Abstract
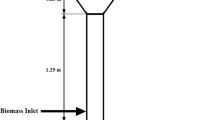
The unsteady characteristics of bubble dynamics inside the air reactor within the first 0–40 s of reforming has always been studied for defining the design criteria of the reactor. In the study, a temporal aspect of the hydrodynamics and chemical kinetics in the reactor of a chemical looping reforming system in form of volume fraction contours of solid species and molar fraction of H2O has been numerically simulated by considering manganese (Mn) and iron (Fe) based metal oxides as oxygen carriers. The Finite Volume Method based approach has been employed to simulate the steam reactor model by encompassing it as a fluidized bed reactor. The granular flow under kinetic theory has been employed using a multiphase Eulerian-based approach for both gas and solid phases in the form of a shrinking core model. An influence of various operating parameters such as particle size of the oxygen carriers, steam inlet velocity, and temperature of the steam reactor on an overall conversion rate of iron-based oxide (FeO) and manganese-based oxide (MnO). The maximum steam conversion rate for FeO and MnO was observed at 32% and 34% at 0.6 m/s steam velocity, 48% and 60% at a maximum temperature of 1273 K, and 47% and 64% at a particle size of 100 μm, respectively.
Similar content being viewed by others
Abbreviations
- a :
-
Acceleration (m·s−2)
- a x :
-
Volume fraction for phase x (—)
- D :
-
Mass diffusion flux (kg·m−2·s−1)
- F :
-
Chemical reaction formation rate (s−1)
- g :
-
Gravity (m·s−2)
- G :
-
Interaction force between phases (N)
- ΔH f :
-
Heat released for fuel (kJ·mol−1)
- h :
-
Enthalpy (kJ·kg−1·K−1)
- i :
-
Spices
- k :
-
Thermal conductivity (W·m−1·K−1)
- ṁ xy :
-
Mass transfer from the xth phase to the yth phase (kg·s−1)
- P :
-
Pressure (N·m−2)
- Q :
-
Heat transfer (kJ)
- R :
-
Heterogeneous reaction rate (—)
- T a :
-
Source term in continuity equation
- \({\overrightarrow v _x}\) :
-
Velocity of the phase x (m·s−1)
- \({\overrightarrow v _{yx}},{\overrightarrow v _{xy}}\) :
-
Interphase velocity (m·s−1)
- \(\overrightarrow G \) :
-
Force (N)
- \({\overrightarrow G _{yx}}\) :
-
Interactive force between phases x and y (N)
- \({\overrightarrow G _x}\) :
-
External body force for phase x (N)
- \({\overrightarrow G _{\rm{L}}}\) :
-
Lift force (N)
- \({\overrightarrow G _{\rm{v}}}\) :
-
Virtual mass force (N)
- v :
-
Velocity (m·s−1)
- x :
-
Phase
- y :
-
Phase
- z :
-
Phase
- V:
-
Virtual
- L:
-
Lift
- ρ :
-
Density (kg·m−3)
- μ :
-
Dynamic viscosity (N·s·m−2)
- τ :
-
Stress-strain tensor
- Σ :
-
Algebraic sum
- ▽:
-
Derivative operator
- ∂ :
-
Partial differential operator
- θ :
-
Temperature gradient
- AR:
-
Air reactor
- CLR:
-
Chemical looping reforming
- CLSR:
-
Chemical looping steam reforming
- CSP:
-
Concentrated solar power
- FR:
-
Fuel reactor
- FVA:
-
Finite volume approach
- RVSPA:
-
Rapid vacuum pressure swing absorption
- SMR:
-
Steam methane reforming
- SR:
-
Steam reactor
- TGA:
-
Thermal gravimetric analysis
References
Adánez, J., de Diego, L. F., García-Labiano, F., Gayán, P., Abad, A., Palacios, J. M. 2004. Selection of oxygen carriers for chemical-looping combustion. Energ Fuel, 18: 371–377.
Antzara, A., Heracleous, E., Bukur, D. B., Lemonidou, A. A. 2015. Thermodynamic analysis of hydrogen production via chemical looping steam methane reforming coupled with in situ CO2 capture. Int J Greenh Gas Con, 32: 115–128.
Bhavsar, S., Tackett, B., Veser, G. 2014. Evaluation of iron- and manganese-based mono- and mixed-metallic oxygen carriers for chemical looping combustion. Fuel, 136: 268–279.
Bhosale, R., AlMomani, F., Takalkar, G. 2020. Thermodynamic analysis of solar-driven chemical looping steam methane reforming over Cr2O3/Cr redox pair. Int J Hydrog Energ, 45: 10370–10380.
Bischi, A., Langørgen, Ø., Morin, J. X., Bakken, J., Ghorbaniyan, M., Bysveen, M., Bolland, O. 2012. Hydrodynamic viability of chemical looping processes by means of cold flow model investigation. Appl Energ, 97: 201–216.
Capocelli, M., Luberti, M., Inno, S., D’Antonio, F., Di Natale, F., Lancia, A. 2019. Post-combustion CO2 capture by RVPSA in a large-scale steam reforming plant. J CO2 Util, 32: 53–65.
Chiesa, P., Lozza, G., Malandrino, A., Romano, M., Piccolo, V. 2008. Three-reactors chemical looping process for hydrogen production. Int J Hydrog Energ, 33: 2233–2245.
Christopher, K., Dimitrios, R. 2012. A review on exergy comparison of hydrogen production methods from renewable energy sources. Energ Environ Sci, 5: 6640.
Clift, R., Grace, J. R. 1985. Continuous bubbling and slugging. Fluidization, 73–132.
Coelho, B., Oliveira, A. C., Mendes, A. 2010. Concentrated solar power for renewable electricity and hydrogen production from water—a review. Energ Environ Sci, 3: 1398.
Deng, Z., Xiao, R., Jin, B., Song, Q. 2009. Numerical simulation of chemical looping combustion process with CaSO4 oxygen carrier. Int J Greenh Gas Con, 3: 368–375.
Dindi, A., Quang, D. V., Vega, L. F., Nashef, E., Abu-Zahra, M. R. M. 2019. Applications of fly ash for CO2 capture, utilization, and storage. J CO2 Util, 29: 82–102.
Fan, L. S., Zeng, L., Wang, W., Luo, S. 2012. Chemical looping processes for CO2 capture and carbonaceous fuel conversion—prospect and opportunity. Energ Environ Sci, 5: 7254–7280.
Gelderbloom, S. J., Gidaspow, D., Lyczkowski, R. W. 2003. CFD simulations of bubbling/collapsing fluidized beds for three Geldart groups. AIChE J, 49: 844–858.
Gidaspow, D. 1994. Multiphase Flow and Fluidization: Continuum and Kinetic Theory Description. Academic Press.
Gunn, D. J. 1978. Transfer of heat or mass to particles in fixed and fluidised beds. Int J Heat Mass Tran, 21: 467–476.
Harichandan, A. B., Shamim, T. 2014. CFD analysis of bubble hydrodynamics in a fuel reactor for a hydrogen-fueled chemical looping combustion system. Energ Convers Manage, 86: 1010–1022.
He, L., Parra, J. M. S., Blekkan, E. A., Chen, D. 2010. Towards efficient hydrogen production from glycerol by sorption enhanced steam reforming. Energ Environ Sci, 3: 1046.
Hossain, M. M., de Lasa, H. I. 2008. Chemical-looping combustion (CLC) for inherent CO2 separations—a review. Chem Eng Sci, 63: 4433–4451.
Huang, Z., He, F., Zhao, K., Feng, Y., Zheng, A., Chang, S., Zhao, Z., Li, H. 2014. Natural iron ore as an oxygen carrier for biomass chemical looping gasification in a fluidized bed reactor. J Therm Anal Calorim, 116: 1315–1324.
Isarapakdeetham, S., Kim-Lohsoontorn, P., Wongsakulphasatch, S., Kiatkittipong, W., Laosiripojana, N., Gong, J., Assabumrungrat, S. 2020. Hydrogen production via chemical looping steam reforming of ethanol by Ni-based oxygen carriers supported on CeO2 and La2O3 promoted Al2O3. Int J Hydrog Energ, 45: 1477–1491.
Johansson, E., Mattisson, T., Lyngfelt, A., Thunman, H. 2006. A 300 W laboratory reactor system for chemical-looping combustion with particle circulation. Fuel, 85: 1428–1438.
Kang, K. S., Kim, C. H., Bae, K. K., Cho, W. C., Kim, S. H., Park, C. S. 2010. Oxygen-carrier selection and thermal analysis of the chemical-looping process for hydrogen production. Int J Hydrog Energ, 35: 12246–12254.
Khan, M. N., Shamim, T. 2017. Thermodynamic screening of suitable oxygen carriers for a three reactor chemical looping reforming system. Int J Hydrog Energ, 42: 15745–15760.
Khan, M. N., Shamim, T. 2019. Techno-economic assessment of a chemical looping reforming combined cycle plant with iron and tungsten based oxygen carriers. Int J Hydrog Energ, 44: 11525–11534.
Kothari, R., Buddhi, D., Sawhney, R. L. 2008. Comparison of environmental and economic aspects of various hydrogen production methods. Renew Sust Energ Rev, 12: 553–563.
Larring, Y., Pishahang, M., Sunding, M. F., Tsakalakis, K. 2015. Fe-Mn based minerals with remarkable redox characteristics for chemical looping combustion. Fuel, 159: 169–178.
Lisbona, P., Martínez, A., Romeo, L. M. 2013. Hydrodynamical model and experimental results of a calcium looping cycle for CO2 capture. Appl Energ, 101: 317–322.
Lu, H., Gidaspow, D., Bouillard, J., Liu, W. 2003. Hydrodynamic simulation of gas-solid flow in a riser using kinetic theory of granular flow. Chem Eng J, 95: 1–13.
Lun, C. K. K., Savage, S. B., Jeffrey, D. J., Chepurniy, N. 1984. Kinetic theories for granular flow: Inelastic particles in Couette flow and slightly inelastic particles in a general flowfield. J Fluid Mech 140: 223–256.
Mattisson, T., Järdnäs, A., Lyngfelt, A. 2003. Reactivity of some metal oxides supported on alumina with alternating methane and oxygen application for chemical-looping combustion. Energ Fuel, 17: 643–651.
Mattisson, T., Lyngfelt, A., Cho, P. 2001. The use of iron oxide as an oxygen carrier in chemical-looping combustion of methane with inherent separation of CO2. Fuel, 80: 1953–1962.
Medeiros, R., Melo, V., Melo, D. M. A., Macedo, H. P., Moure, G. T., Adánez-Rubio, I., Melo, M., Adánez, J. 2020. Double perovskite (La2−xCa-Bax)NiO4 oxygen carriers for chemical looping reforming applications. Int J Hydrog Energ, 45: 1681–1696.
Momirlan, M., Veziroglu, T. 1999. Recent directions of world hydrogen production. Renew Sust Energ Rev, 3: 219–231.
Mosavati, B., Mosavati, M., Kowsary, F. 2013. Solution of radiative inverse boundary design problem in a combined radiating-free convecting furnace. Int Commun Heat Mass Trans, 45: 130–136.
Mosavati, B., Mosavati, M., Kowsary, F. 2016. Inverse boundary design solution in a combined radiating-free convecting furnace filled with participating medium containing specularly reflecting walls. Int Commun Heat Mass Trans, 76: 69–76.
Nazir, S. M., Morgado, J. F., Bolland, O., Quinta-Ferreira, R., Amini, S. 2018. Techno-economic assessment of chemical looping reforming of natural gas for hydrogen production and power generation with integrated CO2 capture. Int J Greenh Gas Con, 78: 7–20.
Ranganathan, R. V., Jony, B., Fondriest, S. M., Liu, Z., Wang, R., Uddi, M. 2019. Plasma-catalysis chemical looping CH4 reforming with water splitting using ceria supported Ni based La-perovskite nano-catalyst. J CO2 Util, 32: 11–20.
Richter, H. J., Knoche, K. F. 1983. Reversibility of combustion processes. ACS Sym Ser, 71–85.
Rydén, M., Leion, H., Mattisson, T., Lyngfelt, A. 2014. Combined oxides as oxygen-carrier material for chemical-looping with oxygen uncoupling. Appl Energ, 113: 1924–1932.
Schaeffer, D. G. 1987. Instability in the evolution equations describing incompressible granular flow. J Differ Equations 66: 19–50.
Shimura, K., Yoshida, H. 2011. Heterogeneous photocatalytic hydrogen production from water and biomass derivatives. Energ Environ Sci, 4: 2467.
Siriwardane, R., Tian, H., Richards, G., Simonyi, T., Poston, J. 2009. Chemical-looping combustion of coal with metal oxide oxygen carriers. Energ Fuel, 23: 3885–3892.
Siriwardane, R., Tian, H., Simonyi, T., Poston, J. 2013. Synergetic effects of mixed copper-iron oxides oxygen carriers in chemical looping combustion. Fuel, 108: 319–333.
Sorgenfrei, M., Tsatsaronis, G. 2014. Design and evaluation of an IGCC power plant using iron-based syngas chemical-looping (SCL) combustion. Appl Energ, 113: 1958–1964.
Stenberg, V., Rydén, M., Mattisson, T., Lyngfelt, A. 2018. Exploring novel hydrogen production processes by integration of steam methane reforming with chemical-looping combustion (CLC-SMR) and oxygen carrier aided combustion (OCAC-SMR). Int J Greenh Gas Con, 74: 28–39.
Syamlal, M., O’Brien, T. J. 1989. Computer simulation of bubbles in a fluidized bed. AIChE Sym Ser, 85: 22–31.
Syamlal, M., Rogers, W., O’Brien, T. J. 1993. MFIX documentation theory guide (No. DOE/METC-94/1004). USDOE Morgantown Energy Technology Center, WV (United States).
Symonds, R. T., Sun, Z., Ashrafi, O., Navarri, P., Lu, D. Y., Hughes, R. W. 2019. Ilmenite ore as an oxygen carrier for pressurized chemical looping reforming: Characterization and process simulation. Int J Greenh Gas Con, 81: 240–258.
Thursfield, A., Murugan, A., Franca, R., Metcalfe, I. S. 2012. Chemical looping and oxygen permeable ceramic membranes for hydrogen production—a review. Energ Environ Sci, 5: 7421.
Zafar, Q., Mattisson, T., Gevert, B. 2006. Redox investigation of some oxides of transition-state metals Ni, Cu, Fe, and Mn supported on SiO2 and MgAl2O4. Energ Fuel, 20: 34–44.
Zhang, H., Xiao, R., Song, M., Shen, D., Liu, J. 2014. Hydrogen production from bio-oil by chemical looping reforming. J Therm Anal Calorim, 115: 1921–1927.
Zhang, X., Jin, H. 2013. Thermodynamic analysis of chemical-looping hydrogen generation. Appl Energ, 112: 800–807.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors have no competing interests to declare that are relevant to the content of this article.
Rights and permissions
About this article
Cite this article
Chavda, A., Mehta, P. & Harichandan, A. Numerical analysis of multiphase flow in chemical looping reforming process for hydrogen production and CO2 capture. Exp. Comput. Multiph. Flow 4, 360–376 (2022). https://doi.org/10.1007/s42757-021-0105-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42757-021-0105-7