Abstract
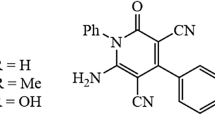
Corrosion inhibition of carbon steel in sulfuric acid solution was performed by three synthesized products named 2-(butylamino)-4-phenylnicotinonitrile (BAPN), 2-(propylamino)-4-phenylnicotinonitrile, and 2-(methylamino)-4-phenylnicotinonitrile using weight-loss method and scanning electron microscopy (SEM). The temperature impact on the inhibition mechanism of the synthesized inhibitors of the carbon steel surface was investigated at various temperatures (20–50 °C) where the inhibitive efficiency diminished with increasing temperatures. The maximum IE of 97.45% was achieved at a temperature of 20 °C, the concentration of BAPN inhibitor of 5×10-4 M, and H2SO4 acid concentration of 0.5 M. The adsorption of inhibitors studied onto the carbon steel surface obeys the Langmuir adsorption isotherm. The SEM surface analysis showed the formation of a protective organic film on the steel surface. The quantum chemical calculations (DFT) supported the experimental results and showed that the inhibition efficiency also depends on the structure of the inhibitor.
Graphic abstract
Three inhibitors products based 2-Aminopyridine derivatives was studied on carbon steel in 0.5 M H2SO4 solution. Results of weight loss measurements and scanning electron microscopy image analyses are reported. In addition, quantum chemical calculations using density functional theory (DFT) were performed to estimate theoretically the reactivity parameters of the inhibitor.





Similar content being viewed by others
References
Abeng F E, Ikpi M E, Anadebe V C and Emori W 2020 Metolazone compound as corrosion inhibitor for api 5l x–52 steel in hydrochloric acid solution Bull. Chem. Soc. Ethiop. 34 407
Nwanonenyi S C, Obasi H C, Chukwujike I C, Chidiebere M A and Oguzie E E 2019 Inhibition of carbon steel corrosion in 1 M H2SO4 using soy polymer and polyvinylpyrrolidone Chem. Afr. 2 277
Chakravarthy M P, Mohana K N and Pradeep Kumar C B 2014 Corrosion inhibition effect and adsorption behaviour of nicotinamide derivatives on mild steel in hydrochloric acid solution Int. J. Ind. Chem. 5 1
Popova A, Christov M and Vasilev A 2015 Mono- and dicationic benzothiazolic quaternary ammonium bromides as mild steel corrosion inhibitors. Part III: influence of the temperature on the inhibition process Corros. Sci. 94 70
Ostapenko G I, Gloukhov P A and Bunev A S 2014 Document investigation of 2-cyclohexenylcycl- ohexanone as steel corrosion inhibitor and surfactant in hydrochloric acid Corros. Sci. 82 265
Özcan M, Toffoli D, Üstünel H and Dehri T 2014 Insights into surface-adsorbat interactions in corrosion inhibition processes at the molecular level Corros. Sci. 80 482
Al-Amiery A, Kassim F, Kadhum A and Mohamad A 2016 Synthesis and characterization of a novel eco-friendly corrosion inhibition for mild steel in 1 M hydrochloric acid Sci. Rep. 6 1
Al-Amiery A A, Kadhum A A, Alobaidy A A and Abu Bakar M 2014 Novel corrosion inhibitor for mild steel in HCl Materials 7 662
Mehiaoui N, Kibou Z, Gallavardin T, Leleu S, Franck X, Mendes R F, et al. 2021 Novel bis-(3-cyano-2-pyridones) derivatives: synthesis and fluorescent properties Res. Chem. Intermed. 2021 1
Mehiaoui N, Kibou Z, Berrichi A and Choukchou-Braham N 2020 Novel synthesis of 3-cyano-2-pyridones derivatives catalyzed by Au–Co/TiO2 Res. Chem. Intermed. 46 5263
Kibou Z, Cheikh N, Choukchou-Braham N and Villemin D 2016 A rapid synthesis of highly functionalized 2-pyridones and 2- aminopyridines via a microwave-assisted multicomponent reaction J. Mater. Environ. Sci. 7 3061
Kibou Z, Cheikh N, Choukchou-Braham N, Villemin D and Lohier J-F 2016 Easy Solventless Synthesis of new mono and bis amino5Hchromeno[3,4-c] pyridin-5-one derivatives from cyanoenaminocoumarin Tetrahedron 72 1653
Abd El-Maksoud S A and Fouda A S 2005 Some pyridine derivatives as corrosion inhibitors for carbon steel in acidic medium Mater. Chem. Phys. 93 84
Ansari K R, Quraishi M A and Singh A 2015 Corrosion inhibition of mild steel in hydrochloric acid by some pyridine derivatives: an experimental and puantum chemical study J. Ind. Eng. Chem. 25 89
Verma C, Olasunkanmi L O, Quadri T W, Sherif E and Ebenso E 2018 Gravimetric, electrochemical, surface morphology, DFT, and Monte Carlo simulation studies on three N-substituted 2-aminopyridine derivatives as corrosion inhibitors of mild steel in acidic medium J. Phys. Chem. C 122 11870
Nouali F, Kibou Z, Boukoussa B, Choukchou-Braham N, Bengueddach A, Villemin D and Hamacha R 2020 Efficient multicomponent synthesis of 2-aminopyridines catalysed by basic mesoporous materials Res. Chem. Intermed. 46 3179
Attar T, Benchadli A and Choukchou-Braham E 2019 Corrosion inhibition of carbon steel in perchloric acid by potassium iodide Int. J. Adv. Chem. 7 35
Attar T, Larabi L and Harek Y 2014 Inhibition effect of potassium iodide on the corrosion of carbon steel (XC 38) in acidic medium Int. J. Adv. Chem. 2 139
Danaee I, Ramesh K S, Rashv Aveic M and Vijayan M 2020 Electrochemical and quantum chemical studies on corrosion inhibition performance of 2,2’-(2-Hydroxyethylimino)bis[N-(alphaalpha-dimethylphenethyl)-N-methylacetamide] on mild steel corrosion in 1M HCl solution J. Mater. Res. 23 1
Pearson R 1988 Absolute electronegativity and hardness: application to inorganic chemistry Inorg. Chem. 27 734
Benhadria N, Messaoudi B and Attar T 2020 The study of the correlation between the detection limit and the energy stability of two antimony complexes by means of conceptual DFT M. J. Chem. 22 111
Attar T, Messaoudi B and Benhadria N 2020 DFT theoretical study of some thiosemicarbazide derivatives with copper Chem. Chem. Technol. 14 20
Musa A Y, Kadhum A A, Mohamed A B and Takriff M S 2011 Molecular dynamics and quantum chemical calculation studies on 4,4-dimethyl-3-thiosemicarbazide as corrosion inhibitor in 2.5 M H2SO4 Mater. Chem. Phys. 129 660
Alaoui K, El Kacimi Y and Galai M 2020 New triazepine carboxylate derivatives: correlation between corrosion inhibition property and chemical structure Int. J. Ind. Chem. 11 23
Guo L, Safi Z S, Kaya S and Shi W 2018 Anticorrosive effects of some thiophene derivatives against the corrosion of iron: a computational study Front. Chem. 6 1
Ait Addi B 2020 Tin corrosion inhibition by molybdate ions in 0.2 M maleic acid solution: electrochemical and surface analytical study Mediterr. J. Chem. 10 465
Li X H, Deng S D and Fu H 2012 Inhibition of the corrosion of steel in HCl, H2SO4 solutions by bamboo leaf extract Corros. Sci. 62 163
Khadom A A and Abd A N 2018 Xanthium strumarium leaves extracts as a friendly corrosion inhibitor of low carbon steel in hydrochloric acid: kinetics and mathematical studies S. Afr. J. Chem. Eng. 25 13
Mobin M, Zehra S and Aslam R 2016 L-Phenylalanine methyl ester hydrochloride as a green corrosion inhibitor for mild steel in hydrochloric acid solution and the effect of surfactant additive RSC Adv. 6 5890
Abdel-Gaber A M, Rahal H T and Beqai F T 2020 Eucalyptus leaf extract as a ecofriendly corrosion inhibitor for mild steel in sulfuric and phosphoric acid solutions Int. J. Ind. Chem. 11 1
Benchadli A, Attar T and Choukchou-Braham E 2019 Inhibition of carbon steel corrosion in perchloric acid solution by povidone iodine Phys. Chem. Res. 7 837
Dagdag O, Safi Z, Hsissou R, Erramli H, ElBouchti M, Wazzan N, et al. 2019 Epoxy pre-polymers as new and effective materials for corrosion inhibition of carbon steel in acidic medium: computational and experimental studies Sci. Rep. 9 1
Attar T, Larabi L and Harek Y 2014 The inhibition effect of potassium iodide on the corrosion of pure iron in sulfuric acid Adv. Chem. 2014 1
Grudic V, Boskovic I, Radonjic D, Jacimovic Z and Knezevic B 2019 The electrochemical behavior of Al alloys in NaCl solution in the presence of pyrazole derivative IJCCE 38 127
Akinbulumo O A, Odejobi O J and Odekanle E L 2020 Thermodynamics and adsorption study of the corrosion inhibition of mild steel by Euphorbia heterophylla L. extract in 1.5 M HCl Res. Mater. 5 100074
Dohare P, Quraishi M A and Obot I B 2018 A combined electrochemical and theoretical study of pyridine-based Schiff bases as novel corrosion inhibitors for mild steel in hydrochloric acid medium J. Chem. Sci. 130 8
Attar T, Benchadli A, Mellal T, Dali Youcef B and Choukchou-Braham E 2021 Use of experimental designs to evaluate the influence of methyl green dye as a corrosion inhibitor for carbon steel in perchloric acid M. J. Chem. 23 60
Attar T, Benchadli A, Messaoudi B, Benhadria N and Choukchou-Braham E 2020 Experimental and theoretical studies of eosin Y dye as corrosion inhibitors for carbon steel in perchloric acid solution Bull. Chem. React. Eng. 15 454
Negm A M, Fouda A S, Abdelwahab S M, Teraze A Y and Shalof R 2019 Cooling system pipeline corrosion behavior after reusing of reverse osmosis reject plant water as feed water source Egypt. J. Chem. 62 257
Gao H, Xie N, Zhang J, Sun J, Zhang J and Jin Z 2020 Synthesis and application of carboxyethylthiosuccinic acid by thiolene click reaction: as a novel rust remover with corrosion inhibition properties J. Chem. Sci. 132 56
Al-Amiery A, Taghried A S, Alazawi K F, Shaker L M, Kadhum A A and Takriff M S 2020 Quantum chemical elucidation on corrosion inhibition efficiency of Schiff base: DFT investigations supported by weight loss and SEM techniques Int. J. Low-Carbon Technol. 15 202
Guocai T and Weizhong Z (2020) Theoretical study of the structure and property of ionic liquids as corrosion inhibitor, density functional theory calculations, Sergio Ricardo De Lazaro, Luis Henrique Da Silveira Lacerda and Renan Augusto Pontes Ribeiro IntechOpen, doi:https://doi.org/10.5772/intechopen.92768
Mashuga M E, Olasunkanmi L, Adekunle A S, Yesudass S, Kabanda M M and Ebenso E E 2015 Adsorption, thermodynamic and quantum chemical studies of 1-hexyl-3-methyl-imidazolium based ionic liquids as corrosion inhibitors for mild steel in HCl Mater. 8 3607
Elshafie A M, Gad E M and Abdel Halim A 2018 Theoretical approach for the performance of 4-mercapto-1-alkylpyridin-1-ium bromide as corrosion inhibitors using DFT Egypt. J. Pet. 27 695
Abd El-Lateef H M 2015 Experimental and computational investigation on the corrosion inhibition characteristics of mild steel by some novel synthesized imines in hydrochloric acid solutions Corros. Sci. 92 104
Akbas E, Celik S, Ergan E and Levent A 2019 Synthesis, characterization, quantum chemical studies and electrochemical performance of new 4,7-dihydrotetrazolo[1,5-a]pyrimidine derivatives J. Chem. Sci. 131 30
Arrousse N, Salim R, Al Houari G, El Hajjaji F, Zarrouk A, Rais Z, et al. 2020 Experimental and theoretical insights on the adsorption and inhibition mechanism of (2E)-2-(acetylamino)-3-(4-nitrophenyl) prop-2-enoic acid and 4-nitrobenzaldehyde on mild steel corrosion J. Chem. Sci. 132 112
Ansari K R and Quraishi M A 2015 Experimental and computational studies of naphthyridine derivatives as corrosion inhibitor for N80 steel in 15% hydrochloric acid Phys. E 69 322
Zhou Y, Guo L, Zhang S, Kaya S, Luo X and Xiang B 2017 Corrosion control of mild steel in 0.1 M H2SO4 solution by benzimidazole and its derivatives: an experimental and theoretical study RSC Adv. 7 23961
Chandrabhan V, Quraishi M A and Neeraj Kumar G 2018 2-(4-{[4-Methyl-6-(1-methyl-1H-1,3-benzodiazol-2-yl)-2-propyl-1H-1,3-benzodiazol-1-yl] methyl} phenyl) benzoic acid as green corrosion inhibitor for mild steel in 1M hydrochloric acid Ain. Shams Eng. J. 9 1225
Acknowledgements
The authors wish to thank Directorate-General for Scientific Research and Technological Development (DGRSDT) and the University of Tlemcen for their financial support.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Attar, T., Nouali, F., Kibou, Z. et al. Corrosion inhibition, adsorption and thermodynamic properties of 2-aminopyridine derivatives on the corrosion of carbon steel in sulfuric acid solution. J Chem Sci 133, 109 (2021). https://doi.org/10.1007/s12039-021-01971-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12039-021-01971-w