Abstract
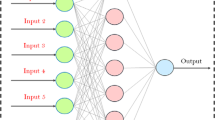
Present work reports the results of the corrosion current density of mild steel in acidic solution as a function of acid concentration, inhibitor concentration, and temperature. Phenylthiourea was used as concentration a corrosion inhibitor. The work is focused on estimating the optimum mathematical equation and the ANN architecture. Corrosion rate (corrosion current density) was the response or the dependent variable, while inhibitor concentration (0.1, 0.75, and 1 g/l), acid concentrations (1, 3, and 5 M), and different temperatures (303, 313, 323, and 333 K) were the factors or the independent variables of the models. Five mathematical equations and five ANN architectures were suggested. Models were developed and estimated with the aid of a computer program. Linear, Linear – Logarithmic, Logarithmic – Linear, polynomial – individual effect, and polynomial – interaction effect model were estimated. Results show that the polynomial – interaction effect equation was able to accurately predict the measured data with acceptable correlation coefficient (R2 = 0.9803). Linear Model, Multi-Layer Perceptrons, and Radial Basis Function were trained and tested. Multi-Layer Perceptrons with three inputs—multi hidden layers—three outputs (MLP 3:3-6-1:1) was the best ANN with significant performance (R2 = 0.9529). Sensitivity analysis was carried out to study the effect of unavailability of each variable. Both mathematical and ANN analyses showed that the temperature has the most significant influence on the corrosion current density than the other two variables. The temperature individual effect coefficient was 840.6 as compared with 88.5 and − 170.5 for acid concentration and inhibitor concentration, respectively. Interaction effects between variables were also addressed.








Similar content being viewed by others
References
Benabdellah M, Touzani R, Dafali A, Hammouti B, El Kadiri S (2007) Ruthenium–ligand complex, an efficient inhibitor of steel corrosion in H3PO4 media. Mater Lett 61:1197–1204
Verma C, Olasunkanmi LO, Obot IB, Ebenso EE, Quraishi MA (2016) 2, 4-Diamino-5- (phenylthio)-5 H-chromeno [2, 3-b] pyridine-3-carbonitriles as green and effective corrosion inhibitors: gravimetric, electrochemical, surface morphology and theoretical studies. RSC Adv 6:53933–53948
Khadom AA, Musa AY, Kadhum AA, Mohamad AB, Takriff MS (2010) Adsorption kinetics of 4-amino-5-phenyl-4H-1, 2, 4-triazole-3-thiol on mild steel surface inhibitor. Port Electrochim Acta 28:221–230
Khadom AA, Yaro AS (2011) Protection of low carbon steel in phosphoric acid by potassium iodide. Prot Met Phys Chem Surf 2011:662–669
Musa AY, Kadhum AH, Mohamad AB, Takriff MS, Daud AR, Kamarudin SK (2010) On the inhibition of mild steel corrosion by 4-amino-5-phenyl-4H-1, 2, 4-trizole-3-thiol. Corros Sci 52:526–533
Ahmed SK, Ali WB, Khadom AA (2019) Synthesis and characterization of new triazole derivatives as corrosion inhibitors of carbon steel in acidic medium. J Bio Tribo Corros 5:15
Abod BM, Al-Alawy RM, Khadom AA et al (2019) Experimental and theoretical studies for tobacco leaf extract as an eco-friendly inhibitor for steel in saline water. J Bio Tribo Corros 5:75
Noor EA (2009) Evaluation of inhibitive action of some quaternary N-heterocyclic compounds on the corrosion of Al–Cu alloy in hydrochloric acid. Mater Chem Phys 114:533–541
Yaro AS, Khadom AA, Ibraheem HF (2011) Peach juice as an anti-corrosion inhibitor of mild steel. Anti-Corros Methods Mater 58(3):116–124
Khadom AA, Ahmed NA, Nagham AA (2017) Xanthium strumarium leaves extracts as a friendly corrosion inhibitor of low carbon steel in HCl: kinetics and mathematical studies. S Afr J Chem Eng 25:13–21
Fadhil AA, Ismael MH, Farhan SN et al (2019) Corrosion of crude oil distillation column: kinetics and mathematical views. J Bio Tribo Corros 5:80
Naseri E, Hajisafari M, Kosari A, Talari M, Hosseinpour S, Davoodi A (2018) Inhibitive effect of Clopidogrel as a green corrosion inhibitor for mild steel; statistical modeling and quantum Monte Carlo simulation studies. J Mol Liq 269:193–202
Anadebe VC, Onukwuli OD, Omotioma M, Okafor NA (2019) Experimental, theoretical modeling and optimization of inhibition efficiency of pigeon pea leaf extract as anti-corrosion agent of mild steel in acid environment. Mater Chem Phys 233:120–132
Suleiman IY, Mohammed AT, Sirajo MZ, Ochu SR (2018) Synergistic effect and statistical model of Terminalia avicennioides as anti-corrosion inhibitor of steel pipelines in acidic environment. J Bio Tribo Corros 4:48
Badiea AM, Mohana KN (2008) Effect of fluid velocity and temperature on the corrosion mechanism of low carbon steel in industrial water in the absence and presence of 2-hydrazino benzothiazole. Korean J Chem Eng 25(6):1292–1299
Dua V (2011) An artificial neural network approximation based decomposition approach for parameter estimation of system of ordinary differential equations. Comput Chem Eng 35:545
Rashidi AM (2012) A galvanostatic modeling for preparation of electrodeposited nanocrystalline coatings by control of current density. J Mater Sci Technol 28:1071
Moral H, Aksoy A, Gokcay CF (2008) Modeling of the activated sludge process by using artificial neural networks with automated architecture screening. Comput Chem Eng 32:2471
Fahmi I, Cremaschi S (2012) Process synthesis of biodiesel production plant using artificial neural networks as the surrogate models. Comput Chem Eng 46:105
Ahmad AL, Azid IA, Yusof AR, Seetharamu KN (2004) Emission control in palm oil mills using artificial neural network and genetic algorithm. Comput Chem Eng 28:2709
Platt JA (1991) A resource-allocating network for function interpolation. Neural Comput 3:213
Salami ES, Ehetshami M, Karimi-Jashni A et al (2016) A mathematical method and artificial neural network modeling to simulate osmosis membrane’s performance. Model Earth Syst Environ 2:1–11
Khadom AA, Yaro AS, Altaie AS, Abdul Amir HK (2009) Electrochemical, activations and adsorption studies for the corrosion of low carbon steel in acidic media. Port Electrochem Acta 27:699–712
Hassan KH, Khadom AA, Kurshed NH (2016) Experimental and mathematical studies for corrosion reaction of mild steel—sulfuric acid—friendly inhibitor system. Eur J Sci Res 139:163–170
Mahmood AK, Khadom AA (2016) Erosion-corrosion of low-carbon steel in the absence and presence of slurry in saline water: kinetic and mathematical views. J Fail Anal Prev 16:1071–1081
Sousa SV, Martins FG, Alvim-Ferraz MC, Pereira MC (2007) Multiple linear regression and artificial neural networks based on principal components to predict ozone concentrations. Environ Model Softw 22:97
Khadom AA, Yaro AS, Kadum AAH, AlTaie AS, Musa AY (2009) The effect of temperature and acid concentration on corrosion of low carbon steel in hydrochloric acid media. Am J Appl Sci 6(7):1403–1409
Khadom AA (2015) Kinetics and synergistic effect of iodide ion and naphthylamine for the inhibition of corrosion reaction of mild steel in hydrochloric acid. React Kinet Mech Catal 115:463–481
Khadom AA, Abd AN, Ahmed NA (2018) Potassium iodide as a corrosion inhibitor of mild steel in hydrochloric acid: kinetics and mathematical studies. J Bio Tribo Corros 4:17
Hassan KH, Khadom AA, Kurshed NH (2016) Citrus aurantium leaves extracts as a sustainable corrosion inhibitor of mild steel in sulfuric acid. S Afr J Chem Eng 22:1–5
Musa AY, Khadom AA, Kadhum AAH et al (2012) The role of 4-amino-5-phenyl-4H-1,2,4-triazole-3-thiol in the inhibition of nickel–aluminum bronze alloy corrosion: electrochemical and DFT studies. Res Chem Intermed 38:91–103
Acknowledgments
Authors would like to thank College of Engineering in the University of Diyala for continues support and facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest arising from the involvement of other parties either internal or external to the University.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khadom, A.A., Mahdi, M.S. & Mahood, H.B. Mathematical Regression and Artificial Neural Network for Prediction of Corrosion Inhibition Process of Steel in Acidic Media. J Bio Tribo Corros 6, 92 (2020). https://doi.org/10.1007/s40735-020-00390-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-020-00390-7