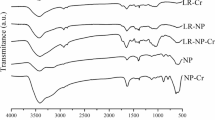
Abstract
Alhagi graecorum (AG) is an invasive plant with a massive/robust root structure that can grow up to 12 feet into the ground. The present study exploited the rich cellulosic content in this ‘AG’ root for the synthesis of a novel biosorbent (‘MA’). This low-cost biosorbent, with high carboxyl content of 447.22 (m eq/100 g sample) was utilized for aqueous zinc ion sequestration. The surface functional groups and textural characteristics required for an efficient heavy metal binding were identified on ‘MA’ using Fourier transform infrared spectroscopy and scanning electron microscopy. Sip isotherm emerged as the model of best fit for equilibrium studies; hence, Zn (II) ion sorption onto ‘MA’ is believed to occur via a hybrid blend of homogeneous monolayer and heterogeneous multilayer adsorption. Meanwhile, the Elovich (SNE = 1.0429), intraparticle diffusion (SNE = 1.0205) and pseudo-first order (SNE = 1.0455) provided the best fitting for 200, 400 and 600 mg/L adsorption system, respectively. The maximum adsorption capacity of 188.67 mg/g was recorded at optimum adsorption conditions, with the predominance of the electrostatic and electron donor–acceptor interaction mechanism. The abundant surface oxygenous functional groups on ‘MA’ positively influenced its adsorption capacity, thus making it a promising biosorbent for aqueous Zn (II) uptake.
Graphical abstract











Similar content being viewed by others
Availability of data and materials
All data generated or analysed during this study are included in this manuscript draft.
References
Abdelraheem WH, Rabia MK, Ismail NM (2016) Evaluation of copper speciation in the extract of Eichhornia crassipes using reverse and forward/CLE voltammetric titrations. Arab J Chem 9:S1670–S1678
Abdelraheem WH, Komy ZR, Ismail NM (2017) Electrochemical determination of Cu2+ complexation in the extract of E. crassipes by anodic stripping voltammetry. Arab J Chem 10:S1105–S1110
Abonyi M, Aniagor C, Menkiti M (2019) Effective dephenolation of effluent from petroleum industry using ionic-liquid-induced hybrid adsorbent. Arab J Sci Eng 44(12):10017–10029
Adigun O, Oninla V, Babarinde NA (2019) Application of sugarcane leaves as biomass in the removal of cadmium (II), lead (II) and zinc (II) ions from polluted water. Int J Energy Water Resour 3(2):141–152
Ahmad MF, Haydar S, Quraishi TA (2013) Enhancement of biosorption of zinc ions from aqueous solution by immobilized Candida utilis and Candida tropicalis cells. Int Biodeter Biodegrad 83:119–128
Ali I, Alharbi OM, Alothman ZA, Badjah AY (2018) Kinetics, thermodynamics, and modeling of amido black dye photodegradation in water using Co/TiO2 nanoparticles. Photochem Photobiol 94(5):935–941
Ali I, Alharbi OM, ALOthman ZA, Alwarthan A, Al-Mohaimeed AM (2019) Preparation of a carboxymethylcellulose-iron composite for uptake of atorvastatin in water. Int J Biol Macromol 132:244–253
Al-Shaalan NH, Ali I, ALOthman ZA, Al-Wahaibi LH, Alabdulmonem H (2019) High performance removal and simulation studies of diuron pesticide in water on MWCNTs. J Mol Liq 289:111039
Aniagor C, Menkiti M (2018) Kinetics and mechanistic description of adsorptive uptake of crystal violet dye by lignified elephant grass complexed isolate. J Environ Chem Eng 6(2):2105–2118
Aniagor C, Menkiti M (2019) Synthesis, modification and use of lignified bamboo isolate for the renovation of crystal violet dye effluent. Appl Water Sci 9(4):77
Aniagor CO, Menkiti MC (2020) Relational description of an adsorption system based on isotherm, adsorption density, adsorption potential, hopping number and surface coverage. Sigma 38(3):1073–1098
Aniagor CO, Igwegbe CA, Ighalo JO, Oba SN (2021a) Adsorption of doxycycline from aqueous media: a review. J Mol Liq. https://doi.org/10.1016/j.molliq.2021.116124:p.116124
Aniagor CO, Elshkankery M, Fletcher A, Morsy OM, Abdel-Halim E, Hashem A (2021b) Equilibrium and kinetic modelling of aqueous cadmium ion and activated carbon adsorption system. Water Conserv Sci Eng 6:95–104
Aniagor CO, Afifi M, Hashem A (2021c) Heavy metal adsorptive application of hydrolyzed corn starch. J Polym Res 28(11):1–10
Awual MR (2016) Solid phase sensitive palladium (II) ions detection and recovery using ligand based efficient conjugate nanomaterials. Chem Eng J 300:264–272
Awual MR, Alharthi NH, Hasan MM, Karim MR, Islam A, Znad H, Hossain MA, Halim ME, Rahman MM, Khaleque MA (2017) Inorganic-organic based novel nano-conjugate material for effective cobalt (II) ions capturing from wastewater. Chem Eng J 324:130–139
Awual MR, Hasan MM, Asiri AM, Rahman MM (2019a) Novel optical composite material for efficient vanadium (III) capturing from wastewater. J Mol Liq 283:704–712
Awual MR, Hasan MM, Islam A, Rahman MM, Asiri AM, Khaleque MA, Sheikh MC (2019b) Offering an innovative composited material for effective lead (II) monitoring and removal from polluted water. J Clean Prod 231:214–223
Badruddoza AZM, Shawon ZBZ, Tay WJD, Hidajat K, Uddin MS (2013) Fe3O4/cyclodextrin polymer nanocomposites for selective heavy metals removal from industrial wastewater. Carbohyd Polym 91(1):322–332
Barakat M (2011) New trends in removing heavy metals from industrial wastewater. Arab J Chem 4(4):361–377
Chen X, Lv F, Lin Y, Wang Z, Meng L, Zhang Q, Zhang W, Li L (2018) Structure evolution of polyethylene-plasticizer film at industrially relevant conditions studied by in-situ X-ray scattering: the role of crystal stress. Eur Polym J 101:358–367
Dada A, Olalekan A, Olatunya A, Dada O (2012) Langmuir, Freundlich, Temkin and Dubinin-Radushkevich isotherms studies of equilibrium sorption of Zn2+ unto phosphoric acid modified rice husk. IOSR J Appl Chem 3(1):38–45
Delgado N, Capparelli A, Navarro A, Marino D (2019) Pharmaceutical emerging pollutants removal from water using powdered activated carbon: study of kinetics and adsorption equilibrium. J Environ Manage 236:301–308
Ebrahimi A, Ehteshami M, Dahrazma B (2015) Isotherm and kinetic studies for the biosorption of cadmium from aqueous solution by Alhaji maurorum seed. Process Saf Environ Prot 98:374–382
Fadzil F, Ibrahim S, Hanafiah MAKM (2016) Adsorption of lead (II) onto organic acid modified rubber leaf powder: batch and column studies. Process Saf Environ Prot 100:1–8
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156(1):2–10
Freundlich H (1907) Über die adsorption in lösungen. Z Phys Chem 57(1):385–470
Guo X, Wang J (2019) A general kinetic model for adsorption: theoretical analysis and modeling. J Mol Liq 288:111100
Hashem A, Badawy SM (2015) Sesbania sesban L. biomass as a novel adsorbent for removal of Pb (II) ions from aqueous solution: non-linear and error analysis. Green Process Synth 4(3):179–190
Hashem A, Hussein HA, Sanousy MA, Adam E, Saad EE (2011) Monomethylolated thiourea–sawdust as a new adsorbent for removal of Hg (II) from contaminated water: equilibrium kinetic and thermodynamic studies. Polym-Plast Technol Eng 50(12):1220–1230
Hashem A, Al-Anwar A, Nagy NM, Hussein DM, Eisa S (2016) Isotherms and kinetic studies on adsorption of Hg (II) ions onto Ziziphus spina-christi L. from aqueous solutions. Green Process Synth 5(2):213–224
Hashem A, Badawy S, Farag S, Mohamed L, Fletcher A, Taha G (2020a) Non-linear adsorption characteristics of modified pine wood sawdust optimised for adsorption of Cd (II) from aqueous systems. J Environ Chem Eng 8:103966
Hashem A, Nasr M, Fletcher A, Mohamed LA (2020b) Aminated acrylic fabric waste derived sorbent for Cd (II) ion removal from aqueous solutions: mechanism, equilibria and kinetics. J Polym Environ 29:1–12
Hashem A, Fletcher A, Younis H, Mauof H, Abou-Okeil A (2020c) Adsorption of Pb (II) ions from contaminated water by 1, 2, 3, 4-butanetetracarboxylic acid-modified microcrystalline cellulose: isotherms, kinetics, and thermodynamic studies. Int J Biol Macromol 164:3193–3203
Hashem A, Aniagor C, Hussein D, Farag S (2021) Application of novel butane-1, 4-dioic acid-functionalized cellulosic biosorbent for aqueous cobalt ion sequestration. Cellulose 28(6):3599–3615
Hashem A, Aniagor CO, Nasr M, Abou-Okeil A (2021) Efficacy of treated sodium alginate and activated carbon fibre for Pb(II) adsorption. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2021.02.067
Hashem A, Aniagor CO, Morsy OM, Abou-Okeil A, Aly A (2022) Apricot seed shell: an agro-waste biosorbent for acid blue193 dye adsorption. Biomass Convers Biorefinery:1–14
Huang W, Hu Y, Li Y, Zhou Y, Niu D, Lei Z, Zhang Z (2018) Citric acid-crosslinked β-cyclodextrin for simultaneous removal of bisphenol A, methylene blue and copper: the roles of cavity and surface functional groups. J Taiwan Inst Chem Eng 82:189–197
Ighalo JO, Tijani IO, Ajala J, Ayandele FO, Eletta O, Adeniyi AG (2020) Competitive biosorption of Pb (II) and Cu (II) by functionalised micropogonias undulates scales. Recent Innov Chem Eng 13:1–12
Igwegbe CA, Onukwuli OD, Ighalo JO, Okoye PU (2020a) Adsorption of cationic dyes on Dacryodes edulis seeds activated carbon modified using phosphoric acid and sodium chloride. Environ Process 7(4):1151–1171
Igwegbe CA, Oba SN, Aniagor CO, Adeniyi AG, Ighalo JO (2020b) Adsorption of ciprofloxacin from water: a comprehensive review. J Industr Eng Chem 93(57):77
Jagaba A, Kutty S, Khaw S, Lai C, Isa M, Baloo L, Lawal I, Abubakar S, Umaru I, Zango Z (2020) Derived hybrid biosorbent for zinc (II) removal from aqueous solution by continuous-flow activated sludge system. J Water Process Eng 34:101152
Kapoor A, Yang R (1989) Correlation of equilibrium adsorption data of condensible vapours on porous adsorbents. Gas Sep Purif 3(4):187–192
Khair U, Fahmi H, Al Hakim S, Rahim R (2017) Forecasting error calculation with mean absolute deviation and mean absolute percentage error. J Phys Conf Ser 930:012002
Khalil M, Hashem A, Hebeish A (1990) Carboxymethylation of maize starch. Starch-Stärke 42(2):60–63
Khan A, Ataullah R, Al-Haddad A (1997) Equilibrium adsorption studies of some aromatic pollutants from dilute aqueous solutions on activated carbon at different temperatures. J Colloid Interface Sci 194(1):154–165
King P, Anuradha K, Lahari SB, Kumar YP, Prasad V (2008) Biosorption of zinc from aqueous solution using Azadirachta indica bark: equilibrium and kinetic studies. J Hazard Mater 152(1):324–329
Komy ZR, Abdelraheem WH, Ismail NM (2013) Biosorption of Cu2+ by Eichhornia crassipes: Physicochemical characterization, biosorption modeling and mechanism. J King Saud Univ Sci 25(1):47–56
Kumar KV, Porkodi K, Rocha F (2008) Comparison of various error functions in predicting the optimum isotherm by linear and non-linear regression analysis for the sorption of basic red 9 by activated carbon. J Hazard Mater 150(1):158–165
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. Part I. Solids. J Am Chem Soc 38(11):2221–2295
Li D, Henschen J, Ek M (2017) Esterification and hydrolysis of cellulose using oxalic acid dihydrate in a solvent-free reaction suitable for preparation of surface-functionalised cellulose nanocrystals with high yield. Green Chem 19(23):5564–5567
Liu X, Chen Z-Q, Han B, Su C-L, Han Q, Chen W-Z (2018) Biosorption of copper ions from aqueous solution using rape straw powders: optimization, equilibrium and kinetic studies. Ecotoxicol Environ Saf 150:251–259
Luef E, Prey T, Kubicek CP (1991) Biosorption of zinc by fungal mycelial wastes. Appl Microbiol Biotechnol 34(5):688–692
Marquardt DW (1963) An algorithm for least-squares estimation of nonlinear parameters. J Soc Ind Appl Math 11(2):431–441
Matouq M, Jildeh N, Qtaishat M, Hindiyeh M, AlSyouf MQ (2015) The adsorption kinetics and modeling for heavy metals removal from wastewater by Moringa pods. J Environ Chem Eng 3(2):775–784
Mattson JA, Mark HB Jr, Malbin MD, Weber WJ Jr, Crittenden JC (1969) Surface chemistry of active carbon: specific adsorption of phenols. J Colloid Interface Sci 31(1):116–130
Menkiti M, Aniagor C (2018) Parametric studies on descriptive isotherms for the uptake of crystal violet dye from aqueous solution onto lignin-rich adsorbent. Arab J Sci Eng 43(5):2375–2392
Menkiti M, Abonyi M, Aniagor C (2018a) Process equilibrium, kinetics, and mechanisms of ionic-liquid induced dephenolation of petroleum effluent. Water Conserv Sci Eng 3(3):205–220
Menkiti M, Aniagor C, Agu C, Ugonabo V (2018b) Effective adsorption of crystal violet dye from an aqueous solution using lignin-rich isolate from elephant grass. Water Conserv Sci Eng 3(1):33–46
Mohamed LA, Aniagor CO, Taha GM, Abou-Okeil A, Hashem A (2021) Mechanistic investigation of the mass transfer stages involved during the adsorption of aqueous lead onto Scopulariopsis brevicompactum fungal biomass. Environ Chall 5:100373
Ng J, Cheung W, McKay G (2002) Equilibrium studies of the sorption of Cu (II) ions onto chitosan. J Colloid Interface Sci 255(1):64–74
Noh JS, Schwarz JA (1990) Effect of HNO3 treatment on the surface acidity of activated carbons. Carbon 28(5):675–682
Oba SN, Ighalo JO, Aniagor CO, Igwegbe CA (2021) Removal of ibuprofen from aqueous media by adsorption: a comprehensive review. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2021.146608:p.146608
Paduraru C, Tofan L, Teodosiu C, Bunia I, Tudorachi N, Toma O (2015) Biosorption of zinc (II) on rapeseed waste: equilibrium studies and thermogravimetric investigations. Process Saf Environ Prot 94:18–28
Ranasinghe S, Navaratne A, Priyantha N (2018) Enhancement of adsorption characteristics of Cr (III) and Ni (II) by surface modification of jackfruit peel biosorbent. J Environ Chem Eng 6(5):5670–5682
Redlich O, Peterson DL (1959) A useful adsorption isotherm. J Phys Chem 63(6):1024–1024
Rivas F, Beltrán F, Gimeno O, Frades J, Carvalho F (2006) Adsorption of landfill leachates onto activated carbon: equilibrium and kinetics. J Hazard Mater 131(1–3):170–178
Saha P, Chowdhury S (2011) Insight into adsorption thermodynamics. Thermodynamics 16:349–364
Salazar-Rabago J, Leyva-Ramos R (2016) Novel biosorbent with high adsorption capacity prepared by chemical modification of white pine (Pinus durangensis) sawdust. Adsorption of Pb (II) from aqueous solutions. J Environ Manag 169:303–312
Sasmal S, Goud VV, Mohanty K (2012) Characterization of biomasses available in the region of North-East India for production of biofuels. Biomass Bioenerg 45:212–220
Sayahi H, Asadabadi S (2022) Anchoring carboxylated pineapple peel cellulose and EDTA onto magnetic (3-aminopropyl) triethoxysilane to effective removal of copper (II) and zinc (II) from polluted water. Cellulose 30:1–23
Schweitzer L, Noblet J (2018) Water contamination and pollution. Green Chem. https://doi.org/10.1016/b978-0-12-809270-5.00011-x
Serguchev YA, Beletskaya IP (1980) Oxidative decarboxylation of carboxylic acids. Russ Chem Rev 49(12):1119
Shahat A, Hassan HM, El-Shahat M, El Shahawy O, Awual MR (2018a) Visual nickel (II) ions treatment in petroleum samples using a mesoporous composite adsorbent. Chem Eng J 334:957–967
Shahat A, Hassan HM, Azzazy HM, El-Sharkawy E, Abdou HM, Awual MR (2018b) Novel hierarchical composite adsorbent for selective lead (II) ions capturing from wastewater samples. Chem Eng J 332:377–386
Temkin M (1940) Kinetics of ammonia synthesis on promoted iron catalysts. Acta Physiochim URSS 12:327–356
Toth J (1971) State equation of the solid-gas interface layers. Acta Chim Hung 69:311–328
Vargas AM, Cazetta AL, Martins AC, Moraes JC, Garcia EE, Gauze GF, Costa WF, Almeida VC (2012) Kinetic and equilibrium studies: adsorption of food dyes Acid Yellow 6, Acid Yellow 23, and Acid Red 18 on activated carbon from flamboyant pods. Chem Eng J 181:243–250
Wang XS, Qin Y, Li ZF (2006) Biosorption of zinc from aqueous solutions by rice bran: kinetics and equilibrium studies. Sep Sci Technol 41(4):747–756
Wang G, Chang Q, Zhang M, Han X (2013) Effect of pH on the removal of Cr (III) and Cr (VI) from aqueous solution by modified polyethyleneimine. React Funct Polym 73(11):1439–1446
Wang L, Shi C, Pan L, Zhang X, Zou J-J (2020) Rational design, synthesis, adsorption principles and applications of metal oxide adsorbents: a review. Nanoscale 12(8):4790–4815
Wołowiec M, Komorowska-Kaufman M, Pruss A, Rzepa G, Bajda T (2019) Removal of heavy metals and metalloids from water using drinking water treatment residuals as adsorbents: a review. Minerals 9(8):487
Yan Y, Xu X, Shi C, Yan W, Zhang L, Wang G (2019) Ecotoxicological effects and accumulation of ciprofloxacin in Eichhornia crassipes under hydroponic conditions. Environ Sci Pollut Res Int 26(29):30348–30355
Zhang D, Xu W, Cai J, Cheng S-Y, Ding W-P (2020) Citric acid-incorporated cellulose nanofibrous mats as food materials-based biosorbent for removal of hexavalent chromium from aqueous solutions. Int J Biol Macromol 149:459–466
Zhou Y, Hu Y, Huang W, Cheng G, Cui C, Lu J (2018) A novel amphoteric β-cyclodextrin-based adsorbent for simultaneous removal of cationic/anionic dyes and bisphenol A. Chem Eng J 341:47–57
Zhuang S, Wang J (2019) Removal of cobalt ion from aqueous solution using magnetic graphene oxide/chitosan composite. Environ Prog Sustain Energy 38(s1):S32–S41
Zhuang S, Zhu K, Wang J (2020) Fibrous chitosan/cellulose composite as an efficient adsorbent for Co (II) removal. J Clean Prod 285:124911
Funding
No funding was available for this research.
Author information
Authors and Affiliations
Contributions
COA contributed to conceptualization, data curation, formal analysis, investigation, validation and writing—original draft, review and editing. DMH and SF performed data curation, formal analysis, visualization, validation and supervision. AH was involved in data curation, formal analysis, visualization, validation, supervision and writing—original draft, review and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no known competing interest.
Ethical approval
This work does not contain any data from human participants or animals. The authors, therefore, claim compliance with the ethical standards.
Consent to participate
This is not applicable, as this study does not involve human subjects.
Consent to publish
This is not applicable, as this study does not involve human subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aniagor, C.O., Hussein, D.M., Farag, S. et al. Application of novel organic acid-modified biosorbent in the sequestration of aqueous zinc ion. Sustain. Water Resour. Manag. 9, 61 (2023). https://doi.org/10.1007/s40899-023-00840-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40899-023-00840-3