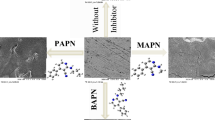
Abstract
5-Amino-3-methyl-1-phenyl-1H-pyrazole-4-carbonitrile (1) was used as a precursor for preparation of 3-methyl-1-phenyl-1,5-dihydro-pyrazolo[3,4-d]pyrimidin-4-one (2) and its derivatives 3–10. The produced compounds were separated, purified, and characterized by FT-IR, 1HNMR, 13C NMR, and mass spectroscopy. The surface properties were studied by measuring the surface tension. The surface tension, critical micelle concentration (CMC), and surface activities were determined. The surface parameters such as surface excess concentration (Γmax), the area per molecule at interface (Amin), and the effectiveness of surface tension reduction (πCMC) were determined from the adsorption isotherms of the prepared compounds. Furthermore, the corrosion inhibition performance of the prepared compounds was evaluated by chemical methods (weight loss) at different inhibitor concentrations and different temperatures. The corrosion inhibition efficiency increased with increase in inhibitor concentration, but decreased with increase in temperature. Thermodynamic activation parameters were computed and discussed to reach the mechanism of the corrosion inhibition process.







Similar content being viewed by others
References
Gupta S, Rodrigues LM, Esteves AP, Oliveira-Campos AMF, José Nascimento MS, Nazareth N, Cidade H, Neves MP, Fernandes E, Pinto M, Cerqueira N, Brás N (2008) Synthesis of N-aryl-5-amino-4-cyanopyrazole derivatives as potent xanthine oxidase inhibitors. Eur J Med Chem 43:771–780
Robins RK (1956) Potential purine antagonists. I. synthesis of some 4,6-substituted pyrazolo[3,4-d]pyrimidines. J Am Chem Soc 78:784–790
Massey V, Komai H, Palmer G, Elion GB (1970) On the mechanism of inactivation of xanthine oxidase by allopurinol and other pyrazolo[3,4-d]pyrimidines. J Biol Chem 245:2837–2844
Helmut R, Martina A, Frank S (2007) Synthesis of dimeric potential anti-gout drugs. Molecules 12:563–575
Shamroukh AH, Rashad AE, Ali HS, Abdel-Megeid FME (2013) Synthesis, reactions and antimicrobial evaluation of β-enaminonitriles of pyrazole. J Heterocyclic Chem 50:758–765
Ghorab MM, Ragab FA, Alqasoumi SI, Alafeefy AM, Aboulmagd SA (2010) Synthesis of some new pyrazolo[3,4-d]pyrimidine derivatives of expected anticancer and radioprotective activity. Eur J Med Chem 45:171–178
Rashad AE, Hegab MI, Abdel-Megeid RE, Fatahala NA, Abdel-Megeid FME (2009) Synthesis and antiviral evaluation of some new pyrazole and fused pyrazolopyrimidine derivatives. Eur J Med Chem 44:1535–1556
Gudmundsson KS, Johns BA, Weatherhead J (2009) Pyrazolopyrimidine and pyrazolotriazines with potent activity against herpesviruses. Bioorg Med Chem Lett 19:5689–5692
Holla BS, Mahalinga MS, Karthekyan MS, Akberali PM, Shetty NS (2006) Synthesis of some novel pyrazolo[3,4-d]pyrimidine derivatives as potential antimicrobial agents. Bioorg Med Chem 14:2040–2047
Ali TE (2009) Synthesis of some novel pyrazolo[3,4-b]pyridine and pyrazolo[3,4-d]pyrimidine derivatives bearing 5,6-diphenyl-1,2,4-triazine moiety as potential antimicrobial agents. Eur J Med Chem 44:4385–4392
Eweas AF, Swelam SA, Fathalla OA, Fawzy NM, Abdel-Moez ShI (2012) Synthesis, anti-microbial evaluation, and molecular modeling of new pyrazolo[3,4-d]pyrimidine derivatives. Med Chem Res 21:3848–3857
Yewale SB, Ganorkar SB, Baheti KG, Shelke RU (2012) Novel 3-substituted-1-aryl-5-phenyl-6-anilinopyrazolo[3,4-d]pyrimidin-4-ones: docking, synthesis and pharmacological evaluation as a potential anti-inflammatory agents. Bioorg Med Chem Lett 22:6616–6620
Vijesh AM, Isloor AM, Telkar S, Peethambar SK, Rai S, Isloor N (2011) Synthesis, characterization and antimicrobial studies of some new pyrazole incorporated imidazole derivatives. Eur J Med Chem 46:3531–3536
Cheng CC, Robins RK (1956) Potential purine antagonists. VI. Synthesis of 1-Alkyl- and 1-Aryl-4-substituted pyrazolo[3,4-d]pyrimidines. J Org Chem 21:1240–1256
Kandeel MM, Mohamed LW, Abd El Hamed MK, Negmeldin AT (2012) Design, synthesis, and antitumor evaluation of novel pyrazolo[3,4-d]pyrimidine derivatives. Scientia Pharm 80:531–545
Davoodnia A, Rahimizadeh M, Rivadeh S, Bakavoli M, Roshani M (2006) Synthesis of new substituted pyrazolo[3,4-d]pyrimidin-4-ones under microwave irradiation. Indian J Heterocycl Chem 16:151–154
Mohamed SF, Youssef MM, Amr AE, Kotb ER (2008) Antimicrobial activities of some synthesized pyridines, oxazines and thiazoles from 3-Aryl-1-(2-naphthyl)prop-2-en-1-ones. Scientia Pharm 76:279–303
Abdallah M, El-Dafrawy AM, Sobhi M, Elwahy AHM, Shaaban MR (2014) Corrosion inhibition of carbon steel in sulphuric acid solutions by novel bisaminothiazole derivatives: chemical, electrochemical and DFT studies. Int J Electrochem Sci 9:2186
Abdel Hameed RS, Al-Shafey HI, Ismail EA, Abu-Nawwas AH (2015) Expired voltaren drugs as corrosion inhibitor for aluminium in hydrochloric acid. Int J Electrochem Sci 10:2098–2109
Abdel Hameed RS, Al Shafey HI, Abu-Nawwas AH (2014) 2-(2,6-Dichloranilino) phenyl acetic acid drugs as eco-friendly corrosion inhibitors for mild steel in 1M HCl. Int J Electrochem Sci 9:6006–6019
Abdel Hameed RS, Alshafey HI, Ali FA, El-Aleem A, Aboul-Magd S, Salah M (2014) Effect of expired drugs as corrosion inhibitors for carbon steel in 1M HCl solution. Int J Pharm Sci Rev Res 27(1):146–152
Ibrahim MM, Abdel Hameed RS, Abu-Nawwas AH, Mohamad SE (2014) Schiff’s bases and their metal complexes as corrosion inhibitors for aluminum alloys in corrosive media. J Organ Chem 10(7):271–281
Abdel Hameed RS, Al-Shafey HI, Ismail EA, Abu-Nawwas AH, ElAzabawy OE (2013) Poly (oxyethylene)terphthylamine as corrosion inhibitors for carbon steel in methanoic acid. Int J Eng Res Appl 3(6):1094–1103
Abdel Hameed RS, Abu-Nawwas AAH, Shehata HA (2013) Nano-composite as corrosion inhibitors for steel alloys in different corrosive media: review article. Adv Appl Sci Res 4(3):126–129
Abdel Hameed RS (2013) Expired drugs as corrosion inhibitors for metals and alloys. Phys Chem 8(4):146–149
Shehata HA, Abdelbary HM, Soliman SA, Salem AM, Atta AM, Abdel-Hameed RS (2012) Evaluation of nonionic surfactants from plastic waste as corrosion inhibitors of carbon steel in 1M HCl. J Mater Sci 8(7):289–302
Abdel Hameed RS, Ismail OM, Eissa FM, Ghanem R (2012) New non ionic polymeric surfactants as corrosion inhibitors for the C-steel alloy in hydrochloric acid corrosive medium. Der Chemica Sinica 3(1):236–248
Abdel Hameed RS, Al-Shafey HI, Abul Magd AS, Shehata HA (2012) Pyrazole derivatives as corrosion inhibitor for C steel in hydrochloric acid medium. J Mater Environ Sci 3(2):294–305
Abd El-Hameed RS (2011) Ranitidine drugs as non-toxic corrosion inhibitor for mild steel in hydrochloric acid medium. Portugaliea Electrochimica Acta 29(4):273–285
Abdel Hameed RS (2011) Aminolysis of polyethylene terephthalate waste as corrosion inhibitor for carbon steel in HCl corrosive medium. Adv Appl Sci Res 2(3):483–499
Griffin WC (1954) Calculation of HLB values of non-ionic surfactants. J Soc Cosmet Chem 5:249
Zaky MF, Badawi AM, El Sabbah I, Abdel Hameed RS, Hendawy ME (2015) Synthesis, characterization and surface activities of cationic polysaccharide (aloe) Schiff base surfactants. J Surfact Deterg 18(3):455–461
Tamaki K (1967) The surface activity of tetrabutylammonium halides in the aqueous solutions. Bull Chem Soc 40:38
Abdullah M, Jahdaly AL, Al-Malyo OA (2015) Corrosion inhibition of carbon steel in hydrochloric acid solution using non-ionic surfactants derived from phenol compounds. Int J Electrochem Sci 10:1754–2740
Hazazi OA, Abdallah M (2013) Prazole compounds as inhibitors for corrosion of aluminum in hydrochloric acid. Int J Electrochem Sci 8:8138
Szauer T, Brandt A (1981) Adsorption of oleates of various amines on iron in acidic solution. Electrochim Acta 26:1253
Abd El-Hameed RS, Al-Shafey HI, Farghaly OA (2012) Corrosion of mild steel in NaCl solutions and effect of recycled plastic waste inhibitors. Res Rev Electrochem 3(2):41–49
Abdel Hameed RS (2018) Cationic surfactant-Zn+2 system as mixed corrosion inhibitors for carbon steel in sodium chloride corrosive medium. Portugaliae Electrochimica Acta 36:1–19
Abdel Hameed RS, Abdallah M (2018) Inhibiting properties of some heterocyclic amide derivatives as potential nontoxic corrosion inhibitors for carbon steel in 1.0 M sulfuric acid. Surf Eng Appl Electrochem J 54:599–606
Abdel Hameed RS (2019) schiff' bases as corrosion inhibitor for aluminum alloy in hydrochloric acid medium. Tenside, Surfactants, Deterg 56(3):209–215
Abdel Hameed RS (2017) Solvent free glycolysis of plastic waste as green corrosion inhibitor for carbon steel in sulfuric acid. J New Mater Electrochem Syst 20:141–149
Aljourani J, Raeissi K, Golozar MA (2009) Benzimidazole and its derivatives as corrosion inhibitors for mild steel in 1 M HCl solution. Corros Sci 51:1836
Abdel Hameed RS, Alfakeera M, Abdallah M (2020) Propoxylated fatty esters as safe inhibitors for corrosion of zinc in hydrochloric acid, protection of metals and physical chemistry of surfaces. Prot Metals Phys Chem Surfaces 65(1):225–232. https://doi.org/10.1134/S2070205120010025
AbdelHameed RS, Abdallah M (2018) Corrosion inhibition of carbon steel in 1 M hydrochloric acid using some pyrazolo[3,4-d]pyrimidnone derivatives. Pro Metals Phys Chem Surf 54(1):113–121
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abdel Hameed, R.S., Al-Bagawi, A.H., Shehata, H.A. et al. Corrosion Inhibition and Adsorption Properties of Some Heterocyclic Derivatives on C-Steel Surface in HCl. J Bio Tribo Corros 6, 51 (2020). https://doi.org/10.1007/s40735-020-00345-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-020-00345-y