.2020.122316
Zhao M, Ma X, Liao X, Cheng S, Liu Q, Wang H, Zheng H, Li X, Luo X, Zhao J, Li F, Xing B (2021) Characteristics of algae-derived biochars and their sorption and remediation performance for sulfamethoxazole in marine environment. Chem Eng J 430:133092. https://doi.org/10.1016/j.cej.2021.133092
Zheng H, Liu B, Liu G, Cai Z, Zhang C (2019) Biochar from biomass and waste. In: Ok YS, Tsang DCW, Bolan N, Novak JM (eds) Potential toxic compounds in biochar: knowledge gaps between biochar research and safety. Elsevier, Amsterdam, pp 349–384
Zheng H, Wang X, Chen L, Wang Z, Xia Y, Zhang Y, Wang H, Luo X, Xing B (2018a) Enhanced growth of halophyte plants in biochar-amended coastal soil: roles of nutrient availability and rhizosphere microbial modulation. Plant Cell Environ 41(3):517–532. https://doi.org/10.1111/pce.12944
Zheng H, Wang X, Luo X, Wang Z, Xing B (2018b) Biochar-induced negative carbon mineralization priming effects in a coastal wetland soil: roles of soil aggregation and microbial modulation. Sci Total Environ 610–611:951–960. https://doi.org/10.1016/j.scitotenv.2017.08.166
Zheng H, Wang Z, Deng X, Zhao J, Luo Y, Novak J, Herbert S, Xing B (2013) Characteristics and nutrient values of biochars produced from giant reed at different temperatures. Bioresour Technol 130:463–471. https://doi.org/10.1016/j.biortech.2012.12.044
Zhou J, Zang H, Loeppmann S, Gube M, Kuzyakov Y, Pausch J (2020a) Arbuscular mycorrhiza enhances rhizodeposition and reduces the rhizosphere priming effect on the decomposition of soil organic matter. Soil Biol Biochem 140:107641. https://doi.org/10.1016/j.soilbio.2019.107641
Zhou M, Butterbach-Bahl K, Vereecken H, Bruggemann N (2017) A meta-analysis of soil salinization effects on nitrogen pools, cycles and fluxes in coastal ecosystems. Glob Change Biol 23(3):1338–1352. https://doi.org/10.1111/gcb.13430
Zhou X, Smaill SJ, Gu X, Clinton PW (2020b) Manipulation of soil methane oxidation under drought stress. Sci Total Environ 757:144089. https://doi.org/10.1016/j.scitotenv.2020.144089
Zhu H, Yang J, Yao R, Wang X, Xie W, Zhu W, Liu X, Cao Y, Tao J (2020) Interactive effects of soil amendments (biochar and gypsum) and salinity on ammonia volatilization in coastal saline soil. CATENA 190:104527. https://doi.org/10.1016/j.catena.2020.104527
Zhu X, Chen B, Zhu L, Xing B (2017) Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: a review. Environ Pollut 227:98–115. https://doi.org/10.1016/j.envpol.2017.04.032
Acknowledgements
Not applicable.
Funding
This work was supported by National Science Fund for Distinguished Young Scholars of Shandong Province (ZR2021JQ13), National Natural Science Foundation of China (42077115), Key R&D Program of Shandong Province, China (2022SFGC0302), Key R&D project of Shaanxi Province (2022NY-054), Fundamental Research Funds for the Central Universities (202261068), and USDA Hatch program (MAS 00549).
Author information
Authors and Affiliations
Contributions
HZ and BX developed the concept for this review and evaluated and revised the manuscript; HZ led the data and literature synthesis, figure design and writing; QL and YY extracted the data from literature; QL performed the data analysis with the help of ZJ. QL and KM wrote the first draft of the manuscript. QL led the drawing and revision of figures, and KM, YY, and MS contributed to the revision of the figures. XL, XL, ZJ, and FL contributed to the revision of final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling editor: Xiaoyuan Yan.
Supplementary Information
Additional file 1:
Supplementary Information.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, Q., Meki, K., Zheng, H. et al. Biochar application in remediating salt-affected soil to achieve carbon neutrality and abate climate change. Biochar 5, 45 (2023). https://doi.org/10.1007/s42773-023-00244-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42773-023-00244-8