Abstract
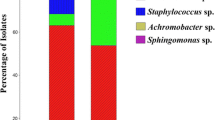
This work aimed to characterize antagonistic bacteria from the field-grown barley rhizosphere, and evaluate their potential for growth promotion and biocontrol of Fusarium wilt on watermelon caused by Fusarium oxysporum f. sp. Niveum (FON). Seven bacteria were isolated and screened for plant growth promoting and antagonistic traits. Based on the results of phenotypic characterization and 16S rRNA gene sequencing, the isolates were identified to be related to Bacillus methylotrophicus (DMK-1), Bacillus amyloliquefaciens subsp. plantarum (DMK-7-2), Bacillus cereus (DMK-12), Pseudomonas brassicacearum subsp. brassicacearum (DMK-2), Pseudomonas veronii (DMK-3), Paenibacillus polymyxa (DMK-8), and Ensifer adhaerens (DMK-17). All the isolates were positive for the production of indole-3-acetic acid (IAA) and ammonia (NH3), while negative for the production of hydrogen cyanide (HCN). Six bacteria strains (except DMK-17) were able to phosphate solubilization. All the bacteria strains, except DMK-8, were able to produce iron siderophore complexes, and possessed the proteolytic activity. Greenhouse experiment indicated six strains can decrease diseased percentage caused by FON. All the isolates enhanced plant biomass, six strains increased root volume, six strains increased root system activity in greenhouse test. Inoculation of mixtures of seven plant growth promoting rhizobacteria could be more effective in plant growth promotion and biocontrol of Fusarium wilt in watermelon.



Similar content being viewed by others
References
Yu JQ, Shou SY, Qian YR, Zhu ZJ, Hu WH (2000) Autotoxic potential in cucurbit crops. Plant Soil 223:147–151
Yao H, Jiao X, Wu F (2006) Effects of continuous cucumber cropping and alternative rotations under protected cultivation on soil microbial community diversity. Plant Soil 284:195–203
Zhou X, Yu G, Wu F (2011) Effects of intercropping cucumber with onion or garlic on soil enzyme activities, microbial communities and cucumber yield. Eur J Soil Biol 47(5):279–287
Zhang F, Li L (2003) Using competitive and facilitative interactions in intercropping systems enhance crop productivity and nutrient-use efficiency. Plant Soil 248:305–312
Ren L, Su S, Yang X, Xu Y, Huang Q, Shen Q (2008) Intercropping with aerobic rice suppressed Fusarium wilt in watermelon. Soil Biol Biochem 40(3):834–844
Xiao X, Cheng Z, Meng H, Khan MA, Li H (2012) Intercropping with garlic alleviated continuous cropping obstacle of cucumber in plastic tunnel. Acta Agric Scand Sect B Soil Plant Sci 62:696–705
Darch T, Giles CD, Blackwell MSA, George TS, Brown LK, Menezes-Blackburn D, Shand CA, Stutter MI, Lumsdon DG, Mezeli MM, Wendler R, Zhang H, Wearing C, Cooper P, Haygarth PM (2018) Inter- and intra-species intercropping of barley cultivars and legume species, as affected by soil phosphorus availability. Plant Soil 427:125–138
Li XG, Wang XX, Dai CC, Zhang TL, Xie XG, Ding CF, Wang HW (2014) Effects of intercropping with Atractylodes lancea and application of bio-organic fertiliser on soil invertebrates, disease control and peanut productivity in continuous peanut cropping field in subtropical China. Agrofor Syst 88:41–52
Boudreau MA (2013) Disease in intercropping systems. Annu Rev Phytopathol 51:499–519
Bhattacharyya PN, Jha DK (2012) Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol 28:1327–1350
Tabassum B, Khan A, Tariq M, Ramzan M, Khan MSI, Shahid N, Aaliya K (2017) Bottlenecks in commercialisation and future prospects of PGPR. Appl Soil Ecol 121:102–117
Grady EN, MacDonald J, Liu L, Richman A, Yuan ZC (2016) Current knowledge and perspectives of Paenibacillus: a review. Microb Cell Factories 15:203
Fan ZY, Miao CP, Qiao XG, Zheng YK, Chen HH, Chen YW, Xu LH, Zhao LX, Guan HL (2016) Diversity, distribution, and antagonistic activities of rhizobacteria of Panax notoginseng. J Ginseng Res 40:97–104
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160(1):47–56
Smibert RM, Krieg NR (1994) Phenotypic characterization. In: Gerhardt P, Murray RGE, Wood WA, Krieg NR (eds) Methods for general and molecular bacteriology. American Society of Microbiology, Washington DC, pp 607–654
Schippers B, Bakker AW, Bakker PAHM, Van Peer R (1990) Beneficial and deleterious effects of HCN-producing pseudomonads on rhizosphere interactions. Plant Soil 129(1):75–83
Cappuccino JC, Sherman N (1992) Microbiology: a laboratory manual, 3rd edn. Benjamin/Cummings Pub. Co, New York
Nautiyal CS (1999) An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett 170(1):265–270
Loper JE, Schroth MN (1986) Influence of bacterial sources on indole-3-acetic acid on root elongation of sugarbeet. Phytopathology 76:386–389
Comas LH, Eissenstat DM, Lakso AN (2000) Assessing root death and root system dynamics in a study of grape canopy pruning. New Phytol 147:171–178
Chauhan AK, Maheshwari DK, Kim K, Bajpai VK (2016) Termitarium-inhabiting Bacillus endophyticus TSH42 and Bacillus cereus TSH77 colonizing Curcuma longa L.: isolation, characterization, and evaluation of their biocontrol and plant-growth-promoting activities. Can J Microbiol 62:880–892
Etesami H, Alikhani HA (2016) Rhizosphere and endorhiza of oilseed rape (Brassica napus L.) plant harbor bacteria with multifaceted beneficial effects. Biol Control 94:11–24
Romero FM, Marina M, Pieckenstain FL (2016) Novel components of leaf bacterial communities of field-grown tomato plants and their potential for plant growth promotion and biocontrol of tomato diseases. Res Microbiol 167:222–233
Wan T, Zhao H, Wang W (2017) Effect of biocontrol agent Bacillus amyloliquefaciens SN16-1 and plant pathogen Fusarium oxysporum on tomato rhizosphere bacterial community composition. Biol Control 112:1–9
Rotolo C, De Miccolis Angelini RM, Dongiovanni C et al (2018) Use of biocontrol agents and botanicals in integrated management of Botrytis cinerea in table grape vineyards. Pest Manag Sci 74(3):715–725
Verma SK, White JF (2018) Indigenous endophytic seed bacteria promote seedling development and defend against fungal disease in browntop millet (Urochloa ramosa L.). J Appl Microbiol 124(3):764–778
Ling N, Xue C, Huang Q, Yang X, Xu Y, Shen Q (2010) Development of a mode of application of bioorganic fertilizer for improving the biocontrol efficacy to Fusarium wilt. BioControl 55:673–683
Montes C, Altimira F, Canchignia H, Castro Á, Sánchez E, Miccono M, Tapia E, Sequeida Á, Valdés J, Tapia P, González C, Prieto H (2016) A draft genome sequence of Pseudomonas veronii R4: a grapevine (Vitis vinifera L.) root- associated strain with high biocontrol potential. Stand Genomic Sci 11:76
Novinscak A, Gadkar VJ, Joly DL, Filion M (2016) Complete genome sequence of Pseudomonas brassicacearum LBUM300, a disease-suppressive bacterium with antagonistic activity toward fungal, oomycete, and bacterial plant pathogens. Genome Announc 4(1):e01623–e01615
Wu H, Yang X, Fan J et al (2008) Suppression of Fusarium wilt of watermelon by a bio-organic fertilizer containing combinations of antagonistic microorganisms. Biocontrol 54:287–295
Ling N, Zhang W, Tan S, Huang Q, Shen Q (2012) Effect of the nursery application of bioorganic fertilizer on spatial distribution of Fusarium oxysporum f. sp. niveum and its antagonistic bacterium in the rhizosphere of watermelon. Appl Soil Ecol 59:13–19
Zahid M, Abbasi MK, Hameed S, Rahim N (2015) Isolation and identification of indigenous plant growth promoting rhizobacteria from Himalayan region of Kashmir and their effect on improving growth and nutrient contents of maize (Zea mays L.). Front Microbiol 6:207
Ahemad M, Kibret M (2014) Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. Journal of King Saud University - Science 26:1–20
Patten CL, Glick BR (1996) Bacterial biosynthesis of indole-3-acetic acid. Can J Microbiol 42:207–220
Haas D, Défago G (2005) Biological control of soil-borne pathogens by Fluorescent Pseudomonas. Nat Rev Microbiol 3:307–319
Domenech J, Reddy MS, Kloepper JW, Ramos B, Gutierrez-Mañero J (2006) Combined application of the biological product LS213 with Bacillus, Pseudomonas or Chryseobacterium for growth promotion and biological control of soil-borne diseases in pepper and tomato. BioControl 51:245–258
Raupach GS, Kloepper JW (1998) Mixtures of plant growth-promoting rhizobacteria enhance biological control of multiple cucumber pathogens. Phytopathology 88:1158–1164
Jetiyanon K, Kloepper JW (2002) Mixtures of plant growth-promoting rhizobacteria for induction of systemic resistance against multiple plant diseases. Biol Control 24:285–291
Mazzola M (2004) Assessment and management of soil microbial community structure for disease suppression. Annu Rev Phytopathol 42:35–59
An M, Zhou X, Wu F, Ma Y, Yang P (2011) Rhizosphere soil microorganism populations and community structures of different watermelon cultivars with differing resistance to Fusarium oxysporum f. sp. Niveum. Can J Microbiol 57:355–365
Xiong W, Guo S, Jousset A, Zhao Q, Wu H, Li R, Kowalchuk GA, Shen Q (2017) Bio-fertilizer application induces soil suppressiveness against Fusarium wilt disease by reshaping the soil microbiome. Soil Biol Biochem 114:238–247
Acknowledgments
The authors are very grateful to Siqin Chao, Yuting Han, Yingxuan Wang, and Xiaoyue Zhang for their valuable assistance in laboratory and greenhouse experiment.
Funding
This research was supported by the Fundamental Research Funds for the Central Universities of China (Grant No. 2013PY092).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Luiz Roesch
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, W. Components of rhizospheric bacterial communities of barley and their potential for plant growth promotion and biocontrol of Fusarium wilt of watermelon. Braz J Microbiol 50, 749–757 (2019). https://doi.org/10.1007/s42770-019-00089-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-019-00089-z