Abstract
The influence of Cr or Ti additions to Cu40Mn25Al20Fe5Ni5 high entropy alloy (HEA) on the microstructure and corrosion behavior in different concentrations of HNO3 solution were investigated. The microstructures of the Cu-rich HEAs are characterized and analyzed. Microstructural analysis of the HEAs indicates the formation of a dendritic structure. Also, the phases are identified by using X-ray diffraction (XRD). The corrosion behavior of the investigated HEAs in the HNO3 solution was studied. The corrosion rate (CR) for Cu40Mn25Al20Fe5Ni5Ti5 HEA (Ti5 HEA) has a lower value than Cu40Mn25Al20Fe5Ni5Cr5 HEA (Cr5 HEA). The morphology of the corroded HEAs was investigated by using SEM images, EDX analysis, and mapping for elemental distribution. Cr addition led to the formation of a Cr2O3 protective film on the dendrites. However, the Ti5 HEA has a good surface morphology with a homogenous distribution than Cr5 HEA which is associated with a decrease in the corrosion rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
High entropy alloys (HEAs) have been an interesting field in the last two decades.1,2,3 HEAs consist of five or more elements which make them different from the traditional multi-components in all properties and used sometimes as reinforced materials.4,5,6 The majority of studies on HEAs focused on Fe-based HEAs but there are few investigations on Cu-based HEAs.2 Therefore, this research discusses novel non-equiatomic Cu40Mn25Al20Fe5Ni5Cr5 HEA (Cr5 HEA) and Cu40Mn25Al20Fe5Ni5Ti5 HEA (Ti5 HEA) with a comparison of the Cr/Ti additions effect on the corrosion resistance. A single-phase or multiphase can be designed to obtain the HEAs microstructure according to the required properties for any application.7,8,9,10 CoCrCuFeNi, CoMoFeNiCu, Cu40Mn25Al20Fe5Co5Ni5 HEAs are composed of FCC solid solution and their microstructures are identified as dendrite and interdendritic structures.11 HEAs improve their corrosion resistance in H2SO4, HNO3, and HCl acids due to the adding elements such as Cr, Ni, Mo, and/or Cu.12,13,14 Ni addition with other metals improves the mechanical, oxidation, and corrosion resistance at elevated temperatures as in Ni-base superalloys.15 As cast Cu45Mn25Al15Fe5Cr5Ni5 HEA has good corrosion behavior in 3% NaCl solution by avoiding of intermetallic formation of Al-Ni compounds.16 Minimizing segregation enhances the corrosion behavior such as adding Cr.16 The Ti addition contains BCC structure on AlCoCrFeNiTix which has good mechanical properties.17 The addition of Cu to FeCoNiCrCux HEA increases the localized corrosion because of Cu-rich interdendritic and Cu-depleted dendrite formation.18 The corrosion behavior of Al0.8CrFeCoNiCux HEA coating is pitting and intergranular corrosion.19 The Ti is an active metal that tends to contain intermetallic compounds.20,21,22,23
To prevent galvanic corrosion in the Cu-Fe-based dual-phase immiscible MEA, one approach is to increase the corrosion potential of the Fe-rich phase by adding Cr, which has been widely used in steels to improve corrosion resistance by forming a Cr passivation layer.24,25,26 Because Cr is immiscible with Cu,27 Cr should dissolve selectively into the Fe-rich phase in the Cu-Fe-based immiscible MEA. This allows the corrosion potentials of the Cu- and Fe-rich phases to be balanced. The formation of Cr-oxide layers on the Fe-rich phase initially converts the less noble phase from Fe-rich to Cu-rich. As a consequence, during the initial corrosion stage, fast Cu corrosion and the formation of the Cu-oxide layer take place. Meanwhile, an Al-Fe-Mn-rich passivation layer protects the Cu-rich phase's surface. Due to the outward diffusion of Cr through the existing Cr-oxide layer, the Cr-oxide layer forms on top of the inner Cu-oxide layer. The Cr-oxide layer formation changes the corrosion behavior of the alloy from active to passive.28
This work aims to develop two different non-equiatomic Cu-rich high entropy alloys and study their characterizations. Cu40Mn25Al20Fe5Ni5Cr5 HEA (Cr5 HEA) and Cu40Mn25Al20Fe5Ni5Ti5 HEA (Ti5 HEA) corrosion behavior in (0.5, 1.0, 5, and 10%) HNO3 solution was investigated. Studying the effect of obtained microstructures from casting on the corrosion behavior by weight-loss (WL) and potentiodynamic polarization (PP) measurements. This investigation was confirmed by using SEM, EDX analysis, and mapping for elemental distribution.
Experimental Work
Materials
Cu40Mn25Al20Fe5Ni5Cr5 HEA (Cr5 HEA) and Cu40Mn25Al20Fe5Ni5Ti5 HEA (Ti5 HEA) were developed via the casting route. The size of each heat was designed to be 200gm, and pure elements of Cu, Al, Ni, Mn, Fe, Ti, and Cr were used as raw materials for the casting process of the two HEAs. The ingots were prepared via arc melting technique under vacuum, and the ingots were melted and solidified in water-cooled copper mold. Each heat was remelted at least three times to ensure homogeneity. After finishing the casting process, the pressure inside the melting chamber is equalized by the atmospheric pressure, then the furnace is opened to extract the samples. HEAs ingots were cut via a wire cutting machine. The chemical composition of the casted ingot was analyzed. The chemical composition was determined via XRF mobile analyzer. The performance required for quick alloy grade identification and precise chemistry of a wide range of materials is delivered by the X-MET8000 range of handheld X-ray fluorescence (HHXRF) analyzers (solid and powder metals, polymers, wood, solutions, soil, ores, minerals, etc). Optical microscopy (OM), scanning electron microscopy (SEM), and X-ray diffraction (XRD) were used to characterize the microstructure and phase identification. The chemical compositions of the two casted HEAs are shown in Table 1.
Electrochemical Corrosion Characterization
The corrosion resistance of the HEAs was evaluated by chemical immersion test, and electrochemical techniques to assess the impact of the addition of Cr or Ti on the corrosion behavior of Cu40Mn25Al20Fe5Ni5 HEA. In a glass vessel, 20 ml of 0.5, 1, 5, and 10% HNO3 were used to measure the weight loss (WL) of the HEAs.
The WL was immersed for 120 h at 20 ± 1°C. HEAs were ground up to a 1000-grit emery paper then polished by alumina paste, degreased in acetone, washed in doubly distilled water, dried, and then weighed on an analytical scale. The weighted HEAs are dispersed in 20 mL of HNO3 solutions of various concentrations. Following immersion, the samples were taken out, cleaned with acetone and distilled water, dried, and weighed. The test was repeated several times, with the average value of WL being recorded. After that, the average corrosion rate (CR) can be calculated.29
where K, T, A, ∆W, and D are a constant (8.76 × 104), time in h, section area (cm2), loss of sample weight (grams), and density (g/cm3), respectively.
With a scan rate of 0.5 mV/s, the PP curves were used to obtain the corrosion potential and corrosion current density in the region of − 0.8 to 0.8 V. The cell consisted of a working electrode made of test specimens, an auxiliary electrode made of platinum wire, and a reference electrode made of SCE (Hg/Hg2Cl2-Sat. KCl) coupled to a typical electrolytic cell with a capacity of 25 ml. It is possible to calculate the CR per year of Cr5 HEA and Ti5 HEA.1 From the analysis of PP curves, characteristic electrochemical characteristics such as corrosion potential (Ecorr) and corrosion current density (icorr) can be derived. Faraday's law can be used to calculate the rate of corrosion (CR) Eqn. 2:
where z is the ionic charge, M is the metal atomic, d is the density g/cm3, and Icorr is the current density of corrosion expressed in a unit of mA/cm2. In the presence of different concentrations of HNO3, the intersection of the linear cathodic and anodic branches of Tafel plots was used to calculate the values of current and potential of corrosion (Icorr and Ecorr).
SEM and EDX analysis were used to analyze the surface morphology of the corroded HEAs, as well as mapping for elemental distribution.
Results and Discussion
Microstructure and Chemical Analysis
The microstructures of the two examined HEAs are demonstrated in Figure 1, which indicate the founding of two phases. As seen in Figure 1, the Cr5 HEA has a dendritic structure. However, the microstructure of the Ti5 HEA consists of a matrix with dispersed cuboidal particles. Moreover, the micrographs reveal the existence of flower-like precipitates. Surprisingly, the EDX analysis of Cr5 HEA reveals no remarkable difference in the chemical analysis of both the dendritic and inter-dendritic zones. On the other hand, the chemical analysis of Ti5 HEA demonstrates three different zones. Firstly, the copper-rich matrix, which depleted in Fe, Ti, and Ni. This result can be attributed to the fact that the positive enthalpy of mixing between Cu-Fe and Cu-Ti pairs. Therefore, the segregation phenomenon is expected to be promoted, resulting in the existence of both copper-rich and lean phases. Figure 2 and Table 2 represent the EDX analysis of the investigated alloys. Moreover, the chemical analysis of spot 4 suggests that the formed precipitates might be C14 laves phase Ti (Fe1−x Mnx)2. Finally, the chemical analysis of the cuboidal particles reveals a high Al content compared to the other two phases. Nonetheless, this chemical analysis suggests that these particles could be solid solutions, not intermetallic ones. The elemental distribution of the Cr5 HEA is presented in Figure 3. Figure 4 illustrates the EDX results for Ti5 HEA, whereas Figure 5 provides the elemental distribution of Ti5 HEA.
The elemental distribution of the Cr5 HEA is presented in Figure 3. The figure shows the complete distribution of all elements all over the Cr5 HEA and appears to be a dendritic structure. However, the elemental distribution of elements all over Ti5 HEA shows the existence of Ti cuboids as given in Figure 5.
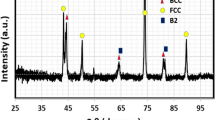
Figure 6 shows the XRD pattern of Cr5 HEA, where it can be seen that there are two BCC solid solutions. Moreover, there are two minor peaks associated with the ordered AlCu2Mn phase. Unlike the previously investigated Cr5 HEA, increasing Al content from 15 to 20 is accompanied by the appearance of super-lattice minor peaks. For the Ti5 HEA, a similar diffraction pattern with two minor super-lattice peaks is present, as seen in Figure 7. However, there is an interesting feature; the peaks of the two solid solutions are overlapped as shown in Figure 8. This could be attributed to maintaining nearly the same lattice constant.
Electrochemical Corrosion Characterization
Weight-Loss Measurement
Figure 9 and Table 3 show the influence of immersion time for HEAs immersed in different concentrations of HNO3 acid for 120 h. An increase in concentration increases the WL per area of Cr5 and Ti5 HEAs. Also, the WL increases with an increase in all conditions. The CR values for Cr5 HEA immersed in various concentrations of HNO3 acid for 120 h are listed in Table 4 and Figure10. The CR of Cr5 HEA for 10% HNO3 is 18.641 mm/y whereas it is 12.016 mm/y for Ti5 HEA. The CR of Ti5 HEA is lower than the CR of Cr5 HEA. The chemical composition of Cr5 HEA and Ti5 HEA, as well as the stability of the oxide coating, influence their corrosion behavior in the investigated conditions.1 The visual images of HEAs in different concentrations of nitric acid, after 120 h of immersion testing experiments are shown in Figure 11.
Potentiodynamic Polarization
The PP curves of Cr5 HEA and Ti5 HEA in numerous concentrations of HNO3 acid are provided in Figure 12. The current change in HNO3 diluted solutions is connected to the applied potential, E. The electrochemical parameters for the HEAs are listed in Table 5. It was noticed from this table that decreasing the polarization resistance with increased acid concentration increases the CR of Cr5 HEA. The CR depends on the composition and the passive film structure produced in the solution.29 At low concentrations, the CR of the Cr5 HEA is good because of a formation of a stable passive film in the acid media. The corrosion current density values and CR for Cr5 HEA reach the maximum values in 10% HNO3 acid with 0.3557 mA/cm2 and 4.19 mm/y, respectively. Where the corrosion current density values and CR for Ti5 HEA approached the maximum values in 10% HNO3 solution with 0.1344 mA/cm2 and 1.583 mm/y, respectively. Generally, the Ti5 HEA has a better corrosion resistance than Cr5 HEA. The findings of the immersion test and the PP curves are in agreement with one another, according to the CR values provided in Table 5. Al2CrFeNiCoCuTix alloys' corrosion resistance is improved by the addition of Ti.12,30
Corrosion Mechanism
The corrosion mechanism was that of the anodic dissolution of the investigated HEAs by the strong HNO3 acid used due to the hydrogen content getting higher which led to the alloys oxidizing. The dissolution anodic characteristic of the HEAs and HNO3 acid is very complex. The anodic reaction for Cu is commonly thought to be as follows31:
where Cu(I)ads are the specimen that is absorbed into the copper surface and not diffused into the solution.
Increasing the Al content in the HEA might lead to increasing the possibility of pitting corrosion.32 The Cr addition in the FeCoNi-based HEA has revealed good corrosion resistance; however, excessive Cr addition leads to deterioration in corrosion resistance due to Cr-induced segregation.33 In a 0.5 M HNO3 solution, the Ti addition greatly enhances the corrosion behavior of Al2CrFeCoCuNiTix (x = 0, 0.5, 1.0, 1.5, and 2.0 molar fraction).28,30,34 The Ti improves the corrosion resistance of Ti5 HEA in Cu-based HEA more than Cr does in Cr5 HEA.
Surface Morphology
The surface morphologies and EDX results of the corroded Cr5 HEAs in 0.5% and 1% HNO3 are provided in Figure 13a, b, respectively. While the results for 5% and 10% HNO3 are shown in Figure 14a, b, respectively. The EDX analysis of corroded Cr5 HEAs in at.% is listed in Table 6. The elemental distribution of the corroded Cr5 HEAs in different concentrations of HNO3 is depicted in Figure 14. As shown in Figure 15 and Table 6, the structure of the dendrite has higher corrosion resistance which mainly is Cr-and Mn-rich. This agrees with the elemental distribution in Figure 15. Adding Cr led to protective passive film formation on the surface, which prevents further corrosion of the alloys underneath.35,36 From mapping, oxygen exists with Cr which may be due to the formation of Cr2O3 protective film on the dendrites.
The surface morphology and the EDX result of the corroded Ti5 HEAs for 10% HNO3 are provided in Figure 16. The EDX result in at.% is 44.2%O, 12.5%Al, 11.8%Ti, 7.5%Mn, 10.8%Fe, 6.0%Ni and 7.2%Cu. The elemental distribution of the corroded Ti5 HEAs is seen in Figure 17. The Ti5 HEA has a good surface morphology with a homogenous distribution than Cr5 HEA which is associated with a decrease in the corrosion rate.
Conclusion
In the present work, novel Cu40Mn25Al20Fe5Ni5Cr5 HEA (Cr5 HEA) and Cu40Mn25Al20Fe5Ni5Ti5 HEA (Ti5 HEA) were synthesized via the casting route. The Cr5 HEA and Ti5 HEA show BCC structures. The EDX results and microstructural analysis of the HEAs indicate the formation of a dendritic structure. The corrosion properties of the HEAs were studied by chemical (WL) and electrochemical techniques (PP). They are investigated in various concentrations of 0.5, 1, 5, and 10% HNO3 acid for various times up to 120 h. The CR increases with increasing the HNO3 acid concentrations. The corrosion behavior of Cr5 HEA and Ti5 HEA using immersion and PP tests was studied. The CR of Ti5 HEA is lower than for Cr5 HEA. The Ti5 HEA demonstrated a promising corrosion behavior in HNO3. The appearance of dendrites of the corroded Cr5 HEA resists the corrosion. The lower CR is approximately 12.016 mm/y for Ti5 HEA in a concentration of 10% HNO3 solution. Cr addition led to the formation of a Cr2O3 protective film on the dendrites. However, the Ti5 HEA has a good surface morphology with a homogenous distribution than Cr5 HEA which associated to decrease the corrosion rate.
Data Availability
The authors confirm that this article contains all the data supporting the findings of this study.
References
H.A. Aly, K.A. Abdelghafar, G.A. Gaber, L.Z. Mohamed, Fabrication, characterization, and corrosion behavior of a New Cu40Mn25Al20Fe5Co5Ni5 High-entropy alloy in HNO3 solution, J. Mater. Eng. Perform (2021)
G.A. Gaber, S.A. Abolkassem, O.A. Elkady, M. Tash, L.Z. Mohamed, ANOVA and DOE of comparative studies of Cu/Mn effect on corrosion features of CoCrFeNi high entropy alloy immersed in different acidic media, Chem. Papers, 2 (2022)
Y. Qiu, S. Thomas, M.A. Gibson, H.L. Fraser, N. Birbilis, Corrosion of high entropy alloys, Mater. Degrad 15 (2017)
Q. Li, X. Bao. C. Fan. et al. The Influence of Multi-Phase Intermetallic Compounds on the Microstructure and Tribological Behaviors of 6061 Al Alloy by Adding AlCoCrFeNi High-Entropy Alloy. Inter Metalcast 16, 871–877 (2022). https://doi.org/10.1007/s40962-021-00631-6
Q. Li, X. Bao, S. Zhao et al. The Influence of AlFeNiCrCoTi High-Entropy Alloy on Microstructure, Mechanical Properties and Tribological Behaviors of Aluminum Matrix Composites. Inter Metalcast 15, 281–291 (2021). https://doi.org/10.1007/s40962-020-00462-x
S. Hedya, L. Mohamed, G. Gaber, O. Elkady, H. Megahed, S. Abolkassem, Effect of Si/Ti additions on physico-mechanical and chemical properties of FeNiCrCo high entropy alloys manufactured by powder metallurgy technique. Trans. Nonferrous Met. Soc. China 32 (2022)
M.C. Gao, J. Qiao, High entropy alloys (HEAs). Metals 8, 108 (2018)
E.P. George, D. Raabe, R.O. Ritchie, High-entropy alloys. Nat. Rev. Mater. 4(8), 515–534 (2019)
J.B. Cheng, X.B. Liang, B.S. Xu, Effect of Nb addition on the structure and mechanical behaviors of CoCrCuFeNi high-entropy alloy coatings. Surf. Coat. Technol. 240, 184 (2014)
M. Fu, X. Ma, K. Zhao, X. Li, D. Su, High-entropy materials for energy-related applications, iScience, 24, 102177 (2021)
Q.-D. Qin, J.-B. Qu, Y. Hu, Y. Wu, X. Su, Microstructural characterization and oxidation resistance of multicomponent equiatomic CoCrCuFeNi–TiO high-entropy alloy. Inter. J. Miner. Metall. Mater. 25(11), 1286 (2018). https://doi.org/10.1007/s12613-018-1681-9
Y. Shi, B. Yang, P.K. Liaw, corrosion-resistant high-entropy alloys: a review, Metals, 7, 43, 8 (2017)
H. Luo, Z. Li, A.M. Mingers, D. Raable, Corrosion behavior of an equiatomic CoCrFeMnNi high-entropy alloy compared with 304 stainless steel in sulfuric acid solution. Corros. Sci. 134(15), 131–139 (2018)
X. Zhang, J. Guo, X. Zhang, Y. Song, Z. Li, X. Xing, D. Kong, Influence of remelting and annealing treatment on corrosion resistance of AlFeNiCoCuCr high entropy alloy in 3.5% NaCl solution, J. Alloys Compd., 775, 565–570 (2019)
M.F. Moreira, L.B. Fantin, C.R.F. Azevedo, Microstructural characterization of Ni-base superalloy As-cast single crystal (CMSX-4). Inter Metalcast 15, 676–691 (2021). https://doi.org/10.1007/s40962-020-00496-1
K.A. Abdelghafar, M.M. Ibrahim, M.A. Shoeib, M.A. Waly, Evaluation of microstructural and corrosion resistance of as-cast Cu45Mn25Al15Fe5Cr5Ni5 HEA. Mater. Res. Express 7, 016579 (2020)
Y.J. Zhou, Y. Zhang, Y.L. Wang, G.L. Chen, Solid solution alloys of AlCoCrFeNiTix with excellent room-temperature mechanical properties. Appl Phys Lett 90, 181904 (2007)
Y.J. Hsu, W.C. Chiang, J.K. Wu, Corrosion behavior of FeCoNiCrCux high-entropy alloys in 3.5% sodium chloride solution, Mater. Chem. Phys. 92, 112–7 (2005)
Y. Li, Y. Shi, E. Olugbade, Microstructure, mechanical, and corrosion resistance properties of Al0.8CrFeCoNiCux high-entropy alloy coatings on aluminum by laser cladding, Mater. Res. Express (2020)
X.F. Wang, Y. Zhang, Y. Qiao, G.L. Chen, Novel microstructure and properties of multicomponent CoCrCuFeNiTix alloys. Intermetallics 15, 357–362 (2007)
B. Ren, R.F. Zhao, Z.X. Liu, S.K. Guan, H.S. Zhang, Microstructure and properties of Al0.3CrFe1.5MnNi0.5Tix and Al0.3CrFe1.5MnNi0.5Six high-entropy alloys. Rare Metal 33, 149–154 (2014)
D. Choudhuri, T. Alam, T. Borkar, B. Gwalani, A.S. Mantri, S.G. Srinivasan, M.A. Gibson, R. Banerjee, Formation of a Huesler-like L21 phase in a CoCrCuFeNiAlTi high-entropy alloy. Scr. Mater. 100, 36–39 (2015)
L. Jiang, Y. Lu, Y. Dong, T. Wang, Z. Cao, T. Li, Annealing effects on the microstructure and properties of bulk high-entropy CoCrFeNiTi0.5 alloy casting ingot. Intermetallics 44, 37–43 (2014)
P. Keller, H.-H. Strehblow, XPS investigations of electrochemically formed passive layers on Fe/Cr-alloys in 0.5 M H2SO4, Corros. Sci., 46, 1939–1952 (2004)
T. Olugbade, C. Liu, J. Lu, Enhanced passivation layer by Cr diffusion of 301 stainless steel facilitated by SMAT. Adv. Eng. Mater. 21, 1900125 (2019)
D. Hamm, K. Ogle, C.-O.A. Olsson, S. Weber, D. Landolt, Passivation of Fe–Cr alloys studied with ICP-AES and EQCM. Corros. Sci. 44, 1443–1456 (2002)
H. Fuxiang, M. Jusheng, N. Honglong, G. Zhiting, L. Chao, G. Shumei, Y. Xuetao, W. Tao, L. Hong, L. Huafen, Analysis of phases in a Cu–Cr–Zr alloy. Scr. Mater. 48, 97–102 (2003)
J. Moon, Y. Choi, T. Sasaki, M. Joo, H. Shin, J.S. Lee, T. Ohkubo, K. Hono, S.M. Baek, H.S. Kim, Corrosion-resistant Cu-Fe-based immiscible medium-entropy alloy with tri-layer passivation. Corros. Sci. 193, 109888 (2021)
S.A. Abolkassem, L.Z. Mohamed, G.A. Gaber, O.A. Elkady, Microstructure and corrosion behavior of FeNiCoCrCu and FeNiCoCrMn high entropy alloys manufactured by powder metallurgy in different acid media, J. Mater. Res. Technol., 1 (2021)
X.W. Qiu, Y.P. Zhang, C.G. Liu, Effect of Ti content on structure and properties of Al2CrFeNiCoCuTix high-entropy alloy coatings. J. Alloys Compd. 585, 282–286 (2014)
F. Gapsari, H. Andoko, A. Wijaya, Corrosion behavior of brass in nitric acid. Metabk 57(4), 333–336 (2018)
L.Z. Mohamed, M.A.H. Gepreel, A. Abdelfatah, Corrosion behavior of Al12Cr12Fe35Mn21Ni20 high entropy alloy in different acidic media. Chem. Papers 75, 6265–6274 (2021)
W. Chai, T. Lu, Y. Pan, Corrosion behaviors of FeCoNiCrx (x = 0, 0.5, 1.0) multiprincipal element alloys: role of Cr-induced segregation, Intermetallics, 116, 106654 (2020)
Y. Qiu, M.A. Gibson, H.L. Fraser, N. Birbilis, Corrosion characteristics of high entropy alloys. Mater. Sci. Technol. 31, 1235–1243 (2015)
Y.Y. Chen, U.T. Hong, H.C. Shih, J.W. Yeh, T. Duval, Electrochemical kinetics of the high entropy alloys in aqueous environments—a comparison with type 304 stainless steel. Corros. Sci. 47, 2679–2699 (2005)
Z. Tang, L. Huang, W. He, P. Liaw, Alloying and processing effects on the aqueous corrosion behavior of high-entropy alloys. Entropy 16, 895–911 (2014)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research received no external funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Consent for Publication
We here give our consent to publish the paper in this journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mohamed, L.Z., Abdelghafar, K.A., Aly, H.A. et al. Comparative Studies of Cr/Ti Additions for Cu40Mn25Al20Fe5Ni5 HEA on Microstructure and Corrosion Behavior in HNO3 Solution. Inter Metalcast 17, 1791–1805 (2023). https://doi.org/10.1007/s40962-022-00891-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40962-022-00891-w