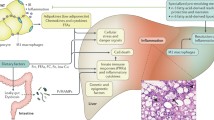
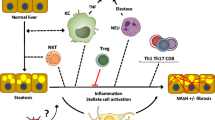
Abstract
Purpose of Review
About 15–25% of patients with simple steatosis of nonalcoholic fatty liver disease progresses to nonalcoholic steatohepatitis (NASH), and the underlying mechanism for this progression has not been elucidated. NASH ultimately could progress to cirrhosis, an irreversible condition.
Recent Findings
Farnesoid X receptor (FXR) has been studied for its role in modulating inflammation, and the expression of FXR is down-regulated during NASH development. FXR deficiency has shown to progress and exacerbate NASH development, and FXR activation has been protective against liver inflammation associated with NASH. The expression of factors in both the adaptive and innate immune response in the liver are regulated in a FXR-dependent and -independent manner.
Summary
Therefore, understanding key signaling pathways of liver inflammation in NASH is important to determine essential components that predispose, progress, or exacerbate NASH. FXR has been identified as a therapeutic target for NASH to prevent liver inflammation.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • of importance, •• of major importance
Li T, Apte U. Bile acid metabolism and signaling in cholestasis, inflammation, and cancer. Adv Pharmacol. 2015;74:263–302.
Matsubara T, Li F, Gonzalez FJ. FXR signaling in the enterohepatic system. Mol Cell Endocrinol. 2013;368(1–2):17–29.
Zhu Y, et al. Fatty liver diseases, bile acids, and FXR. Acta Pharm Sin B. 2016;6(5):409–12.
Zhu Y, Li F, Guo GL. Tissue-specific function of farnesoid X receptor in liver and intestine. Pharmacol Res. 2011;63(4):259–65.
Yuan ZQ, Li KW. Role of farnesoid X receptor in cholestasis. J Dig Dis. 2016;17(8):501–9.
Manley S, Ding W. Role of farnesoid X receptor and bile acids in alcoholic liver disease. Acta Pharm Sin B. 2015;5(2):158–67.
Sinal CJ, et al. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102(6):731–44.
Ganz M, Szabo G. Immune and inflammatory pathways in NASH. Hepatol Int. 2013;7(Suppl 2):771–81.
Meli R, Mattace Raso G, Calignano A. Role of innate immune response in non-alcoholic fatty liver disease: metabolic complications and therapeutic tools. Front Immunol. 2014;5:177.
Bieghs V, Trautwein C. The innate immune response during liver inflammation and metabolic disease. Trends Immunol. 2013;34(9):446–52.
Sutti S, Bruzzi S, Albano E. The role of immune mechanisms in alcoholic and nonalcoholic steatohepatitis: a 2015 update. Expert Rev Gastroenterol Hepatol. 2016;10(2):243–53.
Satapathy SK, Sanyal AJ. Epidemiology and natural history of nonalcoholic fatty liver disease. Semin Liver Dis. 2015;35(3):221–35.
Caligiuri A, Gentilini A and Marra F. Molecular pathogenesis of NASH. Int J Mol Sci. 2016; 17(9).
Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114(4):842–5.
Ibrahim SH, et al. Animal models of nonalcoholic steatohepatitis: eat, delete, and inflame. Dig Dis Sci. 2016;61(5):1325–36.
Bjursell M, et al. Ageing Fxr deficient mice develop increased energy expenditure, improved glucose control and liver damage resembling NASH. PLoS One. 2013;8(5):e64721.
Kong B, et al. Farnesoid X receptor deficiency induces nonalcoholic steatohepatitis in low-density lipoprotein receptor-knockout mice fed a high-fat diet. J Pharmacol Exp Ther. 2009;328(1):116–22.
Szabo G, Iracheta-Vellve A. Inflammasome activation in the liver: focus on alcoholic and non-alcoholic steatohepatitis. Clin Res Hepatol Gastroenterol. 2015;39(Suppl 1):S18–23.
Miura K, et al. Toll-like receptor 2 and palmitic acid cooperatively contribute to the development of nonalcoholic steatohepatitis through inflammasome activation in mice. Hepatology. 2013;57(2):577–89.
Rivera CA, et al. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol. 2007;47(4):571–9.
Miura K, et al. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology. 2010;139(1):323–34. e7
Csak T, et al. Both bone marrow-derived and non-bone marrow-derived cells contribute to AIM2 and NLRP3 inflammasome activation in a MyD88-dependent manner in dietary steatohepatitis. Liver Int. 2014;34(9):1402–13.
Wang YG, et al. The involvement of NLRX1 and NLRP3 in the development of nonalcoholic steatohepatitis in mice. J Chin Med Assoc. 2013;76(12):686–92.
Ganz M, et al. Progression of non-alcoholic steatosis to steatohepatitis and fibrosis parallels cumulative accumulation of danger signals that promote inflammation and liver tumors in a high fat-cholesterol-sugar diet model in mice. J Transl Med. 2015;13:193.
Magee N, Zou A, Zhang Y. Pathogenesis of nonalcoholic steatohepatitis: interactions between liver parenchymal and nonparenchymal cells. Biomed Res Int. 2016;2016:5170402.
Sutti S, et al. Is there a role for adaptive immunity in nonalcoholic steatohepatitis? World J Hepatol. 2015;7(13):1725–9.
Sutti S, et al. Adaptive immune responses triggered by oxidative stress contribute to hepatic inflammation in NASH. Hepatology. 2014;59(3):886–97.
Leroux A, et al. Toxic lipids stored by Kupffer cells correlates with their pro-inflammatory phenotype at an early stage of steatohepatitis. J Hepatol. 2012;57(1):141–9.
Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1(6):a001651.
Wang YD, et al. Farnesoid X receptor antagonizes nuclear factor kappaB in hepatic inflammatory response. Hepatology. 2008;48(5):1632–43.
Daly C, Rollins BJ. Monocyte chemoattractant protein-1 (CCL2) in inflammatory disease and adaptive immunity: therapeutic opportunities and controversies. Microcirculation. 2003;10(3–4):247–57.
Ito M, et al. Longitudinal analysis of murine steatohepatitis model induced by chronic exposure to high-fat diet. Hepatol Res. 2007;37(1):50–7.
Haukeland JW, et al. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J Hepatol. 2006;44(6):1167–74.
• Li L, et al. Activation of farnesoid X receptor downregulates monocyte chemoattractant protein-1 in murine macrophage. Biochem Biophys Res Commun. 2015;467(4):841–6. This manuscript provides a direct role of FXR in the adpative immune response and anti-inflammatory pathways with the down-regulation of Mcp-1.
Zhang S, et al. Farnesoid X receptor agonist WAY-362450 attenuates liver inflammation and fibrosis in murine model of non-alcoholic steatohepatitis. J Hepatol. 2009;51(2):380–8.
Pathil A, et al. Ursodeoxycholyl lysophosphatidylethanolamide improves steatosis and inflammation in murine models of nonalcoholic fatty liver disease. Hepatology. 2012;55(5):1369–78.
Balasubramaniyan N, Ananthanarayanan M, Suchy FJ. Nuclear factor-kappaB regulates the expression of multiple genes encoding liver transport proteins. Am J Physiol Gastrointest Liver Physiol. 2016;310(8):G618–28.
Kim MS, et al. Repression of farnesoid X receptor during the acute phase response. J Biol Chem. 2003;278(11):8988–95.
Kemper JK, et al. FXR acetylation is normally dynamically regulated by p300 and SIRT1 but constitutively elevated in metabolic disease states. Cell Metab. 2009;10(5):392–404.
Lee J, et al. Genomic analysis of hepatic farnesoid X receptor binding sites reveals altered binding in obesity and direct gene repression by farnesoid X receptor in mice. Hepatology. 2012;56(1):108–17.
• Kim DH, et al. A dysregulated acetyl/SUMO switch of FXR promotes hepatic inflammation in obesity. EMBO J. 2015;34(2):184–99. The manuscript demonstrates that acetylation/sumolyation of FXR alters transcriptional pathways related to inflammation. SUMO-modified FXR is identified to be involved in the transrepression of NF-κB and inhibits pro-inflammatory gene expression thus showing FXR has a role in anti-inflammation. This created a direct connection between FXR, NF-κB, and inflammation, which supports emerging research in FXR protection against inflammation associated with NASH.
Gruys E, et al. Acute phase reaction and acute phase proteins. J Zhejiang Univ Sci B. 2005;6(11):1045–56.
Yoneda M, et al. High-sensitivity C-reactive protein is an independent clinical feature of nonalcoholic steatohepatitis (NASH) and also of the severity of fibrosis in NASH. J Gastroenterol. 2007;42(7):573–82.
Ndumele CE, et al. Hepatic steatosis, obesity, and the metabolic syndrome are independently and additively associated with increased systemic inflammation. Arterioscler Thromb Vasc Biol. 2011;31(8):1927–32.
Zhang S, et al. Suppression of interleukin-6-induced C-reactive protein expression by FXR agonists. Biochem Biophys Res Commun. 2009;379(2):476–9.
•• Porez G, et al. The hepatic orosomucoid/alpha1-acid glycoprotein gene cluster is regulated by the nuclear bile acid receptor FXR. Endocrinology. 2013;154(10):3690–701. This is the first publication to demonstrate that members in the lipocalin gene family are putative FXR target genes, specifically Lcn13 was the most downregulated gene in FXR KO mice and Orm3 was the most up-regulated gene in FXR KO mice. This manuscript supports further research into FXR-mediated regulation of lipocalins involved in the acute phase response.
Zhan L, et al. Genome-wide binding and transcriptome analysis of human farnesoid X receptor in primary human hepatocytes. PLoS One. 2014;9(9):e105930.
• Ye D, et al. Lipocalin-2 mediates non-alcoholic steatohepatitis by promoting neutrophil-macrophage crosstalk via the induction of CXCR2. J Hepatol. 2016;65(5):988–97. This manuscript demonstrates the direct role of lipocalin 2 in NASH, and mechanistically supports previous publications with obeservations of the role of lipocalin 2 in NASH of murine and human models. In addition, it demonstrates the importance of acute phase proteins in inflammation related to NASH.
Auguet T, et al. Liver lipocalin 2 expression in severely obese women with non alcoholic fatty liver disease. Exp Clin Endocrinol Diabetes. 2013;121(2):119–24.
Semba T, et al. The FLS (fatty liver Shionogi) mouse reveals local expressions of lipocalin-2, CXCL1 and CXCL9 in the liver with non-alcoholic steatohepatitis. BMC Gastroenterol. 2013;13:120.
Wieser V, et al. Lipocalin 2 drives neutrophilic inflammation in alcoholic liver disease. J Hepatol. 2016;64(4):872–80.
Cai Y, et al. The detrimental role played by Lipocalin-2 in alcoholic fatty liver in mice. Am J Pathol. 2016;186(9):2417–28.
Suzuki K, et al. Molecular evolution of epididymal lipocalin genes localized on mouse chromosome 2. Gene. 2004;339:49–59.
Cho KW, et al. Lipocalin-13 regulates glucose metabolism by both insulin-dependent and insulin-independent mechanisms. Mol Cell Biol. 2011;31(3):450–7.
Sheng L, et al. Lipocalin 13 protein protects against hepatic steatosis by both inhibiting lipogenesis and stimulating fatty acid beta-oxidation. J Biol Chem. 2011;286(44):38128–35.
Goodwin B, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6(3):517–26.
Watanabe M, et al. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Investig. 2004;113(10):1408–18.
Chen F, et al. Liver receptor homologue-1 mediates species- and cell line-specific bile acid-dependent negative feedback regulation of the apical sodium-dependent bile acid transporter. J Biol Chem. 2003;278(22):19909–16.
Smalling RL, et al. Genome-wide transcriptome analysis identifies novel gene signatures implicated in human chronic liver disease. Am J Physiol Gastrointest Liver Physiol. 2013;305(5):G364–74.
Myronovych A, et al. The role of small heterodimer partner in nonalcoholic fatty liver disease improvement after sleeve gastrectomy in mice. Obesity (Silver Spring). 2014;22(11):2301–11.
Neuschwander-Tetri BA, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385(9972):956–65.
Musso G, Cassader M, Gambino R. Non-alcoholic steatohepatitis: emerging molecular targets and therapeutic strategies. Nat Rev Drug Discov. 2016;15(4):249–74.
Pathil A, et al. The synthetic bile acid-phospholipid conjugate ursodeoxycholyl lysophosphatidylethanolamide suppresses TNFalpha-induced liver injury. J Hepatol. 2011;54(4):674–84.
Kim JK, et al. Omega-3 polyunsaturated fatty acid and ursodeoxycholic acid have an additive effect in attenuating diet-induced nonalcoholic steatohepatitis in mice. Exp Mol Med. 2014;46:e127.
Acknowledgements
The authors would like to acknowledge the NIH Grant, GM104037, to support Dr. Grace L. Guo’s research and the NIH/NIEHS Training Grant, T32ES007148, to support Dr. Laura E. Armstrong’s post-doctoral training.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Laura E. Armstrong and Grace L. Guo declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Liver & Xenobiotic Metabolism
Rights and permissions
About this article
Cite this article
Armstrong, L.E., Guo, G.L. Role of FXR in Liver Inflammation During Nonalcoholic Steatohepatitis. Curr Pharmacol Rep 3, 92–100 (2017). https://doi.org/10.1007/s40495-017-0085-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40495-017-0085-2