Abstract
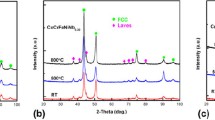
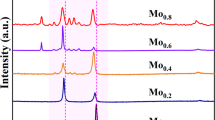
The influence of grain refinement by high-pressure torsion (HPT) on the corrosion behavior of CoCrFeNi alloys with varied Cr content was investigated in aqueous 0.5 M H2SO4 and 3.5% NaCl solutions. The results were compared with CoCrFeMnNi alloys. Both the alloys showed a single fcc phase after vacuum melting and HPT. Protective passivation capability and resistance to general corrosion in H2SO4 was higher than that of Fe–Cr alloys and became higher with increasing Cr contents, indicating that high corrosion resistance of Cr-containing HEAs is attributed to the incorporation of Cr with other supporting elements. However, the impact of the nanocrystalline structure by HPT on the general corrosion behavior in the H2SO4 solution was negligibly small while the resistance to the local attack as pitting in NaCl was improved for the CoCrFeNi alloy. This is contrasted with the CoCrFeMnNi alloy, which exhibited negligibly small change by HPT in both NaCl and H2SO4 solutions. X-ray photoelectron spectroscopy indicated the Cr enrichment in passive films, but its degree is smaller regardless of grain size and Mn content, as compared with that of Fe–Cr alloys reported in the literature. The small change in the Cr enrichment in the passive film and the resulting corrosion behavior by grain refinement through HPT could be attributed to an intrinsic nature of HEAs such as sluggish diffusion and intrinsic lattice distortion. The improved resistance to pitting corrosion in CoCrFeNi alloys could be explained by the homogenization effect of the ultrahigh strain deformation, which may not take effect in alloys containing the solute Mn.









Similar content being viewed by others
Data availability
The raw processed data required to reproduce these findings cannot be shared at this time as the data also forms part of an ongoing study.
References
Ye YF, Wang Q, Lu J, Liu CT, Yang Y (2016) High-entropy alloy: challenges and prospects. Mater Today 19:349–362. https://doi.org/10.1016/j.mattod.2015.11.026
Qiu Y, Gibson YMA, Fraser HI, Birbilis N (2015) Corrosion characteristics of high entropy alloys. Mater Sci Technol 31:1235–1243. https://doi.org/10.1016/j.mtcomm.2020.101979
Ye Q, Feng K, Li Z, Lu F, Li R, Huang J, Wu Y (2017) Microstructure and corrosion properties of CrMnFeCoNi high entropy alloy coating. Appl Surf Sci 396:420–1426. https://doi.org/10.1016/j.apsusc.2016.11.176
Luo H, Li Z, Mingers AM, Raabe D (2018) Corrosion behavior of an equiatomic CoCrFeMnNi high-entropy alloy compared with 304 stainless steel in sulfuric acid solution. Corros Sci 134:131–139. https://doi.org/10.1016/j.corsci.2018.02.031
Torbati-Sarraf H, Shabani M, Jablonski PD, Pataky GJ, Poursaee A (2019) The influence of incorporation of Mn on the pitting corrosion performance of CrFeCoNi high entropy alloy at different temperatures. Mater Des 184:108170. https://doi.org/10.1016/j.matdes.2019.108170
Wang L, Mercier D, Zanna S, Seyeux A, Laurent-Brocq M, Perrière I, Guillot I, Marcus P (2020) Study of the surface oxides and corrosion behavior of an equiatomic CoCrFeMnNi high entropy alloy by XPS and ToF-SIMS. Corros Sci 167:108507. https://doi.org/10.1016/j.corsci.2020.108507
Yang J, Wu J, Zhang CY, Zhang SD, Yang BJ, Emori W, Wang JQ (2020) Effects of Mn on the electrochemical corrosion and passivation behavior of CoFeNiMnCr high-entropy alloy system in H2SO4 solution. J Alloys Compd 819:152943. https://doi.org/10.1016/j.jallcom.2019.152943
Hsu KM, Lin CS (2021) Microstructural and electrochemical characterization of the passive film on a 50 kg hot rolled FeCrNiCoMn high entropy alloy. Mater Today Commun 26:101979. https://doi.org/10.1016/j.mtcomm.2020.101979
Koch CC, Ovid’ko IA, Seal S, Veprek S, (2007) Corrosion of structural nanomaterials. In: Kock CC (ed) Structural nanocrystalline materials. Cambridge University Press, Cambridge, pp 317–340
Ralston KD, Birbilis N (2010) Effect of grain size on corrosion: a review. Corrosion 66:075005. https://doi.org/10.5006/1.3462912
Wang Y, Jin J, Zhang M, Wang X, Gong P, Zhang J, Liu J (2021) Effect of the grain size on the corrosion behavior of CoCrFeMnNi HEAs in a 0.5 M H2SO4 solution. J Alloys Compd 858:157712. https://doi.org/10.1016/j.jallcom.2020.157712
Han Z, Ren W, Yang J, Tian A, Du Y, Liu G, Wei R, Zhang G, Chen Y (2020) The corrosion behavior of ultra-fine grained CoNiFeCrMn high-entropy alloys. J Alloys Compd 816:152583. https://doi.org/10.1016/j.jallcom.2019.152583
Singh D, Aggarwal S, Grandhi S, Rahul R, Parida S, Bakshi SR, Kumar R (2022) Synthesis and characterization of nanocrystalline and microcrystalline high entropy alloys and study of their corrosion behavior. Trans Indian Inst Metals 75:2091–2097. https://doi.org/10.1007/s12666-022-02576-8
Xue L, Ding Y, Pradeep KG, Case R, Castaneda H, Paredes M (2022) The grain size effect on corrosion property of Al2Cr5Cu5Fe53Ni35 high-entropy alloy in marine environment. Corros Sci 208:110625. https://doi.org/10.1016/j.corsci.2022.110625
Edalati K et al (2022) Nanomaterials by severe plastic deformation: review of historical developments and recent advances. Mater Res Lett 10:163–256. https://doi.org/10.1080/21663831.2022.2029779
Edalati K, Horita Z (2016) A review on high-pressure torsion (HPT) from 1935 to 1988. Mater Sci Eng A 652:325–352. https://doi.org/10.1016/j.msea.2015.11.074
Edalati K (2019) Metallurgical alchemy by ultra-severe plastic deformation via high-pressure torsion process. Mater Trans 60:1221–1229. https://doi.org/10.2320/matertrans.MF201914
Schuh B, Mendez-Martin F, Völker B, George EP, Clemens H, Pippan R, Hohenwarter A (2015) Mechanical properties, microstructure and thermal stability of a nanocrystalline CoCrFeMnNi high-entropy alloy after severe plastic deformation. Acta Mater 96:258–268. https://doi.org/10.1016/j.actamat.2015.06.025
Shahmir H, He J, Lu Z, Kawasaki M, Langdon TG (2016) Effect of annealing on mechanical properties of a nanocrystalline CoCrFeNiMn high-entropy alloy processed by high-pressure torsion. Mater Sci Eng A 676:294–303. https://doi.org/10.1016/j.msea.2016.08.118
Lee DH, Lee JA, Zhao Y, Lu Z, Suh JY, Kim JY, Ramamurty U, Kawasaki M, Langdon TG, Jang JI (2017) Annealing effect on plastic flow in nanocrystalline CoCrFeMnNi high-entropy alloy: a nanomechanical analysis. Acta Mater 140:443–451. https://doi.org/10.1016/j.actamat.2017.08.057
Skrotzki Q, Pukenas A, Joni B, Odor E, Ungar T, Hohenwarter A, Pippan R, George EP (2017) Microstructure and texture evolution during severe plastic deformation of CrMnFeCoNi high-entropy alloy. IOP Conf Ser Mater Sci Eng 194:012028. https://doi.org/10.1088/1757-899X/194/1/012028
Mohammadi A, Edalati P, Arita M, Bae JW, Kim HS, Edalati K (2022) Microstructure and defect effects on strength and hydrogen embrittlement of high-entropy alloy CrMnFeCoNi processed by high-pressure torsion. Mater Sci Eng A 844:143179. https://doi.org/10.1016/j.msea.2022.143179
Mohammadi A, Edalati P, Arita M, Bae JW, Kim HS, Edalati K (2023) High strength and high ductility of a severely deformed high-entropy alloy in the presence of hydrogen. Corros Sci 216:111097. https://doi.org/10.1016/j.corsci.2023.111097
Shahmir H, Nili-Ahmadabadi M, Shafiee A, Langdon TG (2018) Effect of a minor titanium addition on the superplastic properties of a CoCrFeNiMn high-entropy alloy processed by high-pressure torsion. Mater Sci Eng A 718:468–476. https://doi.org/10.1016/j.msea.2018.02.002
Shahmir H, He J, Lu Z, Kawasaki M, TGLangdon, (2017) Evidence for superplasticity in a CoCrFeNiMn high-entropy alloy processed by high-pressure torsion. Mater Sci Eng A 685:342–348. https://doi.org/10.1016/j.msea.2017.01.016
Miyamoto H (2016) Corrosion of ultrafine grained materials by severe plastic deformation, an overview. Mater Trans 57:559–572. https://doi.org/10.2320/matertrans.M2015452
Miyamoto H, Yuasa M, Rifai M, Fujiwara H (2019) Corrosion behavior of severely deformed pure and single-phase materials. Mater Trans 60:1243–1255. https://doi.org/10.2320/matertrans.MF201935
Gupta RK, Birbilis N (2015) The influence of nanocrystalline structure and processing route on corrosion of stainless steel: a review. Corros Sci 92:1–15. https://doi.org/10.1016/j.corsci.2014.11.041
Raman RKS, Gupta RK (2009) Oxidation resistance of nanocrystalline vis-à-vis microcrystalline Fe–Cr alloys. Corros Sci 51:316–321. https://doi.org/10.1016/j.corsci.2008.10.020
Raman RKS, Gupta RK, Koch CC (2010) Resistance of nanocrystalline vis-a-vis microcrytalline Fe–Cr alloys to environmental degradation and challenge to their synthesis. Philos Mag 90:3233–3260. https://doi.org/10.1080/14786435.2010.484402
Pisarek M, Kȩdzierzawski P, Janik-Czachor M, Kurzydłowski KJ (2009) Effect of hydrostatic extrusion on passivity breakdown on 303 austenitic stainless steel in chloride solution. J Solid State Electrochem 13:283–291. https://doi.org/10.1007/s10008-007-0488-9
Zheng ZJ, Gao Y, Gui Y, Zhu M (2012) Corrosion behaviour of nanocrystalline 304 stainless steel prepared by equal channel angular pressing. Corros Sci 54:60–67. https://doi.org/10.1016/j.corsci.2011.08.049
Gupta RK, Singh Raman RK, Koch CC, Murty BS (2013) Effect of nanocrystalline structure on the corrosion of a Fe20Cr alloy. Int J Electrochem Sci 8:6791–6806. https://doi.org/10.1016/S1452-3981(23)14806-1
Gupta RK, Singh Raman RK, Koch CC (2012) Electrochemical characteristics of nano and microcrystalline Fe–Cr alloys. J Mater Sci 47:6118–6124. https://doi.org/10.1007/s10853-012-6529-5
Meng M, Li Y, Wang F (2006) The corrosion behavior of Fe–10Cr nanocrystalline coating. Electrochim Acta 51:4277–4284. https://doi.org/10.1016/j.electacta.2005.12.015
Pan C, Liu L, Bin Z, Wang F (2012) The electrochemical corrosion behaviour of nanocrystalline 304 stainless steel prepared by magnetron sputtering. J Electrochem Soc 159:C453–C460. https://doi.org/10.1149/2.034211jes
Li S, Ren Z, Dong Y, Ye C, Cheng G, Cong H (2017) Enhanced pitting corrosion resistance of 304 SS in 3.5 wt% NaCl by ultrasonic nanocrystal surface modification. J Electrochem Soc 164:C682–C689. https://doi.org/10.1149/2.1781712jes
Koch CC (2007) Structural nanocrystalline materials: an overview. J Mater Sci 42:1403–1414. https://doi.org/10.1007/s10853-006-0609-3
Hajizadeh K, Maleki-Ghaleh H, Arabi A, Behnamian Y, Aghaie F, Farrokhi A, Hosseini MG, Fathi MH (2015) Corrosion and biological behavior of nanostructured 316L stainless steel processed by severe plastic deformation. Surf Interface Anal 47:978–985. https://doi.org/10.1002/sia.5806
Oh K, Ahn S, Eom K, Jung K, Kwon H (2014) Observation of passive films on Fe–20Cr–xNi (x = 0, 10, 20 wt%) alloys using TEM and Cs-corrected STEM–EELS. Corros Sci 79:34–40. https://doi.org/10.1016/j.corsci.2013.10.023
Hamada E, Yamada K, Nagoshi M, Makiishi N, Sato K, Ishii T, Fukuda K, Ishikawa S, Ujiro T (2010) Direct imaging of native passive film on stainless steel by aberration corrected STEM. Corros Sci 52:3851–3854. https://doi.org/10.1016/j.corsci.2010.08.025
Shimizu H, Yuasa M, Miyamoto H, Edalati K (2022) Corrosion behavior of ultrafine-grained CoCrFeMnNi high-entropy alloys fabricated by high-pressure torsion. Materials 15:1007. https://doi.org/10.3390/ma15031007
Wang C, Yu Y, Yu J, Zhang Y, Wang F, Li H (2020) Effect of the macro-segregation on corrosion behavior of CrMnFeCoNi coating prepared by arc cladding. J Alloys Compd 846:156263. https://doi.org/10.1016/j.jallcom.2020.156263
Hsu KM, Chen SH, Lin CS (2021) Microstructure and corrosion behavior of FeCrNiCoMnx (x = 10, 06, 03, 0) high entropy alloys in 0.5 M H2SO4. Corros Sci 190:109694. https://doi.org/10.1016/j.corsci.2021.109694
Ha HY, Jang MH, Lee TH (2016) Influences of Mn in solid solution on the pitting corrosion behaviour of Fe-23 wt% Cr-based alloys. Electrochim Acta 191:864–875. https://doi.org/10.1016/j.electacta.2016.01.118
Sahu S, Swanson OJ, Li T, Gerard AY, Scully JR, Frankel GS (2020) Localized corrosion behavior of non-equiatomic NiFeCrMnCo multi-principal element alloys. Electrochim Acta 354:136749. https://doi.org/10.1016/j.electacta.2020.136749
Asami K, Hashimoto K, Shimodaira S (1978) An XPS study of the passivity of a series of iron-chromium alloys in sulphuric acid. Corros Sci 18:151–160. https://doi.org/10.1016/S0010-938X(78)80085-7
Hamm D, Ogle K, Olsson COA, Weber S, Landolt D (2002) Passivation of Fe–Cr alloys studied with ICP-AES and EQCM. Corros Sci 44:1443–1456. https://doi.org/10.1016/S0010-938X(01)00147-0
Sieradzki K, Newman RC (1986) A percolation model for passivation in stainless steels. J Electrochem Soc 133:1979–1980. https://doi.org/10.1149/1.2109065
Jargelius-Petterson RFA (1998) Application of the pitting resistance equivalent concept to some highly alloyed austenitic stainless steels. Corrosion 54(2):162–168
Hosoi Y (2007) Localized corrosion of high purity ferritic stainless steels and effects of alloying elements. Zairyo Kankyo 56:439–446
Kirchheim R, Heine B, Fischmeister H, Hofmann S, Knote H, Stolz U (1989) The passivity of iron-chromium alloys. Corros Sci 29:899–917. https://doi.org/10.1016/0010-938X(89)90060-7
Olsson COA, Landolt D (2003) Passive films on stainless steels—chemistry, structure and growth. Electrochim Acta 48:1093–1104. https://doi.org/10.1016/S0013-4686(02)00841-1
Qian S, Newman RC, Cottis RA (1990) Validation of a percolation model for passivation of Fe–Cr alloys: two-dimensional computer simulation. J Electrochem Soc 137:435–439. https://doi.org/10.1149/1.2086458
Wang ZB, Tao NR, Tong WP, Lu J, Lu K (2003) Diffusion of chromium in nanocrystalline iron produced by means of surface mechanical attrition treatment. Acta Mater 51:4319–4329. https://doi.org/10.1016/S1359-6454(03)00260-X
Tsai KY, Tsai MH, Yeh JW (2013) Sluggish diffusion in Co–Cr–Fe–Mn–Ni high-entropy alloys. Acta Mater 61:4887–4897. https://doi.org/10.1016/j.actamat.2013.04.058
Chen BR, Yeh AC, Yeh JW (2016) Effect of one-step recrystallization on the grain boundary evolution of CoCrFeMnNi high entropy alloy and its subsystems. Sci Rep 6:22306. https://doi.org/10.1038/srep22306
Zhou N, Hu T, Huang J, Luo J (2016) Stabilization of nanocrystalline alloys at high temperatures via utilizing high-entropy grain boundary complexions. Scr Mater 124:160–163. https://doi.org/10.1016/j.scriptamat.2016.07.014
Utt D, Stukowski A, Albe K (2020) Grain boundary structure and mobility in high-entropy alloys: a comparative molecular dynamics study on a Σ11 symmetrical tilt grain boundary in face-centered cubic CuNiCoFe. Acta Mater 186:11–19. https://doi.org/10.1016/j.actamat.2019.12.031
Acknowledgements
This work was supported by Grants-in-Aid for Scientific Research on Innovative Area on High Entropy Alloys (Grant Numbers 19H05178, 19H05176 and 21H00155, JP21H00150) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan and by a grant from IKETANI Foundation (2020 0321097-A). The authors would like to thank Professor Haruyuki Inui of Kyoto University for providing us the arc melting apparatus, and thank Professor Takumi Haruna of Kansai University for valuable comments and discussion on the corrosion behavior of our samples. The authors appreciate Dr. Abe and Dr. Ohnuma of the National Institute of Materials Science (NIMS) for conducting CALPHAD and calculating the phase diagrams which was helpful for the alloy design.
Author information
Authors and Affiliations
Contributions
NH was involved in investigation, methodology and original draft, MY contributed to validation, review and editing, HM was involved in conceptualization, funding acquisition, project administration, review and editing and KE contributed to investigation, funding acquisition, review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this manuscript.
Additional information
Handling Editor: Megumi Kawasaki.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hata, N., Yuasa, M., Miyamoto, H. et al. Influence of grain refinement via high-pressure torsion on corrosion behavior of medium- and high-entropy alloys CoCrFeNi and CoCrFeMnNi with various chromium contents. J Mater Sci 59, 5891–5905 (2024). https://doi.org/10.1007/s10853-024-09458-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-024-09458-y