Abstract
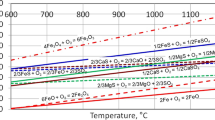
Kinetics of hot metal desulfurization were studied using CaO–SiO2–Al2O3–Na2O–TiO2 slag in the range of 1400–1500 °C on a laboratory scale. The results of kinetic experiments indicate that the desulfurization rate increases as the temperature, Al2O3 content, Na2O content, and TiO2 content increase and basicity increases from 1.01 to 1.75, but decreases when basicity increases from 1.75 to 2.02. The melting effect of slag is promoted as the temperature, Na2O content, and TiO2 content increase and Al2O3 content increases from 12.13 to 17.17 mass%, but worsened as basicity increases and Al2O3 content increases from 17.17 to 22.27 mass%. A kinetic model of hot metal desulfurization has been developed to calculate the mass transfer coefficient and the mass transfer resistance of sulfur in slag. The mass transfer coefficient of sulfur increases as the temperature, Al2O3 content, Na2O content, and TiO2 content increase and basicity decreases. Total mass transfer coefficients of sulfur were in the range of (5.02–23.78) × 10−7 m s−1. The activation energy was estimated to be 464.06 kJ mol−1 at the temperature from 1400 to 1450 °C and 176.35 kJ mol−1 at the temperature from 1450 to 1500 °C. The sulfur distribution at the slag–metal interface was observed using a mineral liberation analyzer. The result shows that the mass transfer of sulfur in slag is the controlling step at high temperature during the desulfurization process.








Similar content being viewed by others
References
J.J. Pak, R.J. Fruehan, Metall. Trans. B 22 (1991) 39–46.
Y.L. Zhang, F.S. Li, R.M. Wang, D.D. Tian, Steel Res. Int. 88 (2017) 1600140.
J.H. Park, D.J. Min, H.S. Song, Metall. Mater. Trans. B 35 (2004) 269–275.
J.F. Xu, L.J. Su, D. Chen, J.Y. Zhang, Y. Chen, J. Iron Steel Res. Int. 22 (2015) 1091–1097.
X.L. Tang, Z.T. Zhang, M. Guo, M. Zhang, X.D. Wang, J. Iron Steel Res. Int. 18 (2011) No. 2, 1–17.
K. Yajima, H. Matsuuran, F. Tsukihashi, ISIJ Int. 50 (2010) 191–194.
H. Park, J.Y. Park, G.H. Kim, I. Sohn, Steel Res. Int. 83 (2012) 150–156.
R.Z. Xu, J.L. Zhang, H.S. Zhang, C.J. Liu, X.Y. Fan, Z.Y. Wang, Iron and Steel 52 (2017) No. 9, 104–109.
I. Sohn, W. Wang, H. Matsuura, F. Tsukihashi, D.J. Min, ISIJ Int. 52 (2012) 158–160.
L.L. Yang, H.M. Wang, X. Zhu, G.R. Li, J. Iron Steel Res. Int. 21 (2014) 745–748.
J.Y. Choi, D.J. Kim, H.G. Lee, ISIJ Int. 41 (2001) 216–224.
Z.F. Tong, J.L. Qiao, X.Y. Jiang, ISIJ Int. 57 (2017) 245–253.
Z.S. Ren, X.J. Hu, K.C. Chou, J. Iron Steel Res. Int. 20 (2013) No. 9, 21–25.
X. Tang, C.S. Xu, ISIJ Int. 35 (1995) 367–371.
J.J. Pak, K. Ito, F.J. Fruehan, ISIJ Int. 29 (1989) 318–323.
X.H. Huang, Iron and steel metallurgical principles, Metallurgical Industry Press, Beijing, 2013.
J.F. Xu, F.X. Huang, X.H. Wang, J. Iron Steel Res. Int. 23 (2016) 784–791.
Q.Y. Han, Kinetics of metallurgical process, Metallurgical Industry Press, Beijing, 1983.
J.K. Jung, J.J. Pak, J. Korean Inst. Met. Mater. 38 (2000) 585–590.
S. Seetharaman, Fundamentals of metallurgy, Woodhead Publishing Limited, Cambridge, 2005.
B. Deo, R. Boom, Fundamentals of steelmaking metallurgy, Prentice Hall, New York, 1993.
Acknowledgements
The authors would like to acknowledge the National Key R&D Program of China (No. 2017YFC0210301) and the National Natural Science Foundation of China (No. 51474021) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Kh., Zhang, Yl. & Wu, T. Kinetics of hot metal desulfurization using CaO–SiO2–Al2O3–Na2O–TiO2 slag. J. Iron Steel Res. Int. 26, 1041–1051 (2019). https://doi.org/10.1007/s42243-018-0171-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42243-018-0171-7