Abstract
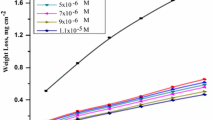
Corrosion inhibition of mild steel in 1 M HCl was investigated in the absence and presence of potassium iodide (KI) as a corrosion inhibitor. The effects of temperature and inhibitor concentration were studied using weight loss technique. The result obtained shown that KI act as an inhibitor for mild steel in HCl and decreases the corrosion rate. The inhibition performance was found to increases with increase in inhibitor concentration and temperature. Higher inhibition efficiency was 94% at higher level of inhibitor concentration and temperature. The adsorption of KI on mild steel surface was found to fellow Langmuir adsorption isotherm. The values of the free energy of adsorption were between − 20 and − 40 kJ/mol that is indication of mixed mode of physical and chemical adsorption. Mathematical models were also suggested to correlate the corrosion rate data with independent variables.







Similar content being viewed by others
References
Popova A (2007) Temperature effect on mild steel corrosion in acid media in presence of azoles. Corros Sci 49:2144–2158
Musa AY, Khadom AA, Kadhum AA (2010) Kinetic approach to mild steel Corrosion Inhibition by 4-amino-5-phenyl-4H-1, 2, 4-triazole-3-thiol. J Taiwan Inst Chem Eng 41:126–128
Khadom AA, Yaro AS, Kadhum AA (2010) Adsorption mechanism of benzotriazole for corrosion inhibition of copper–nickel alloy in hydrochloric acid. J Chil Chem Soc 55:150–152
Bouayed M, Rabaa H, Srhiri A, Saillard J, Ben Bachir A (1999) Experimental and theoretical study of organic corrosion inhibitors on iron in acidic medium. Corros Sci 41:501–517
Quraishi MA, Sardar R (2002) Corrosion inhibition of mild steel in acid solutions by some aromatic oxadiazoles. Mater Chem Phys 78:425–431
Khaled KF (2011) Studies of the corrosion inhibition of copper in sodium chloride solutions using chemical and electrochemical measurements. Mater Chem Phys 125:427–433
Khadom AA, Abdul-Hadi AA (2014) Kinetic and mathematical approaches to the corrosion of mild steel in nitric acid. React Kinet Mech Catal 112:15–26
Khadom AA, Hassan AF, Abod BM (2015) Evaluation of environmentally friendly inhibitor for galvanic corrosion of steel–copper couple inpetroleum waste wate. Process Saf Environ Prot 98:93–101
Khadom AA, Yaro AS, Kadum AA, AlTaie AS, Musa AY (2009) The effect of temperature and acid concentration on corrosion of low carbon steel in hydrochloric acid media. Am J Appl Sci 6:1403–1409
Putilova LN, Balezin SA, Barannik VP (1960) Metallic corrosion inhibitors. Pergamon Press, New York
Zarrouk A, Zarrok H, Salghi R, Hammouti B, Bentiss F, Touir R, Bouachrine M (2013) Evaluation of N-containing organic compound as corrosion inhibitor for carbon steel in phosphoric acid. J Mater Environ Sci 4:177–192
Szauer T, Brandt A (1981) Adsorption of oleates of various amines on iron in acidic solution. Electrochim Acta 26:1253–1256
Khaled KF (2008) New synthesized guanidine derivative as a green corrosion inhibitor for mild steel in acidic solutions. Int J Electrochem Sci 3:462–475
Fouda AS, Wahed HA (2016) Corrosion inhibition of copper in HNO3 solution using thiophene and its derivatives. Arab J Chem 9(Supplement 1):S91–S99
Fekry AM, Ameer MA (2010) Corrosion inhibition of mild steel in acidic media using newly synthesized heterocyclic organic molecules. Int J Hydrog Energy 35:7641–7646
Bentiss F, Lebrini M, Lagrenee M (2005) Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in mild steel/2,5-bis(n-thienyl)-1,3,4-thiadiazoles/hydrochloric acid system. Corros Sci 47:2915–2931
Noor EA, Al-Moubaraki AH (2008) Thermodynamic study of metal corrosion and inhibitor adsorption processes in mild steel/1-methyl-4[4 (-X)-styryl pyridinium iodides/hydrochloric acid systems. Mater Chem Phys 110:145–154
Benabdellah M, Touzani R, Dafali A, Hammouti M, El Kadiri S (2007) Ruthenium–ligand complex, an efficient inhibitor of steel corrosion in H3PO4 media. Mater Lett 61:1197–1204
Mu G, Li X, Liu G (2005) Synergistic inhibition between tween 60 and NaCl on the corrosion of cold rolled steel in 0.5 M sulfuric acid. Corros Sci 47:1932–1952
Prathibha BS, Kotteeswaran P, Bheema Raju V (2012) Study on the inhibition of mild steel corrosion by N, N- dimethylN-(2-phenoxyethyl) dodecan-1-aminiumbromide in HCl Medium. IOSR J Appl Chem 2:61–70
Banerijee G, Malhotra SN (1992) Contribution to adsorption of aromatic amines on mild steel surface from HCl solutions by impedance, UV and Raman spectroscopy. Corrosion 48:10–15
Khadom AA (2015) Kinetics and synergistic effect of halide ion and naphthylamin for inhibition of corrosion reaction of mild steel in hydrochloric acid. React Kinet Mech Catal 115:463–481
Ahmed Z (2006) Principles of corrosion engineering and corrosion control, 1st edn. Butterworth-Heinemann, Oxford
Yaro AA, Al-Jendeel H, Khadom AA (2011) Cathodic protection system of copper–zinc–saline water in presence of bacteria. Desalination 270:193–198
Hassan KH, Khadom AA, Kurshed NH (2016) Experimental and mathematical studies for corrosion reaction of mild steel–sulfuric acid—friendly inhibitor system. Eur J Sci Res 139:163–170
Acknowledgements
The authors would like to thank University of Diyala, Iraq, for continuous support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest arising from the involvement of other parties either internal or external to the University.
Rights and permissions
About this article
Cite this article
Khadom, A.A., Abd, A.N. & Ahmed, N.A. Potassium Iodide as a Corrosion Inhibitor of Mild Steel in Hydrochloric Acid: Kinetics and Mathematical Studies. J Bio Tribo Corros 4, 17 (2018). https://doi.org/10.1007/s40735-018-0133-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-018-0133-4