Abstract
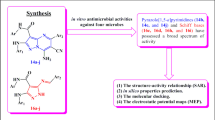
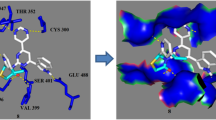
Synthesis of some new pyrazolo[3,4-d]pyrimidine derivatives using readily available starting materials are described. A one-pot multi component cyclocondensation reaction was used to prepare the novel 3-methyl-4-aryl-1-phenyl-1H-pyrazolo[3,4-d]pyrimidine-6-thiol which served as a new starting material for all new compounds in this research. The anti-microbial activities of the selective synthesized compounds have been evaluated. Some of the newly prepared compounds were found to have moderate to strong anti-microbial activity, e.g., compound 4a, 6a, and 8, in comparison to the reference drugs. Molecular modeling of the most three biologically active new compounds 4a, 6a, and 8 compared to the reference drugs tobramycin and fluconazole was carried out using Fieldalign 2.0 software.



Similar content being viewed by others
References
Andreana PR, Liu CC, Schreiber SL (2004) Stereochemical control of the passerini reaction. Org Lett 6(23):4231–4233
Armstrong RW, Combs AP, Tempest PA, Brown SD, Keating TA (1996) Multiple-component condensation strategies for combinatorial library synthesis. Acc Chem Res 29:123–131
Bakavoli M, Bagherzadeh G, Vaseghifar M, Shiri A, Pordel M, Mashreghi M, Pordeli P, Araghi M (2010) Molecular iodine promoted synthesis of new pyrazolo[3,4-d]pyrimidine derivatives as potential anti-bacterial agents. Eur J Med Chem 45(2):647–650
Burke MD, Schreiber SL (2004) A planning strategy for diversity-oriented synthesis. Angew Chem Int Ed Engl 43(1):46–58
Cozzi PG, Rivalta E (2005) Highly enantioselective one-pot, three-component imino-reformatsky reaction. Angew Chem Int Ed Engl 44(23):3600–3603
Denmark SE, Fan Y (2003) The first catalytic, asymmetric α-additions of isocyanides. Lewis-base-catalyzed, enantioselective passerini-type reactions. J Am Chem Soc 125(26):7825–7827
Genin MJ, Biles C, Keiser BJ, Poppe SM, Swaney SM, Tarpley WG, Yagi Y, Romero DL (2000) Novel 1,5-diphenylpyrazole non-nucleoside HIV-1 reverse transcriptase inhibitors with enhanced activity versus the delavirdine-resistant P236L mutant: lead identification and SAR of 3- and 4-substituted derivatives. J Med Chem 43:1034–1040
Gomez L, Hack MD, McClure K, Sehon C, Huang L, Morton M, Li L, Barrett TD, Shankley N, Breitenbucher JG (2007) SAR studies of 1,5-diarylpyrazole-based CCK1 receptor antagonists. Bioorg Med Chem Lett 17(23):6493–6498
Heravi MM, Motamedi R, Bamoharram FF, Seify N (2007) A catalytic method for synthesis of 6-aryl-1H-pyrazolo[3,4-d]pyrimidin-4[5H]-ones by heteropolyacids: H14[NaP5W29MoO110] and H3PMo12O40. Catal Commun 8:1467–1471
Huang YR, Katzenellenbogan JA (2000) Regioselective synthesis of 1,3,5-triaryl-4-alkylpyrazoles: novel ligands for the estrogen receptor. Org Lett 2(18):2833–2836
Jorda R, Havliček L, McNae IW, Walkinshaw MD, Voller J, Sturc A, Navratilova J, Kuzma M, Mistrik M, Bartek J, Strnad M, Krystof V (2011) Ethionamide boosters: synthesis, biological activity, and structure-activity relationships of a series of 1,2,4-oxadiazole EthR inhibitors. J Med Chem 54(8):2980–2993
Lee KY, Kim JM, Kim JN (2003) Regioselective synthesis of 1,3,4,5-tetrasubstituted pyrazoles from Baylis–Hillman adducts. Tetrahedron Lett 44:6737–6740
Liu H, Wang HQ, Liu ZJ (2007) Synthesis and herbicidal activity of novel pyrazolo[3,4-d]pyrimidin-4-one derivatives containing aryloxyphenoxypropionate moieties. Bioorg Med Chem Lett 17(8):2203–2209
Lyga JW, Patera RM, Plummer MJ, Halling BP, Yuhas DA (1994) Synthesis, mechanism of action, and QSAR of herbicidal 3-substituted-2-aryl-4,5,6,7-tetrahydroindazoles. Pestic Sci 42(1):29–36
Penning TD, Talley JJ, Bertenshaw SR, Carter JS, Collins PW, Docter S, Graneto MJ, Lee LF, Malecha JW, Miyashiro JM, Rogers RS, Rogier DJ, Yu SS, Anderson GD, Burton EG, Cogburn JN, Gregory SA, Koboldt CM, Perkins WE, Seibert K, Veenhuizen AW, Zhang YY, Isakson PC (1997) Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benzenesulfonamide (SC-58635, celecoxib). J Med Chem 40 (9):1347–1365
Quiroga J, Trilleras J, Insuasty B, Abonia R, Nogueras M, Marchal A, Cobo J (2008) Microwave-assisted synthesis of pyrazolo[3,4-d]pyrimidines from 2-amino-4, 6-dichloropyrimidine-5-carbaldehyde under solvent-free conditions. Tetrahedron Lett 49:3257–3259
Ramachary DB, Barbas CF (2004) Towards organo-click chemistry: development of organocatalytic multicomponent reactions through combinations of Aldol, Wittig, Knoevenagel, Michael, Diels–Alder and Huisgen cycloaddition reactions. Chem Eur J 10:5323–5331
Ramón DJ, Yus M (2005) Asymmetric multicomponent reactions (AMCRs): the new frontier. Angew Chem Int Ed Engl 44(11):1602–1634
Schenone S, Brullo C, Bruno O, Bondavalli F, Mosti L, Maga G, Crespan E, Carraro F, Manetti F, Tintori C, Botta M (2008) Synthesis, biological evaluation and docking studies of 4-amino substituted 1H-pyrazolo[3,4-d]pyrimidines. Eur J Med Chem 43:2665–2676
Sgouras D, Maragkoudakis P, Petraki K, Gonzalez BM, Eriotou E, Hopoulos S, Kalantzopoulos MG, Tsakalidou E, Mentis A (2004) In vitro and in vivo inhibition of Helicobacter pylori by Lactobacillus casei strain Shirota. Appl Environ Microbiol 70(1):518–526
Swelam SA, Fawzy NM (2004) New synthesis of pyrronolo[2,3-d]pyrimidine indacen-7-one pyrronolo[2,3-d]1,2,3 triazine derivatives. Egypt J Chem 47(3):565–577
Swelam SA, Fathalla OA, Zaki MEA, Aly HF (2004) Synthesis of pyrido[2,1-c] [1,2,4]triazine, 1,2,4 triazolo[4,3-a]pyridine and 2-(substituted-pyrazolyl) nicotino-nitrile and their effect on Biomphalaria alexandrina snail enzymes. Egypt J Chem 47(4):677–692
Swelam SA, Fathalla OA, Zaki MEA (2008) Afindad 53(537):379–385
Terrett NK, Bell AS, Brown D, Ellis P (1996) Sildenafil (VIAGRATM), a potent and selective inhibitor of type 5 cGMP phosphodiesterase with utility for the treatment of male erectile dysfunction. Bioorg Med Chem Lett 6:1819–1824
Tominaga Y, Mastuda Y (1985) Synthesis of heterocyclic compounds using nitro ketene dithioacetal. J Heterocyclic Chem 22:937–949
Wiart C (2007) Goniothalamus species: a source of drugs for the treatment of cancers and bacterial infections. Evid Based Complement Alternat Med 4(3):299–311
Zaki MEA, Fawzy NMS, Swelam A (1999) Synthesis of fused azoles and N-heteroaryl derivatives based on pyrano[2,3-c]pyrazole. Molecules 3:1–8
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eweas, A.F., Swelam, S.A., Fathalla, O.A. et al. Synthesis, anti-microbial evaluation, and molecular modeling of new pyrazolo[3,4-d]pyrimidine derivatives. Med Chem Res 21, 3848–3857 (2012). https://doi.org/10.1007/s00044-011-9911-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-011-9911-y