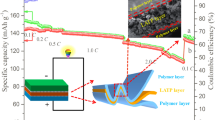
Abstract
With the proliferation of energy storage and power applications, electric vehicles particularly, solid-state batteries are considered as one of the most promising strategies to address the ever-increasing safety concern and high energy demand of power devices. Here, we demonstrate the Al4B2O9 nanorods-modified poly(ethylene oxide) (PEO)-based solid polymer electrolyte (ASPE) with high ionic conductivity, wide electrochemical window, decent mechanical property and nonflammable performance. Specifically, because of the longer-range ordered Li+ transfer channels conducted by the interaction between Al4B2O9 nanorods and PEO, the optimal ASPE (ASPE-1) shows excellent ionic conductivity of 4.35×10−1 and 3.1×10−1 S cm−1 at 30 and 60°C, respectively. It also has good electrochemical stability at 60°C with a decomposition voltage of 5.1 V. Besides, the assembled LiFePO4//Li cells show good cycling performance, delivering 155 mA h g−1 after 300 cycles at 1 C under 60°C, and present excellent low temperature adaptability, retaining over 125 mA h g−1 after 90 cycles at 0.2 C under 30°C. These results verify that the addition of Al4B2O9 nanorods can effectively promote the integrated performance of solid polymer electrolyte.
摘要随
着储能设备和电力驱动产品的激增, 特别是电动汽车的大规模推广应用, 全固态电池被认为是最有可能解决电动设备日益严峻的安全问题和高能量密度需求的策略之一. 本文报道了一种Al4B2O9纳米棒改性的聚环氧乙烷(PEO)基固体聚合物电解质(ASPE), 其具有高离子电导率、 宽电化学窗口、 良好的机械性能和阻燃性能. 具体来说, 因为Al4B2O9纳米棒与PEO之间的相互作用会产生更长距离的Li+传递通道, 所以ASPE-1具有优异的离子电导率, 在30和60°C下, 分别达到3.1×10−4和4.35×10−5 S cm−1. 此外, ASPE-1在60°C时表现出良好的电化学稳定性, 分解电压达5.1 V. 另外, ASPE-1在LiFePO4//Li半电池具有良好的循环倍率性能, 当温度为60°C、 电流密度为1 C时, 经300次循环充放电后, 放电比容量为155 mA h g−1. 当温度为30°C、 电流密度为0.2 C时, 经90次循环充放电后, 放电比容量为125 mA h g−1, 表现出良好的低温性能. 这些实验结果证明Al4B2O9纳米棒可以有效地提高固体聚合物电解质的综合性能.
Similar content being viewed by others
References
Li M, Lu J, Chen Z, et al. 30 years of lithium-ion batteries. Adv Mater, 2018, 30: 1800561
Cao Y, Li M, Lu J, et al. Bridging the academic and industrial metrics for next-generation practical batteries. Nat Nanotechnol, 2019, 14: 200–207
Chen X, Ma Y. Wearable lithium ion batteries based on carbon nanotubes and graphene. Adv Mater Technol, 2018, 3: 1800041
Wang Y, Chen C, Xie H, et al. 3D-printed all-fiber Li-ion battery toward wearable energy storage. Adv Funct Mater, 2017, 27: 1703140
Palacin MR, de Guibert A. Why do batteries fail? Science, 2016, 351: 1253292
Wang J, Yamada Y, Sodeyama K, et al. Fire-extinguishing organic electrolytes for safe batteries. Nat Energy, 2018, 3: 22–29
Lin D, Liu Y, Cui Y. Reviving the lithium metal anode for high-energy batteries. Nat Nanotech, 2017, 12: 194–206
Fan L, Wei S, Li S, et al. Recent progress of the solid-state electrolytes for high-energy metal-based batteries. Adv Energy Mater, 2018, 8: 1702657
Manthiram A, Yu X, Wang S. Lithium battery chemistries enabled by solid-state electrolytes. Nat Rev Mater, 2017, 2: 16103
Nolan AM, Zhu Y, He X, et al. Computation-accelerated design of materials and interfaces for all-solid-state lithium-ion batteries. Joule, 2018, 2: 2016–2046
Kamaya N, Homma K, Yamakawa Y, et al. A lithium superionic conductor. Nat Mater, 2011, 10: 682–686
Keller M, Appetecchi GB, Kim GT, et al. Electrochemical performance of a solvent-free hybrid ceramic-polymer electrolyte based on Li7La3Zr2O12 in P(EO)15LiTFSI. J Power Sources, 2017, 353: 287–297
Meesala Y, Jena A, Chang H, et al. Recent advancements in Li-ion conductors for all-solid-state Li-ion batteries. ACS Energy Lett, 2017, 2: 2734–2751
Sun C, Liu J, Gong Y, et al. Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy, 2017, 33: 363–386
Wang C, Xie H, Ping W, et al. A general, highly efficient, high temperature thermal pulse toward high performance solid state electrolyte. Energy Storage Mater, 2019, 17: 234–241
Sun YZ, Huang JQ, Zhao CZ, et al. A review of solid electrolytes for safe lithium-sulfur batteries. Sci China Chem, 2017, 60: 1508–1526
Sharafi A, Kazyak E, Davis AL, et al. Surface chemistry mechanism of ultra-low interfacial resistance in the solid-state electrolyte Li7La3Zr2O12. Chem Mater, 2017, 29: 7961–7968
Nam YJ, Cho SJ, Oh DY, et al. Bendable and thin sulfide solid electrolyte film: a new electrolyte opportunity for free-standing and stackable high-energy all-solid-state lithium-ion batteries. Nano Lett, 2015, 15: 3317–3323
Bachman JC, Muy S, Grimaud A, et al. Inorganic solid-state electrolytes for lithium batteries: mechanisms and properties governing ion conduction. Chem Rev, 2016, 116: 140–162
Dai J, Yang C, Wang C, et al. Interface engineering for garnet-based solid-state lithium-metal batteries: Materials, structures, and characterization. Adv Mater, 2018, 30: 1802068
Fu KK, Gong Y, Hitz GT, et al. Three-dimensional bilayer garnet solid electrolyte based high energy density lithium metal-sulfur batteries. Energy Environ Sci, 2017, 10: 1568–1575
Dong D, Zhou B, Sun Y, et al. Polymer electrolyte glue: A universal interfacial modification strategy for all-solid-state Li batteries. Nano Lett, 2019, 19: 2343–2349
Chen L, Li Y, Li SP, et al. PEO/garnet composite electrolytes for solid-state lithium batteries: From “ceramic-in-polymer” to “polymer-in-ceramic”. Nano Energy, 2018, 46: 176–184
Yang K, Zhang Z, Liao Z, et al. Organic ionic plastic crystal-polymer solid electrolytes with high ionic conductivity and mechanical ability for solid-state lithium ion batteries. ChemistrySelect, 2018, 3: 12595–12599
Zhang W, Nie J, Li F, et al. A durable and safe solid-state lithium battery with a hybrid electrolyte membrane. Nano Energy, 2018, 45: 413–419
Hu J, Wang W, Zhou B, et al. Poly(ethylene oxide)-based composite polymer electrolytes embedding with ionic bond modified nanoparticles for all-solid-state lithium-ion battery. J Membrane Sci, 2019, 575: 200–208
Yuan B, Luo G, Liang J, et al. Self-assembly synthesis of solid polymer electrolyte with carbonate terminated poly(ethylene glycol) matrix and its application for solid state lithium battery. J Energy Chem, 2019, 38: 55–59
Zhou W, Wang Z, Pu Ys, et al. Double-layer polymer electrolyte for high-voltage all-solid-state rechargeable batteries. Adv Mater, 2019, 31: 1805574
Tan SJ, Zeng XX, Ma Q, et al. Recent advancements in polymer-based composite electrolytes for rechargeable lithium batteries. Electrochem Energ Rev, 2018, 1: 113–138
Xi J. Electrochemistry study on PEO-LiClO4-ZSM5 composite polymer electrolyte. Chin Sci Bull, 2004, 49: 785
Yao P, Zhu B, Zhai H, et al. PVDF/palygorskite nanowire composite electrolyte for 4 V rechargeable lithium batteries with high energy density. Nano Lett, 2018, 18: 6113–6120
Ji X, Zeng H, Gong X, et al. A Si-doped flexible self-supporting comb-like polyethylene glycol copolymer (Si-PEG) film as a polymer electrolyte for an all solid-state lithium-ion battery. J Mater Chem A, 2017, 5: 24444–24452
Lopez J, Sun Y, Mackanic DG, et al. A dual-crosslinking design for resilient lithium-ion conductors. Adv Mater, 2018, 30: 1804142
Grewal MS, Tanaka M, Kawakami H. Bifunctional poly(ethylene glycol) based crosslinked network polymers as electrolytes for all-solid-state lithium ion batteries. Polym Int, 2019, 68: 684–693
Huang Z, Pan Q, Smith DM, et al. Plasticized hybrid network solid polymer electrolytes for lithium-metal batteries. Adv Mater Interfaces, 2019, 6: 1801445
Wei Z, Zhang Z, Chen S, et al. UV-cured polymer electrolyte for LiNi0.85Co0.05Al0.1O2//Li solid state battery working at ambient temperature. Energy Storage Mater, 2019, 22: 337–345
Zhang D, Zhang L, Yang K, et al. Superior blends solid polymer electrolyte with integrated hierarchical architectures for all-solidstate lithium-ion batteries. ACS Appl Mater Interfaces, 2017, 9: 36886–36896
Weston J, Steele B Effects of inert fillers on the mechanical and electrochemical properties of lithium salt-poly(ethylene oxide) polymer electrolytes. Solid State Ion, 1982, 7: 75–79
Appetecchi GB, Croce F, Dautzenberg G, et al. Dry composite polymer electrolytes for lithium batteries. MRS Proc, 1997, 496: 511–516
Croce F, Settimi L, Scrosati B Superacid ZrO2-added, composite polymer electrolytes with improved transport properties. Electrochem Commun, 2006, 8: 364–368
Niu C, Liu J, Chen G, et al. Anion-regulated solid polymer electrolyte enhances the stable deposition of lithium ion for lithium metal batteries. J Power Sources, 2019, 417: 70–75
Lin D, Yuen PY, Liu Y, et al. A silica-aerogel-reinforced composite polymer electrolyte with high ionic conductivity and high modulus. Adv Mater, 2018, 30: 1802661
Liu Y, Lee JY, Hong L Functionalized SiO2 in poly(ethylene oxide)-based polymer electrolytes. J Power Sources, 2002, 109: 507–514
Xiong HM, Wang ZD, Xie DP, et al. Stable polymer electrolytes based on polyether-grafted ZnO nanoparticles for all-solid-state lithium batteries. J Mater Chem, 2006, 16: 1345–1349
Reddy MJ, Chu PP, Kumar JS, et al. Inhibited crystallization and its effect on conductivity in a nano-sized Fe oxide composite PEO solid electrolyte. J Power Sources, 2006, 161: 535–540
Zhu K, Liu Y, Liu J. A fast charging/discharging all-solid-state lithium ion battery based on PEO-MIL-53(Al)-LiTFSI thin film electrolyte. RSC Adv, 2014, 4: 42278–42284
Shen L, Wu HB, Liu F, et al. Creating lithium-ion electrolytes with biomimetic ionic channels in metal-organic frameworks. Adv Mater, 2018, 30: 1707476
Liu L, Lyu J, Mo J, et al. Flexible, high-voltage, ion-conducting composite membranes with 3D aramid nanofiber frameworks for stable all-solid-state lithium metal batteries Sci China Mater, 2020, 63: 703–718
Pan Q, Zheng Y, Kota S, et al. 2D MXene-containing polymer electrolytes for all-solid-state lithium metal batteries Nanoscale Adv, 2019, 1: 395–402
Lin Y, Wang X, Liu J, et al. Natural halloysite nano-clay electrolyte for advanced all-solid-state lithium-sulfur batteries Nano Energy, 2017, 31: 478–485
Tang C, Hackenberg K, Fu Q, et al. High ion conducting polymer nanocomposite electrolytes using hybrid nanofillers. Nano Lett, 2012, 12: 1152–1156
Cao L, Wu H, Yang P, et al. Graphene oxide-based solid electrolytes with 3D prepercolating pathways for efficient proton transport. Adv Funct Mater, 2018, 28: 1804944
Liu W, Lee SW, Lin D, et al. Enhancing ionic conductivity in composite polymer electrolytes with well-aligned ceramic nanowires. Nat Energy, 2017, 2: 17035
Zhang X, Xie J, Shi F, et al. Vertically aligned and continuous nanoscale ceramic-polymer interfaces in composite solid polymer electrolytes for enhanced ionic conductivity. Nano Lett, 2018, 18: 3829–3838
Bae J, Li Y, Zhang J, et al. A 3D nanostructured hydrogel-framework-eerived high-performance composite polymer lithium-ion electrolyte. Angew Chem Int Ed, 2018, 57: 2096–2100
Fu KK, Gong Y, Dai J, et al. Flexible, solid-state, ion-conducting membrane with 3D garnet nanofiber networks for lithium batteries. Proc Natl Acad Sci USA, 2016, 113: 7094–7099
Sheng O, Jin C, Luo J, et al. Mg2B2O5 nanowire enabled multifunctional solid-state electrolytes with high ionic conductivity, excellent mechanical properties, and flame-retardant performance. Nano Lett, 2018, 18: 3104–3112
Zheng M, Wu K, Liang H, et al. Microstructure and mechanical properties of aluminum borate whisker-reinforced magnesium matrix composites. Mater Lett, 2002, 57: 558–564
Zhou D, Mei X, Ouyang J. Ionic conductivity enhancement of polyethylene oxide-LiClO4 electrolyte by adding functionalized multi-walled carbon nanotubes. J Phys Chem C, 2011, 115: 16688–16694
Li M, Wahyudi W, Kumar P, et al. Scalable approach to construct free-standing and flexible carbon networks for lithium-sulfur battery. ACS Appl Mater Interfaces, 2017, 9: 8047–8054
Zhai H, Xu P, Ning M, et al. A flexible solid composite electrolyte with vertically aligned and connected ion-conducting nanoparticles for lithium batteries. Nano Lett, 2017, 17: 3182–3187
Porcarelli L, Shaplov AS, Bella F, et al. Single-ion conducting polymer electrolytes for lithium metal polymer batteries that operate at ambient temperature. ACS Energy Lett, 2016, 1: 678–682
Li C, Qin B, Zhang Y, et al. Single-ion conducting electrolyte based on electrospun nanofibers for high-performance lithium batteries. Adv Energy Mater, 2019, 9: 1803422
Lin D, Liu W, Liu Y, et al. High ionic conductivity of composite solid polymer electrolyte via in situ synthesis of monodispersed SiO2 nanospheres in poly(ethylene oxide). Nano Lett, 2016, 16: 459–465
Angulakshmi N, Kumar RS, Kulandainathan MA, et al. Composite polymer electrolytes encompassing metal organic frame works: a new strategy for all-solid-state lithium batteries. J Phys Chem C, 2014, 118: 24240–24247
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (51804344), the Huxiang Youth Talent Support Program (2019RS2002), the Innovation and Entrepreneurship Project of Hunan Province, China (2018GK5026), the Innovation-Driven Project of Central South University (2020CX027), and Guangdong YangFan Plan for Postdoctor Program.
Author information
Authors and Affiliations
Contributions
Guo X designed and engineered the samples, wrote the paper with support from Wang J. All authors contributed to the general discussion.
Corresponding author
Ethics declarations
Conflict of interest The authors declare that they have no conflict of interest.
Additional information
Xiqiang Guo obtained his BSc degree (2017) in metallurgical engineering, and then pursued his master degree (2017-2020) in Professor Xinhai Li and Associate Professor Jiexi Wang’s group at Central South University (China). He mainly studies the efficient synthesis of 1D nanomaterials and their applications in enhancing the solid polymer electrolytes for lithium-ion batteries. Besides, he has been a visiting student in Zaiping Guo’s group at the University of Wollongong (Australia), where he participated in the separator research for lithium-sulfur batteries.
Jiexi Wang received his BSc degree (2010) in metallurgical engineering and PhD degree (2015) in physical chemistry of metallurgy from Central South University (China). After working as a postdoctoral fellow at Hong Kong University of Science & Technology and The University of Hong Kong, he started his independent research career as an associate professor at Central South University (China) in 2017. His research focuses on the green synthesis and application of nonferrous-based materials and composites for energy storage, such as high-power/high-energy lithium/sodium ion batteries, and supercapacitors. He has published about 100 scientific papers with ∼3,800 citations (h-index=38).
Supplementary information
Rights and permissions
About this article
Cite this article
Guo, X., Peng, W., Wu, Y. et al. Al4B2O9 nanorods-modified solid polymer electrolytes with decent integrated performance. Sci. China Mater. 64, 296–306 (2021). https://doi.org/10.1007/s40843-020-1393-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40843-020-1393-2