Abstract
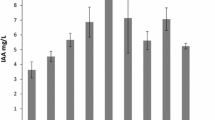
Plant growth-promoting rhizobacteria (PGPR) have the potential to make a significant contribution to the development of sustainable agricultural systems. Generally, PGPRs function in three different ways, summarized as the synthesis of certain compounds for plants, facilitating the uptake of certain nutrients from the soil and protecting plants from diseases. This study aims to isolate plant growth-promoting bacteria from different plant rhizospheres from Ankara province, to reveal their genetic diversity, and to determine their plant growth-promoting properties. The identification of the 69 isolates was made according to the 16S rDNA sequence results and ARDRA analyses were also performed using AluI, HeaIII, and MspI enzymes. Nitrogen fixation, phosphate dissolving, IAA (indole-3-acetic acid) and siderophore production capacities of the 69 bacterial strains including 12 different genera (30 Pseudomonas, 13 Arthrobacter, 7 Bacillus, 4 Phyllobacter, 4 Variovorax, 3 Olivibacter, 3 Enterobacter, 2 Paenarthrobacter, 1 Stenotrophomonas, 1 Flavobacterium, 1 Caulobacter, 1 Paenibacillus) were evaluated in in vitro conditions. Nitrogen fixation capacities of 55 isolates varied between 2.29 and 46.11 µg mL−1 according to micro-kjeldahl method. Among the strains studied, nifH gene was detected only in Paenibacillus polymyxa H8/2 strain. The highest Phosphorus dissolving and IAA production capacity (in tryptophan-added medium) of isolates were 186.52 µg mL−1, and 50.05 μg mL−1 respectively, and 31 of 69 isolates were able to produce siderophore. Regarding antifungal activities, results showed that 31 bacterial isolates had antagonistic activities against at least one of the tested pathogens. Nitrogen fixation and phosphate solubilizing potential of the promising bacterial strains were determined through two-independent pot experiments with wheat and it has been found that they have positive effects on the yield parameters of wheat.




Similar content being viewed by others
References
Cook RJ (2000) Advances in plant health management in the twentieth century. Annu Rev Phytopathol 38:95–116
Gray EJ, Smith DL (2005) Intracellular and extracellular PGPR: commonalities and distinctions in the plant-bacterium signaling processes. Soil Biol Biochem 37:395–412
Solanki MK, Wang Z, Wang FY, Li CN, Lan TJ, Singh RK (2017) Intercropping in sugarcane cultivation influenced the soil properties and enhanced the diversity of vital diazotrophic bacteria. Sugar Tech 19:136–147
Glick BR (1995) The enhancement of plant growth by free living bacteria. Can J Microbiol 41:109–114
Beringer JE, Hirsch PR (1984) Genetic engineering and nitrogen fixation. Biotechnol Genet Eng Rev 1:65–88
Rubio LM, Ludden PW (2002) The gene products of nif regulon. In: Leıgh GJ (ed) Nitrogen Fixation at the Millennium. Elsevier, The Netherlands, pp 101–130
Nannipieri P, Giagnoni L, Landi L, Renella G (2011) Role of phosphatase enzymes in soil. In: Bunemann E, Oberson A, Frossard E (eds) Phosphorus in action: Biological processes in soil phosphorus cycling. Springer, Heidelberg, pp 251–244
Zahir ZA, Arshad M, Frankenberger WT (2004) Plant growth promoting rhizobacteria: applications and perspectives in agriculture. Adv Agron 81:96–168
Spaepen S, Vanderleyden J (2011) Auxin and plant-microbe interactions. Cold Spring Harb Perspect Biol 3:4
Rajkumar M, Ae N, Prasad MNV, Freitas H (2010) Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol 28:142–149
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Schmidt W (1999) Mechanisms and regulation of reduction-based iron uptake in plants. New Phytol 141:1–26
Kandel SL, Firrincieli A, Joubert PM, Okubara PA, Leston ND, McGeorge KM, Mugnozza GS, Harfouche A, Kim SH, Doty SL (2017) An in vitro study of biocontrol and plant growth promotion potential of salicaceae endophytes. Front Microbiol 8:386
Upadhyay SK, Singh DP, Saikia R (2009) Genetic diversity of plant growth promoting rhizobacteria isolated from rhizospheric soil of wheat under saline condition. Curr Microbiol 59(5):489–496
Wilson K (2001) Preparation of genomic DNA from bacteria. Curr Protoc Mol Biol 2:2
Edwards U, Rogall T, Blocker H, Emde M, Böttger EC (1989) Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res 17:7843–7853
Kumar A (2016) Phosphate solubilizing bacteria in agriculture biotechnology: diversity, mechanism and their role in plant growth and crop yield. Int J Adv Res 4(4):116–124
Bremner JM (1965) Chemical and microbiological properties. In: Black CA (ed) Methods of soil analysis. Wisconsin, USA
Poly F, Monrozier LJ, Bally R (2001) Improvement in the RFLP procedure for studying the diversity of nifH genes in communities of nitrogen fixers in soil. Res Microbiol 152:95–103
Mehta S, Nautiyal CS (2001) An efficienet method for qualitative screeening of phosphate solubilizing bacteria. Curr Microbial 43(1):51–60
Perez E, Sulbaran M, Ball MM, Yarzabal LA (2007) Isolation and characterization of mineral phosphate-solubilizing bacteria naturally colonizing a limonitic crust in the south-eastern Venezuelan region. Soil Biol Biochem 39:2905–2914
Ahmad F, Ahmad I, Khan MS (2005) Indole acetic acid production by the ındigenous ısolates of azotobacter and fluorescent pseudomonas in the presence and absence of tryptophan. Turk J Biol 29:29–34
Singh RP, Jha PN (2015) Molecular identification and characterization of rhizospheric bacteria for plant growth promoting ability. Int J Curr Biotechnol 3:12–18
Jetiyanon K, Kloepper JW (2002) Mixtures of plant growth promoting rhizobacteria for induction of systemic resistance against multiple plant diseases. Biol Control 24:285–291
Jin F, Ding Y, Ding W, Reddy MS, Dilantha Fernando WG, Du B (2011) Genetic diversity and phylogeny of antagonistic bacteria against phytophthora nicotianae isolated from tobacco rhizosphere. Int J Mol Sci 12:3055–3071
Ahmad F, Ahmad I, Khan MS (2008) Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol Res 163:173–181
Souza R, Beneduzi A, Ambrosini A, Costa PB, Meyer J, Vargas LK, Schoenfeld R, Passaglia LMP (2013) The effect of plant growth-promoting rhizobacteria on the growth of rice (Oryza sativa L.) cropped in southern Brazilian fields. Plant Soil 366:585–603
Tchakounte GVT, Berger B, Patz S, Fankem H, Ruppel S (2018) Community structure and plant growth-promoting potential of cultivable bacteria isolated from Cameroon soil. Microbiol Res 214:47–59
Young JPW (1992) Phylogenetic classification of nitrogen fixing organisms in Biological Nitrogen Fixation. In: Burris RH, Evans HJ (eds) Stacey G. Springer, New York, pp 43–86
Ding Y, Wang J, Liu Y, Chen S (2005) Isolation and identification of nitrogen-fixing bacilli from plant rhizospheres in Beijing region. J Appl Microbiol 99:1271–1281
Gosal SK, Saroa GS, Vikal Y, Cameotra SS, Pathania N, Bhanot A (2011) Isolation and molecular characterisation of diazotrophic growth-promoting bacteria from wheat rhizospheric soils of Punjab. Soil Research 49:725–732
Wang LY, Li J, Li QX, Chen SF (2014) Paenibacillus beijingensis sp. nov., a nitrogen-fixing species isolated from wheat rhizosphere soil. Antonie Van Leeuwenhoek 104:675–683
Liu X, Li Q, Li Y, Guan G, Chen S (2019) Paenibacillus strains with nitrogen fixation and multiple beneficial properties for promoting plant growth. PeerJ 7:7445
Shen H, He X, Liu Y, Chen Y, Tang J, Guo T (2016) A complex ınoculant of N2-fixing, P- and K-solubilizing bacteria from a purple soil ımproves the growth of kiwi fruit (Actinidia chinensis) plantlets. Front Microbiol 7:841
Li HB, Singh RK, Singh P, Song Q, Xing Y, Yang L, Li Y (2017) Genetic diversity of nitrogen-fixing and plant growth promoting Pseudomonas species ısolated from sugarcane rhizosphere. Front Microbiol 8:1268
Nautiyal CS (1999) An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett 170:265–270
Malik DK, Sindhu SS (2011) Production of indole acetic acid by Pseudomonas sp effect of coinoculation with Mesorhizobium sp Cicer on nodulation and plant growth of chickpea (Cicer arietinum). Physiol Mol Biol Plants 17(1):25–32
Yadav AN, Sachan SG, Verma P, Tyagi SP, Kaushik R, Saxena AK (2015) Culturable diversity and functional annotation of psychrotrophic bacteria from cold desert of Leh Ladakh (India). World J Microbiol Biotechnol 31(1):95–108
Ozdal M, Ozdal OG, Sezen A, Algur OF, Kurbanoglu EB (2017) Continuous production of indole-3-acetic acid by immobilized cells of Arthrobacter agilis 3. Biotech 7:23
Beneduzi A, Peres D, Vargas LK, Bodanese-Zanettini MH, Passaglia LMP (2008) Evaluation of genetic diversity and plant growth promoting activities of nitrogen-fixing bacilli isolated from rice fields in South Brazil. Appl Soil Ecol 39:311–320
Haas D, Défago G (2005) Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat Rev Microbiol 3:307–319
Camele I, Elshafie HS, Caputo L, Sakr SH, De Feo V (2019) Bacillus mojavensis: biofilm formation and biochemical investigation of its bioactive metabolites. J Biol Res 92:8296
Weselowski B, Nathoo N, Eastman AW, Macdonald J, Yuan ZC (2016) Isolation, identification and characterization of Paenibacillus polymyxa CR1 with potentials for biopesticide, biofertilization, biomass degradation and biofuel production. Bmc Microbiol 16:244
DengY LuZ, Lu F, Zhang C, Wang Y, Zhao H, Bi X (2011) Identification of LI-F type antibiotics and di-n-butyl phthalate produced by Paenibacillus polymyxa. J Microbiol Meth 85:175–182
Kotan R, Sahin F, Demirci E, Eken C (2011) Biological control of the potato dry rot caused by Fusarium species using PGPR strains. Biol Control 59(3):390
Pastor NA, Reynoso MM, Tonelli ML, Masciarelli O, Rosas SB, Rovera M (2010) Potentıal bıologıcal control Pseudomonas sp pcı2 agaınst dampıng-off of tomato caused by Sclerotıum rolfsıı. Journal of Plant Pathology 92(3):737–745
Kai M, Effmert U, Berg G (2007) Volatiles of bacterial antagonists inhibit mycelial growth of the plant pathogen Rhizoctonia solani. Arch Microbiol 187:351
Chernın L, Ismaılov Z, Haran S, Chet I (1995) Chitinolytic Enterobacter agglomerans antagonistic to fungal plant pathogens. App Environ Mıcrobiol 61(5):1720–1726
Çakmakcı R, Dönmez F, Erdoğan Ü (2007) The effect of plant growth promoting rhizobacteria on barley seedling growth, nutrient uptake, some soil properties, and bacterial counts. Turk J Agric For 31:189–199
Majeed A, Abbasi MK, Hameed S, Imran A, Rahim N (2015) Isolation and characterization of plant growth-promoting rhizobacteria from wheat rhizosphere and their effect on plant growth promotion. Front Microbiol 6:198
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Özdoğan, D.K., Akçelik, N. & Akçelik, M. Genetic Diversity and Characterization of Plant Growth-Promoting Effects of Bacteria Isolated from Rhizospheric Soils. Curr Microbiol 79, 132 (2022). https://doi.org/10.1007/s00284-022-02827-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-022-02827-3