Abstract
Cerenkov luminescence imaging (CLI) is a novel molecular optical imaging technique based on the detection of optical Cerenkov photons emitted by positron emission tomography (PET) imaging agents. The ability to use clinically approved tumour-targeted tracers in combination with small-sized imaging equipment makes CLI a particularly interesting technique for image-guided cancer surgery. The past few years have witnessed a rapid increase in proof-of-concept preclinical studies in this field, and several clinical trials are currently underway. This article provides an overview of the basic principles of Cerenkov radiation and outlines the challenges of CLI-guided surgery for clinical use. The preclinical and clinical trial literature is examined including applications focussed on image-guided lymph node detection and Cerenkov luminescence endoscopy, and the ongoing clinical studies and technological developments are highlighted. By intraoperatively guiding the oncosurgeon towards more accurate and complete resections, CLI has the potential to transform current surgical practice, and improve oncological and cosmetic outcomes for patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cancer surgery
The International Agency for Research on Cancer (IARC) reports that 14.1 million new cancer cases were diagnosed in 2012 worldwide, with 8.2 million cancer-related deaths. By 2030, these figures will grow to 21.7 million new cases and 13 million deaths, simply due to population growth and ageing [2]. Of the estimated 21.7 million global new cancer patients in 2030, 17.3 million, or approximately 80 %, will need surgery as the main form of treatment [3].
For cancer surgery to have curative intent, complete tumour resection (i.e. excision of all cancer tissue with no residual loco-regional disease) is mandatory. To achieve this, surgeons try to identify a tumour’s extent, and aim to excise the lesion with a surrounding margin of healthy tissue. In an effort to minimise functional loss and/or cosmetic impairment, the goal is to remove the least possible amount of healthy tissue without compromising oncological safety [4].
Palpation and visual inspection—combined with a surgeon’s experience and judgement—are currently the only widely available ‘modalities’ to guide resection. These are frequently inaccurate at discriminating between malignant and normal tissue, resulting in positive tumour margin rates of up to 50 % in some cancers [5–7]. Positive margins are associated with a higher risk of local recurrence and poor prognosis [8–11]. Adjuvant treatments such as radiotherapy, hormone therapy or chemotherapy, and repeat operations to excise residual disease are often indicated to reduce the likelihood of local recurrence, but these treatments can impact on quality of life by causing significant physical and emotional distress, and suboptimal cosmetic outcome [12, 13].
With the above in mind, continuing efforts have been made to assist surgeons in the process of determining which tissue needs to be excised during cancer surgery. Currently used clinical techniques include ultrasonography, specimen radiography, and intraoperative histology and cytology techniques. Although all of these techniques are used to varying degrees in cancer surgery, none has quite solved the Goldilocks problem of margins, due to limitations in sensitivity, specificity, accuracy, or costs [14, 15].
Cerenkov luminescence imaging
Cerenkov luminescence imaging (CLI) is a novel imaging modality that has great potential for image-guided surgery in general, and the issue of surgical margins in particular. CLI is based on the detection of Cerenkov photons emitted by positron emission tomography (PET) imaging agents. Cerenkov photons are emitted by a charged particle (positron or electron) when travelling through a dielectric medium at a velocity greater than the velocity of light in that medium. The Cerenkov phenomenon seems to have been first observed by Marie Curie in the late 19th century. In her biography, she describes observing a pale blue glow from the radium-containing bottles in her laboratory. The first person to systematically describe Cerenkov radiation was Pavel Cerenkov, and together with Il’ja Mikhailovic Frank and Igor Yevgenyevich Tamm who developed the theoretical framework, they won the Nobel Prize in Physics in 1958 for their contribution to the discovery of the Cerenkov effect. In the lay mind, Cerenkov radiation is known as the blue glow in the cooling water basins that surround nuclear reactors.
By detecting the optical photons from PET imaging tracers, CLI combines optical and molecular imaging. Robertson et al. were the first who demonstrated that CLI with PET agents can be used to image cancer in vivo [16], and since then, this technology has rapidly emerged in the field of biomedical imaging. In recent years, several review papers have outlined the various applications of CLI including its use in Cerenkov luminescence imaging dosimetry (CLID), radionuclide therapy monitoring, tumour response monitoring and photoactivation therapy [17–21]. An in-depth explanation of the complex physics underlying Cerenkov radiation and CLI has also been reported [22, 23].
The aim of this review paper is to provide an overview on the use of CLI for image-guided interventions with a specific focus on image-guided cancer surgery. The first section of this paper outlines the characteristics of Cerenkov radiation and CLI. Rather than describing these characteristics using complex physical equations as already done by others, this review provides a simplified explanation with an emphasis on the features that are relevant to image-guided surgery. The second section of this paper contains an overview of the published work in this field to date, and the last section will highlight the ongoing clinical studies and technological developments.
Cerenkov radiation: the basics
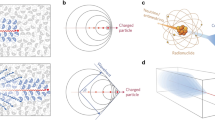
Cerenkov radiation is produced when a charged particle travels through a dielectric medium, i.e. a medium that can be polarised by an electric field, with a speed faster than the speed of light in that medium [24]. When propagating, the charged particle (a positively charged positron or negatively charged electron) induces a local polarisation by displacing the positive and negative charges of the atoms in the medium (Fig. 1). In a situation where the particle’s velocity does not exceed the speed of light in that medium, the polarisation field surrounding the particle is perfectly symmetrical, and there is no electric field at larger distances. The net result is that no Cerenkov radiation is emitted. When the particle’s speed exceeds the speed of light, however, the polarisation becomes asymmetrical along the track of the particle, resulting in a dipole electric field at larger distances from the particle. As the particle passes the electrons of the atoms return to their ground state, thereby emitting the transferred energy as optical photons that are known as Cerenkov radiation. Thus, Cerenkov radiation is produced as secondary emission; it is not the charged particle generating light, but the medium as a reaction to the particle.
A charged particle, in this case an electron, passing through a dielectric medium with a a particle speed (ν) lower than speed of light in that medium (c/η), b a particle speed larger than speed of light in that medium. The condition such that Cerenkov luminescence is produced along the particle’s track requires ν ≥ c/η
For Cerenkov radiation to be emitted, the charged particle needs to exceed a certain energy threshold. This threshold is expressed by \({\text{v }} \ge c/\eta\), where ν is the charged particle’s velocity, c is the speed of light in vacuum, and η is the refraction index of the medium. From this expression, it becomes clear that the Cerenkov threshold is related to the refractive index of the medium. Using the relationship between the velocity of the particle and its energy as described by equations 2 and 3 in Gill et al., it is found that in water with a refractive index of 1.33, the threshold is 0.264 MeV [25]. In soft tissues, the refractive index typically ranges from 1.36 to 1.40, resulting in a threshold for the production of Cerenkov radiation of approximately 0.219–0.250 MeV. These thresholds are lower than the beta particle energies from radionuclides used in PET, and these radionuclides thus emit Cerenkov radiation in both water and tissue [26]. As the charged particle travels through the medium, it loses energy due to interactive processes with its surroundings including absorption and scattering, and eventually, its energy falls below the threshold, and Cerenkov light is no longer produced. For the much heavier alpha particles, the Cerenkov threshold in water and tissue is 1926 and 1673 MeV, respectively [27]. Although none of the energies from existing alpha-emitting radionuclides come near this threshold (typical alpha particle energies range between 3 and 7 MeV), studies have shown emission of Cerenkov photons by alpha emitters [27–29]. There are two explanations for this observation, depending on the type of radionuclide: either photons arise from the short-lived beta-emitting daughter radionuclides of some alpha emitters (e.g. Actinium-225), or they are produced by electrons that arise from Compton scattered high-energy gamma photons. Regardless of the mechanism, Cerenkov radiation from alpha emitters is, thus, produced indirectly by secondary beta particles. The pure gamma-emitter Technetium-99 m (99mTc) is also able to produce optical photons as shown by several groups [30–32]. Although the mechanism of this optical emission is not yet fully understood, it is assumed to be from OH radicals that are excited by the low energy Compton electrons [30] or from gamma excitation of the luminophores that are present in 99mTc based tracers (e.g. the amino acids in 99mTc-macroaggregates albumin) [32]. This form of luminescence is known as radioluminescence and differs from Cerenkov radiation; it has a different wavelength spectrum, and its signal intensity is lower in tissue [33]. The latter may provide additional challenges for its use in image-guided interventions. In the remainder of this review, our focus will, therefore, solely be on Cerenkov radiation.
The number of Cerenkov photons N emitted per distance travelled x can be calculated using equation 1, which is derived from the Frank–Tamm equation [25]:
Here, α is the fine structure constant (1/137), β is the ratio between particle’s velocity and the speed of light in vacuum (ν/c), and the integral is over the interval λ 1 to λ 2. From this equation, it follows that the intensity of the Cerenkov radiation depends on a particle’s velocity, and thus, its energy. Fluorine-18 (18F), the most commonly used radionuclide in PET imaging, has an average and maximum β-energy of 250 and 633 keV, respectively. As a result, only 47 % of the decays produce a positron that exceeds the energy threshold for production of Cerenkov radiation in water [22]. Yttrium-90 (90Y), a radionuclide often used in radiation therapy, has a much higher average and maximum β-energy of 934 keV and 2.28 MeV, respectively, and 90 % of its produced electrons are above the Cerenkov threshold in water. Gill et al. recently studied 47 radionuclides widely used in nuclear medicine, and used Monte Carlo simulations to quantify the expected Cerenkov light yield (photons/decay) for each radionuclide in tissue (η = 1.4) [25]. They found that 18F emits 2.58 photons per decay in tissue; approximately 23 times less than the 58.5 photons per decay emitted by 90Y. The light yield from some commonly used radionuclides in order from high to low is shown in Table 1. Although it is important to realise that the reported light yields do not take into account the wavelength-dependent absorption and wavelength-dependent scattering that would occur in human tissue—this would reduce the number of detectable photons—it is clear that the signal intensity of CLI can be improved significantly using higher-energetic isotopes. However, even with the use of such isotopes, the Cerenkov light yield from a single radioactive decay process is low in comparison to, for example, the light yield from a single fluorescent molecule. Fluorescein and Indocyanine green (ICG), fluorophores used in fluorescence image-guided surgery, emit roughly three orders of magnitude more photons [34]. This low light yield requires strict control of the light environment to obtain a sufficient signal-to-background ratio (SBR) when using CLI in an intraoperative setting as explained below.
Another characteristic of Cerenkov light is its broad emission spectrum that ranges from approximately 350 to 900 nm [16]. The light intensity is inversely proportional to the square of the wavelength (1/λ 2). This is why Cerenkov radiation is strongest towards the blue end of the visible spectrum, and hence why Cerenkov radiation appears blue.
The fundamental resolution of Cerenkov radiation is determined by the distance over which a β-particle emits light. It was found that for 90Y and 18F, this distance is approximately 2 and 0.3 mm, respectively [22]. This shows that lower-energetic tracers have a better physical resolution limit, but the downside is a lower light yield, and thus, sensitivity.
Characteristics of CLI from an image-guided surgery perspective
CLI images can be acquired by detecting the Cerenkov light from PET tracers using ultra-high-sensitivity optical cameras such as electron-multiplying charge-coupled device (EMCCD) cameras. The CLI image can be analysed semiquantitatively in photon radiance. CLI and PET are directly correlated due to both techniques measuring the photons produced by positron-emitting radiopharmaceuticals; PET measures the annihilation photons, and CLI measures the Cerenkov photons. Several studies have shown a strong correlation between CLI and PET for different radiopharmaceuticals in vitro, ex vivo and in vivo, thus demonstrating the feasibility of CLI for molecular imaging of living subjects. An overview of the published literature on the correlation between CLI and PET is provided in Table 2. Results on the correlation between CLI and radiotracer activity are also included in this table.
There are several reasons why CLI has sparked so much interest in the field of biomedical imaging, and why it is a promising technology to guide surgical resection. Firstly, CLI images can be acquired with clinically approved tumour-targeted radiopharmaceuticals that have been used for over two decades in molecular medical imaging [26]. This provides great potential for rapid translation of CLI into clinical practice. Especially, the possibility to use the most commonly used PET radiopharmaceutical 2-deoxy-2-(18F)fluoro-d-glucose (abbreviated 18F-FDG) facilitates wide clinical adoption of CLI, as this is a versatile tracer that can be used in several solid cancers, including lung cancer, colorectal cancer, melanoma, head and neck cancer, breast cancer and oesophageal cancer [35].
The ability to use clinically approved tumour-specific tracers is an important advantage over conventional optical imaging techniques, such as targeted fluorescence imaging, as to date, there are no tumour-specific fluorescent tracers that have been approved by the FDA or EMA [36]. Targeted fluorescence imaging faces a significant commercial hurdle for clinical adoption, because the process of obtaining regulatory and reimbursement approval is costly and lengthy [37], while the revenue of imaging agents is often low compared to therapeutic agents, which makes it a significantly less interesting investment for industry [38, 39].
In addition to the already approved PET tracers, a significant number of new tracers are being developed for market approval including 68Ga-PSMA, 68Ga-DOTATOC, 18F-NaF, 18F-Choline, and 18F-FDOPA [40].
The ability to use the same tracer for both CLI and PET or SPECT enables dual-modality molecular imaging. PET and SPECT provide preoperative information on the location and extent of the tumour, while CLI can be used as an intraoperative adjunct to aid lesion identification and guide surgical resection. The use of the same tracer ensures visualisation of the same structures and facilitates a more accurate comparison between modalities. Depending on the patient pathway and half-life of the tracer, preoperative and intraoperative imaging could be performed using only one tracer injection, or by reinjecting the tracer. By capturing a white-light image with a standard camera at the time of CLI image acquisition, the functional information from the CLI image can be combined with the anatomical and structural information from the photograph, thereby providing the surgeon unprecedented information on the nature, location, and extent of the cancerous tissue.
Beta-emitting radiopharmaceuticals can also be detected by a beta probe or gamma probe [41–43], so these tools could potentially be used in addition to CLI-guided surgery to overcome the limited penetration depth of CLI as a result of absorption and scattering, thereby further ensuring successful tumour resection.
Another advantage of CLI is that the optical imaging systems required to acquire an image can be small in dimension or use fibre-optics or laparoscopic capabilities. Unlike a PET system, this provides the ability to use CLI in an operating theatre or in endoscopy equipment, and examples of such applications are provided in the next section.
CLI faces a number of challenges for routine clinical adoption. As mentioned earlier, Cerenkov luminescence is very faint due to the small number of optical Cerenkov photons emitted by charged particles. In biological applications, the signal intensity is further reduced by strong tissue attenuation from chromophores like (oxy)haemoglobin and light scattering which is more pronounced in the 400–650 nm range [44, 45]. Consequently, the acquisition time required to obtain high-resolution images with a sufficient signal-to-noise ratio (SNR) is longer than with conventional optical imaging. Typical imaging times in preclinical and clinical CLI studies range from 1 to 5 min (Table 3). Although these images are not available in ‘real-time’, these acquisition times are considered feasible for most intraoperative applications. However, when imaging with handheld devices (e.g. endoscopes), it is essential that during image acquisition, the device is not moved as this causes blurring of the image resulting in a reduced image quality. In an in vivo environment, this may prove especially difficult due to bowel activity and breathing artefacts, and motion-correction algorithms may be needed to correct for this.
The weak light intensity also requires a light-tight environment as any leakage of ambient light will overwhelm the CLI signal. Since Cerenkov radiation is strongest in the visible wavelengths, it cannot be spectrally separated from the much brighter ambient lights currently used in operating theatres. Control of the light environment is, therefore, currently achieved by imaging in a light-tight specimen chamber or room with light-sealed doors, or in anatomical areas that provide natural darkness (e.g. gastrointestinal tract).
An often mentioned limitation of optical imaging, in general, is the limited light penetration depth, and thereby, the inability to image deep located tissue. This was nicely illustrated by Chin et al. who calculated the reduction in signal intensity from one 18F-isotope and one ICG molecule in 1 mm of tissue, and found a reduction in signal intensity of 77 and 39 %, respectively [34]. Because Cerenkov light is ‘blue-weighted’ and tissue absorption and scattering are significantly increased for these wavelengths, CLI is mainly applicable for imaging superficially located tissue.
Due to the half-life dependency of radiotracers, the window in which CLI imaging needs to be performed to obtain a sufficient SNR and image quality is limited. Well-designed logistics and close collaboration between nuclear medicine, radiology and surgical departments are, therefore, a prerequisite for the successful implementation of CLI in current clinical and surgical workstreams.
A challenge for CLI-guided surgery in particular is the radiation exposure to patients and theatre staff from using radiopharmaceuticals. For patients, the effective dose from a 300 MBq 18F-FDG injection is approximately 6 mSv; this is comparable to the radiation dose for a typical chest CT scan [46] and much lower than the 20–2500 mSv radiation exposure from diagnostic and interventional fluoroscopy procedures [47]. Staff that work in close proximity of the patient during surgery are also exposed to radiation. The received radiation dose is dependent on the time between injection and the start of the interventional procedure, as well as the duration of the procedure. Various groups have published staff radiation doses from 18F-FDG-guided cancer surgery procedures [48–50], and have shown that the radiation dose received per procedure is generally low. For example, for a 105 min procedure starting approximately 1 h after injection of 370 MBq 18F-FDG, the exposure to the surgeon was 42 μSv [48]. However, depending on the national annual occupational dose limit (50 mSv in the United States, and 20 mSv in most other countries) and type of procedure, the number of procedures an individual can perform per year without exceeding the permissible limits for professional workers may be restricted. Regardless of these limits, there are strict requirements for the use of radioactivity in clinical practice. For example, routine staff monitoring is a requisite for each institution that conducts radiotracer guided procedures, strict regulations need to be followed with regards to clinical waste disposal and handling of radioactive specimens, and staff need to attend radiation safety training prior to participation in any procedure involving radiation [51]. These requirements could hinder adoption of radioguided surgical technologies, especially in small district hospitals that do not have access to nuclear medicine or radiation safety departments. The aforementioned characteristics of Cerenkov radiation and CLI in light of image-guided surgical applications are summarised in Table 1.
Applications of CLI for image-guided surgery and ongoing clinical trials
After it was first described in 2009, CLI has gained significant scientific interest. A search of Embase and Medline performed on 28 December 2015 using the keywords ‘Cerenkov Luminescence Imaging’ provided a total of 103 and 59 articles, respectively. Despite the limitations mentioned in the previous section, various research groups have been successful in using CLI for image-guided surgical interventions. An overview of the results published to date is provided in Table 3. The majority of this work is preclinical, although one clinical study was also published recently. In addition to the tumour types shown in Table 3, CLI-guided surgery could also be applied to other superficial malignancies where precision surgery is essential for preserving organ function, such as neoplasms in the oral cavity and genital tract. However, publications of CLI in these malignancies have not yet emerged.
The published studies show the ability to perform CLI-guided surgical excision of tumours using a variety of radiopharmaceuticals and different CLI embodiments, including standard IVIS optical imaging systems, custom-build flexible fibre endoscope systems, and clinically approved rigid laparoscope and flexible endoscope systems coupled to EMCCD cameras. An example that nicely illustrates CLI-guided tumour excision is shown in Fig. 2.

This research was originally published in Molecular Imaging [1]
89Zr-DFO-trastuzumab CLI-guided tumour excision. a Empty background image acquired prior to surgery. b Image acquired pre-incision and c post-incision after removal of the skin. An elevated tumour radiance is visible in the HER2/neu positive tumour (red circle); 89Zr-DFO-trastuzumab is not taken up in the HER2/neu negative tumour, and this tumour, therefore, does not display an elevated radiance (blue circle). Note the increase in radiance due to a reduction in tissue absorption and scattering after removal of the skin. d Image of the surgical cavity after excision of the HER2/neu positive tumour. An elevated radiance from the excised tumour specimen is visible (red circle). No CLI signal is left at the excision site indicating complete tumour resection. e Image of excised tumour alone. f Image acquired straight after the surgical wound was closed with sutures.
An important advantage of using CLI in an endoscopic setting is that these make use of anatomical dark chambers, so that there is no interference from external light sources. Besides, this technology can also be implemented in other types of endoscopes, such as a bronchoscope or hysteroscope, and future applications of CLI could, for example, focus on lung cancer, endometrial cancer and metastatic lymph nodes in the abdomen, pelvis and thorax.
CLI has also been successfully used for lymph node identification and image-guided lymph node excision using 18F-FDG and 68Ga-labelled superparamagnetic iron oxide particles (SPIONs) [52, 53].
Another interesting application of CLI, although not directly related to image-guided surgery, has been published by Spinelli et al. [54]. They imaged the thyroid gland of a patient treated for hyperthyroidism who received 550 MBq of Iodine-131 (131I). Using an EMCCD camera positioned in a light-tight room, tracer uptake in the thyroid could be visualised with a 2-min exposure time. This application is of clinical interest as imaging the uptake of beta-emitting radiopharmaceuticals could provide a rapid and inexpensive alternative for monitoring radiation doses given to superficial organs.
The successful applications of CLI for image-guided cancer surgery have resulted in several clinical studies that are currently ongoing to evaluate the feasibility of this technique in different tumour types. At Guy’s Hospital (London, UK), a first-in-woman pilot study evaluates intraoperative CLI for measuring tumour resection margins and lymph node status in 30 patients undergoing breast-conserving surgery (BCS) (ClinicalTrials.gov identifier NCT02037269). Patients receive an intravenous standard of care PET dose of 5 MBq/kg 18F-FDG, and excised wide local excision (WLE) specimens and lymph nodes are imaged within 1–3 h post-injection using an investigational intraoperative CLI specimen camera (Lightpoint Medical Ltd, UK) (Fig. 3). The investigational CLI camera consists of a light-tight sample chamber, a radiation-shielded thermoelectrically-cooled EMCCD camera, and a f/0.95 lens. The camera provides 8 × 8 cm field of view and 156 µm intrinsic spatial resolution. Interim results show that elevated radiances are detected in cancer compared to normal breast tissue, and that the radiation exposure to surgical staff is low [55, 56]. The results from comparing CLI resection margin status and lymph node status to the gold-standard, histopathology, are being prepared for publication at the time of writing. An example of a CLI image from a WLE specimen that was scanned intraoperatively in this clinical study is shown in Fig. 4. This image illustrates that CLI provides high-resolution functional information that allows surgeons to accurately assess tumour margins during surgery.
Investigational intraoperative CLI imaging system used in breast-conserving surgery trial. a Computer aided design (CAD) rendering. The red object indicates the location of the tissue specimen within the specimen chamber. b Schematic diagram showing: (1) thermoelectrically-cooled EMCCD camera, (2) f/0.95 lens, (3) hinged reflex mirror, (4) CMOS reference camera for anatomical imaging, (5) specimen holder, (6) lead radiation shielding for EMCCD camera, (7) focal zone, (8) fixed lens for reference camera, (9) filter wheel, (10) LED RGB light array, (11) specimen chamber. The purple line shows the optical paths for the EMCCD camera and the reference camera as determined by the angle of the reflex mirror
Wide local excision specimen from a patient with a 22 mm, grade 2, ER+/HER2− invasive lobular carcinoma. The specimen was incised to expose the primary tumour and margins of excision, and subsequently scanned with the investigational CLI camera. a Cerenkov image, b white-light photograph (black and white) overlaid with Cerenkov signal. An increased radiance from the tumour is visible (white arrows); mean radiance is 544.0 (SD 71.0) photons/s/cm2/sr. The tumour-to-tissue background ratio is 2.44. Phosphorescent signals from the pathology inks used to orientate the specimen prior to incision are also present (open arrows). The posterior margin (blue) and superior margin (green) are visible; both margins were clear (≥5 mm) on CLI and histopathology, respectively
To evaluate the effect of intraoperative 18F-FDG CLI on reoperation rate and quality of life in BCS, a randomised, controlled, multi-centre clinical study is scheduled to commence in mid-2016 (ClinicalTrials.gov identifier NCT02666079). This will run across an anticipated eight study sites in the UK and Germany, and use the CE-marked LightPath™ Imaging System (Lightpoint Medical Ltd, UK).
Another CLI study that is currently being conducted at Guy’s Hospital and University College London Hospital focusses on tumour margin evaluation in prostate cancer (ClinicalTrials.gov identifier NCT02151097). Patients undergoing a therapeutic radical prostatectomy receive a 370 MBq intravenous injection of 18F-Choline, and the margins of the resected prostatectomy specimen are imaged using the investigational intraoperative CLI camera. The initial results show that intraoperative 18F-Choline CLI is a feasible and low-risk procedure [57]. Elevated radiances were present in all three primary tumours (tumour-to-background ratio between 2.49 and 4.90), and CLI imaging did not add additional time to surgery. The assisting surgeon and scrub nurse received the highest body dose; 110–180 and 40–80 μSv, respectively. Work is currently being done to perform CLI imaging with the Gallium-68 (68Ga) labelled prostate specific membrane antigen (PSMA); a tracer that has strong advantages over 18F-Choline. PSMA is a cell surface target that is highly expressed by nearly all prostate cancers, and 68Ga-PSMA is, therefore, highly taken up in prostate cancer cells [58]. The Cerenkov radiance from 68Ga in tissue (η = 1.4) is approximately 17 times higher compared to 18F, which could facilitate a reduction in tracer dose, thereby lowering the radiation exposure to theatre staff. The shorter 68Ga half-life of 68 min means that contaminated surgical instruments and surgical waste can be cleaned and disposed much quicker. Another advantage is that besides imaging the primary tumour, this tracer also holds promise for visualising small lymph node metastases [59].
In addition to imaging resected WLE specimens ex vivo, scanning the post-resection surgical cavity for residual tumour that cannot be identified by visual inspection or palpation could further aid achieving complete excision of cancers. Detection of beta-radiation with handheld betascopes can identify small areas of malignant cells [60], and clinical studies to test the combination of in vivo betascope detection and ex vivo CLI will soon commence in gastrointestinal cancers (ClinicalTrials.gov identifier NCT02446379) and breast cancer (ClinicalTrials.gov identifier NCT02151071).
Another interesting application of CLI that is currently being evaluated is the non-invasive detection of nodal disease in a preoperative clinical setting (ClinicalTrials.gov Identifier NCT01664936). In this observational study, patients with lymphoma, leukaemia and metastatic lymphadenopathy scheduled to undergo standard clinical 18F-FDG PET are included. CLI imaging is performed immediately after the PET-scan in a dark room with a single-photon sensitive camera positioned on a standard photography tripod. The preliminary results of this study from four patients (two lymphoma, one lung cancer and one breast cancer) showed that metastatic lymph nodes in the neck or axilla, located at 1.6 ± 0.5 cm under the skin, had a statistically significant higher Cerenkov signal than negative nodes (P = 0.02), and this finding strongly correlated with the results from PET [61]. Examples of patient population that can benefit from accurate preoperative identification of nodal disease are breast cancer patients with involved lymph nodes. If positive lymph nodes are identified preoperatively on CLI, their treatment could convert from sentinel lymph node biopsy (SLNB) to immediate axillary node clearance (ANC), thus preventing the patient from undergoing an unnecessary surgical procedure. Alternatively, these patients may undergo neoadjuvant chemotherapy followed by SLNB ± ANC. Completion of this study will provide further insight in the real value of preoperative CLI imaging in aiding surgical and medical decision-making.
Future technical developments
CLI has only recently been introduced as a modality for imaging biological tissue, and this technique is, therefore, still in its infancy. In the last decade, advances in optical imaging devices in the biophotonics field have progressed rapidly with the development of highly sensitive, charge-coupled detectors (CCD), and current technological developments focus on further increasing the sensitivity of this imaging technology. This would facilitate a reduction in acquisition time, and a reduction in the administered radiopharmaceutical dose.
Improvements in detection sensitivity can be achieved using more specialised optics and more sensitive detectors. For example, the Schott-75 glass used in the CLI prototype device of Liu et al. transmits only 40 % of light at 500 nm, and impurities in the glass scintillate gamma photons, which increase background noise [62]. The use of fused silica, which transmits further in the violet and ultraviolet wavelengths and has fewer impurities, would significantly improve detection sensitivity.
In non-invasive CLI imaging, improvements in sensitivity may be obtained by using CCD cameras that are optimised to detect Cerenkov radiation in the UV for surface imaging, or in the near-infrared (NIR) for deep imaging. Spinelli et al. showed in a theoretical analysis that a CCD detector with a quantum efficiency peak in the NIR range could enhance the number of detected Cerenkov photons by 35 %, especially for Cerenkov source located deeper inside the tissue [63].
As already described in the section ‘Cerenkov radiation’, Cerenkov light is mainly weighted towards the ultraviolet (UV) and blue part of the spectrum. The high absorption and scattering of these frequencies in biological tissue hampers CLI detection and quantification. To overcome these limitations, current work focusses on shifting the CLI emission spectrum to NIR wavelengths by ways of Cerenkov radiation energy transfer (CRET). Different research groups have done this using fluorescent quantum dots (QDs) or other fluorophores, in vitro and in vivo animal models [64–66]. The broad excitation spectrum that matches the CR spectrum and the narrow emission spectrum make QDs specifically favourable. NIR wavelength light would enable the use of spectral filters to reduce interference from external light source, thus facilitating the use of CLI in the intraoperative suite. However, as with targeted fluorescent probes, nanoparticles have not yet received marked approval, and these approaches can, therefore, not be used clinically yet.
Another interesting development in the field of CLI is the acquisition of three-dimensional (3D) images by means of Cerenkov luminescence tomography (CLT). Different reconstruction approaches have been proposed using multi-view [67, 68] or multi-spectral [69] imaging methods, all showing a good correlation in radiotracer distribution on CLT and PET or SPECT, respectively. Although each method is currently still limited in terms of acquisition time or spatial resolution and has only been used preclinically, the ability of CLT to provide 3D information on the in vivo distribution of radiopharmaceuticals could provide a more accurate depiction of the location and extent of the tumour, thereby aiding the surgeon in more accurate tumour excision.
Conclusions
CLI is a fast-emerging optical imaging technology that has rapidly progressed from bench to bedside. This rapid development has been facilitated by the ability to use clinically approved tumour-targeted PET tracers. Due to its high-resolution, wide applicability across a range of solid cancers and small size imaging equipment, CLI is of particular interest in the field of image-guided surgery. Challenges for the clinical implementation of this technique include the low signal intensity, the requirement for light-tightness, the minute-scale image acquisition times and the logistical issues associated with using radiotracers intraoperatively. Preclinical studies have shown that CLI can be successfully used to guide surgical resection of tumours and lymph nodes, as well as to detect cancerous lesions using Cerenkov luminescence endoscopy. Several clinical studies on the preoperative and intraoperative use of CLI in breast cancer, prostate cancer, gastrointestinal cancer and metastatic lymph nodes are currently underway. Results from these studies, together with ongoing developments in ultra-sensitive camera technology will help drive widespread clinical adoption. By improving the accuracy of surgical resections, CLI has the potential to become a disruptive technology in cancer surgery.
References
Holland JP, Normand G, Ruggiero A, Lewis JS, Grimm J (2011) Intraoperative imaging of positron emission tomographic radiotracers using Cerenkov luminescence emissions. Mol Imaging 10(3):177–186 (171–173)
Bray F, Jemal A, Grey N, Ferlay J, Forman D (2012) Global cancer transitions according to the Human Development Index (2008-2030): a population-based study. Lancet Oncol 13(8):790–801. doi:10.1016/s1470-2045(12)70211-5
Sullivan R, Alatise OI, Anderson BO, Audisio R, Autier P, Aggarwal A, Balch C, Brennan MF, Dare A, D’Cruz A, Eggermont AM, Fleming K, Gueye SM, Hagander L, Herrera CA, Holmer H, Ilbawi AM, Jarnheimer A, Ji JF, Kingham TP, Liberman J, Leather AJ, Meara JG, Mukhopadhyay S, Murthy SS, Omar S, Parham GP, Pramesh CS, Riviello R, Rodin D, Santini L, Shrikhande SV, Shrime M, Thomas R, Tsunoda AT, van de Velde C, Veronesi U, Vijaykumar DK, Watters D, Wang S, Wu YL, Zeiton M, Purushotham A (2015) Global cancer surgery: delivering safe, affordable, and timely cancer surgery. Lancet Oncol 16(11):1193–1224. doi:10.1016/s1470-2045(15)00223-5
Keereweer S, Van Driel PB, Snoeks TJ, Kerrebijn JD, Baatenburg de Jong RJ, Vahrmeijer AL, Sterenborg HJ, Lowik CW (2013) Optical image-guided cancer surgery: challenges and limitations. Clin Cancer Res 19(14):3745–3754. doi:10.1158/1078-0432.ccr-12-3598
Waljee JF, Hu ES, Newman LA, Alderman AK (2008) Predictors of re-excision among women undergoing breast-conserving surgery for cancer. Ann Surg Oncol 15(5):1297–1303. doi:10.1245/s10434-007-9777-x
Iczkowski KA, Lucia MS (2011) Frequency of positive surgical margin at prostatectomy and its effect on patient outcome. Prostate Cancer 2011:673021. doi:10.1155/2011/673021
McMahon J, O’Brien CJ, Pathak I, Hamill R, McNeil E, Hammersley N, Gardiner S, Junor E (2003) Influence of condition of surgical margins on local recurrence and disease-specific survival in oral and oropharyngeal cancer. Br J Oral Maxillofac Surg 41(4):224–231
Menes TS, Tartter PI, Bleiweiss I, Godbold JH, Estabrook A, Smith SR (2005) The consequence of multiple re-excisions to obtain clear lumpectomy margins in breast cancer patients. Ann Surg Oncol 12(11):881–885. doi:10.1245/aso.2005.03.021
Raziee HR, Cardoso R, Seevaratnam R, Mahar A, Helyer L, Law C, Coburn N (2012) Systematic review of the predictors of positive margins in gastric cancer surgery and the effect on survival. Gastric Cancer 15(Suppl 1):S116–124. doi:10.1007/s10120-011-0112-7
Luryi AL, Chen MM, Mehra S, Roman SA, Sosa JA, Judson BL (2015) Treatment factors associated with survival in early-stage oral cavity cancer: analysis of 6830 cases from the National Cancer Data Base. JAMA Otolaryngol Head Neck Surg 141(7):593–598. doi:10.1001/jamaoto.2015.0719
Silberstein JL, Eastham JA (2014) Significance and management of positive surgical margins at the time of radical prostatectomy. Indian J Urol 30(4):423–428. doi:10.4103/0970-1591.134240
Munshi A, Kakkar S, Bhutani R, Jalali R, Budrukkar A, Dinshaw KA (2009) Factors influencing cosmetic outcome in breast conservation. Clin Oncol (R Coll Radiol) 21(4):285–293. doi:10.1016/j.clon.2009.02.001
Zelefsky MJ, Harrison LB, Fass DE, Armstrong JG, Shah JP, Strong EW (1993) Postoperative radiation therapy for squamous cell carcinomas of the oral cavity and oropharynx: impact of therapy on patients with positive surgical margins. Int J Radiat Oncol Biol Phys 25(1):17–21
Butler-Henderson K, Lee AH, Price RI, Waring K (2014) Intraoperative assessment of margins in breast conserving therapy: a systematic review. Breast 23(2):112–119. doi:10.1016/j.breast.2014.01.002
Handgraaf HJ, Boonstra MC, Van Erkel AR, Bonsing BA, Putter H, Van De Velde CJ, Vahrmeijer AL, Mieog JS (2014) Current and future intraoperative imaging strategies to increase radical resection rates in pancreatic cancer surgery. Biomed Res Int 2014:890230. doi:10.1155/2014/890230
Robertson R, Germanos MS, Li C, Mitchell GS, Cherry SR, Silva MD (2009) Optical imaging of Cerenkov light generation from positron-emitting radiotracers. Phys Med Biol 54(16):N355–365. doi:10.1088/0031-9155/54/16/n01
Xu Y, Liu H, Cheng Z (2011) Harnessing the power of radionuclides for optical imaging: Cerenkov luminescence imaging. J Nucl Med 52(12):2009–2018. doi:10.2967/jnumed.111.092965
Thorek D, Robertson R, Bacchus WA, Hahn J, Rothberg J, Beattie BJ, Grimm J (2012) Cerenkov imaging—a new modality for molecular imaging. Am J Nucl Med Mol Imaging 2(2):163–173
Das S, Thorek DL, Grimm J (2014) Cerenkov imaging. Adv Cancer Res 124:213–234. doi:10.1016/b978-0-12-411638-2.00006-9
Spinelli AE, Boschi F (2015) Novel biomedical applications of Cerenkov radiation and radioluminescence imaging. Phys Med 31(2):120–129. doi:10.1016/j.ejmp.2014.12.003
Tanha K, Pashazadeh AM, Pogue BW (2015) Review of biomedical Cerenkov luminescence imaging applications. Biomed Opt Express 6(8):3053–3065. doi:10.1364/boe.6.003053
Mitchell GS, Gill RK, Boucher DL, Li C, Cherry SR (2011) In vivo Cerenkov luminescence imaging: a new tool for molecular imaging. Philos Trans A Math Phys Eng Sci 369(1955):4605–4619. doi:10.1098/rsta.2011.0271
Ma X, Wang J, Cheng Z (2014) Cerenkov radiation: a multi-functional approach for biological sciences. Front Phys. doi:10.3389/fphy.2014.00004
Jelley JV (1955) Cerenkov radiation and its applications. Br J Appl Phys 6(7):227
Gill RK, Mitchell GS, Cherry SR (2015) Computed Cerenkov luminescence yields for radionuclides used in biology and medicine. Phys Med Biol 60(11):4263–4280. doi:10.1088/0031-9155/60/11/4263
Liu H, Ren G, Miao Z, Zhang X, Tang X, Han P, Gambhir SS, Cheng Z (2010) Molecular optical imaging with radioactive probes. PLoS ONE 5(3):e9470. doi:10.1371/journal.pone.0009470
Ackerman NL, Graves EE (2012) The potential for Cerenkov luminescence imaging of alpha-emitting radionuclides. Phys Med Biol 57(3):771–783. doi:10.1088/0031-9155/57/3/771
Ruggiero A, Holland JP, Lewis JS, Grimm J (2010) Cerenkov luminescence imaging of medical isotopes. J Nucl Med 51(7):1123–1130. doi:10.2967/jnumed.110.076521
Boschi F, Meo SL, Rossi PL, Calandrino R, Sbarbati A, Spinelli AE (2011) Optical imaging of alpha emitters: simulations, phantom, and in vivo results. J Biomed Opt 16(12):126011. doi:10.1117/1.3663441
Spinelli AE, Lo Meo S, Calandrino R, Sbarbati A, Boschi F (2011) Optical imaging of Tc-99 m-based tracers: in vitro and in vivo results. J Biomed Opt 16(11):116023. doi:10.1117/1.3653963
Boschi F, Pagliazzi M, Rossi B, Cecchini MP, Gorgoni G, Salgarello M, Spinelli AE (2013) Small-animal radionuclide luminescence imaging of thyroid and salivary glands with Tc99 m-pertechnetate. J Biomed Opt 18(7):76005. doi:10.1117/1.jbo.18.7.076005
Kondakov AK, Gubskiy IL, Znamenskiy IA, Chekhonin VP (2014) Possibilities of optical imaging of the (99 m)Tc-based radiopharmaceuticals. J Biomed Opt 19(4):046014. doi:10.1117/1.jbo.19.4.046014
Pagliazzi M, Boschi F, Spinelli AE (2014) Imaging of luminescence induced by beta and gamma emitters in conventional non-scintillating materials. RSC Adv 4(26):13687–13692. doi:10.1039/C3RA47102K
Chin PT, Welling MM, Meskers SC, Valdes Olmos RA, Tanke H, van Leeuwen FW (2013) Optical imaging as an expansion of nuclear medicine: Cerenkov-based luminescence vs fluorescence-based luminescence. Eur J Nucl Med Mol Imaging 40(8):1283–1291. doi:10.1007/s00259-013-2408-9
Abouzied MM, Crawford ES, Nabi HA (2005) 18F-FDG imaging: pitfalls and artifacts. J Nucl Med Technol 33(3):145–155 (quiz 162–143)
Rosenthal EL, Warram JM, Bland KI, Zinn KR (2015) The status of contemporary image-guided modalities in oncologic surgery. Ann Surg 261(1):46–55. doi:10.1097/sla.0000000000000622
Frangioni JV (2006) Translating in vivo diagnostics into clinical reality. Nat Biotechnol 24(8):909–913. doi:10.1038/nbt0806-909
Agdeppa ED, Spilker ME (2009) A review of imaging agent development. AAPS J 11(2):286–299. doi:10.1208/s12248-009-9104-5
Nunn AD (2006) The cost of developing imaging agents for routine clinical use. Invest Radiol 41(3):206–212. doi:10.1097/01.rli.0000191370.52737.75
Mahajan A, Goh V, Basu S, Vaish R, Weeks AJ, Thakur MH, Cook GJ (2015) Bench to bedside molecular functional imaging in translational cancer medicine: to image or to imagine? Clin Radiol 70(10):1060–1082. doi:10.1016/j.crad.2015.06.082
Franc BL, Mari C, Johnson D, Leong SP (2005) The role of a positron- and high-energy gamma photon probe in intraoperative localization of recurrent melanoma. Clin Nucl Med 30(12):787–791
Gulec SA (2007) PET probe-guided surgery. J Surg Oncol 96(4):353–357. doi:10.1002/jso.20862
Povoski SP, Chapman GJ, Murrey DA Jr, Lee R, Martin EW Jr, Hall NC (2013) Intraoperative detection of (1)(8)F-FDG-avid tissue sites using the increased probe counting efficiency of the K-alpha probe design and variance-based statistical analysis with the three-sigma criteria. BMC Cancer 13:98. doi:10.1186/1471-2407-13-98
Mourant JR, Fuselier T, Boyer J, Johnson TM, Bigio IJ (1997) Predictions and measurements of scattering and absorption over broad wavelength ranges in tissue phantoms. Appl Opt 36(4):949–957
Weissleder R (2001) A clearer vision for in vivo imaging. Nat Biotechnol 19(4):316–317. doi:10.1038/86684
Brix G, Nosske D, Lechel U (2014) Radiation exposure of patients undergoing whole-body FDG-PET/CT examinations: an update pursuant to the new ICRP recommendations. Nuklearmedizin 53(5):217–220. doi:10.3413/Nukmed-0663-14-04
Mahesh M (2001) Fluoroscopy: patient radiation exposure issues. Radiographics 21(4):1033–1045. doi:10.1148/radiographics.21.4.g01jl271033
Heckathorne E, Dimock C, Dahlbom M (2008) Radiation dose to surgical staff from positron-emitter-based localization and radiosurgery of tumors. Health Phys 95(2):220–226. doi:10.1097/01.hp.0000310962.96089.44
Povoski SP, Sarikaya I, White WC, Marsh SG, Hall NC, Hinkle GH, Martin EW Jr, Knopp MV (2008) Comprehensive evaluation of occupational radiation exposure to intraoperative and perioperative personnel from 18F-FDG radioguided surgical procedures. Eur J Nucl Med Mol Imaging 35(11):2026–2034. doi:10.1007/s00259-008-0880-4
Andersen PA, Chakera AH, Klausen TL, Binderup T, Grossjohann HS, Friis E, Palnaes Hansen C, Schmidt G, Kjaer A, Hesse B (2008) Radiation exposure to surgical staff during F-18-FDG-guided cancer surgery. Eur J Nucl Med Mol Imaging 35(3):624–629. doi:10.1007/s00259-007-0532-0
ICRP (2007) Recommendations of the International Commission on Radiological Protection. ICRP Publication 103, vol 37
Thorek DL, Abou DS, Beattie BJ, Bartlett RM, Huang R, Zanzonico PB, Grimm J (2012) Positron lymphography: multimodal, high-resolution, dynamic mapping and resection of lymph nodes after intradermal injection of 18F-FDG. J Nucl Med 53(9):1438–1445. doi:10.2967/jnumed.112.104349
Madru R, Tran TA, Axelsson J, Ingvar C, Bibic A, Stahlberg F, Knutsson L, Strand SE (2013) (68)Ga-labeled superparamagnetic iron oxide nanoparticles (SPIONs) for multi-modality PET/MR/Cherenkov luminescence imaging of sentinel lymph nodes. Am J Nucl Med Mol Imaging 4(1):60–69
Spinelli AE, Ferdeghini M, Cavedon C, Zivelonghi E, Calandrino R, Fenzi A, Sbarbati A, Boschi F (2013) First human Cerenkography. J Biomed Opt 18(2):20502. doi:10.1117/1.jbo.18.2.020502
Grootendorst M, Purushotham A (2015) Clinical feasibility of intraoperative 18F-FDG Cerenkov Luminescence Imaging in breast cancer surgery. J Nucl Med 56:S3
Grootendorst MR, Kothari A, Cariati M, Hamed H, Douek M, Kovacs T, Cook G, Allen S, Sibley-Allen C, Britten A, Pawa A, Nimmo F, Vyas K, Tuch D, Pinder S, Purushotham A (2015) P094. Clinical feasibility of Cerenkov Luminescence Imaging (CLI) for intraoperative assessment of tumour excision margins and sentinel lymph node metastases in breast-conserving surgery. Eur J Surg Oncol 41(6):S53. doi:10.1016/j.ejso.2015.03.132
Michel C, Freeman A, Jameson C, Waddington W, Tuch D, Harboe M, Cathcart P (2015) P7: intra-operative margin detection using Cerenkov Luminescence Imaging during radical prostatectomy; Initial results from the PRIME study. Eur J Surg Oncol 41(11):S271. doi:10.1016/j.ejso.2015.08.112
Afshar-Oromieh A, Avtzi E, Giesel FL, Holland-Letz T, Linhart HG, Eder M, Eisenhut M, Boxler S, Hadaschik BA, Kratochwil C, Weichert W, Kopka K, Debus J, Haberkorn U (2015) The diagnostic value of PET/CT imaging with the (68)Ga-labelled PSMA ligand HBED-CC in the diagnosis of recurrent prostate cancer. Eur J Nucl Med Mol Imaging 42(2):197–209. doi:10.1007/s00259-014-2949-6
Afshar-Oromieh A, Haberkorn U, Schlemmer HP, Fenchel M, Eder M, Eisenhut M, Hadaschik BA, Kopp-Schneider A, Rothke M (2014) Comparison of PET/CT and PET/MRI hybrid systems using a 68 Ga-labelled PSMA ligand for the diagnosis of recurrent prostate cancer: initial experience. Eur J Nucl Med Mol Imaging 41(5):887–897. doi:10.1007/s00259-013-2660-z
King MT, Carpenter CM, Sun C, Ma X, Le QT, Sunwoo JB, Cheng Z, Pratx G, Xing L (2015) Beta-Radioluminescence imaging: a comparative evaluation with Cerenkov luminescence imaging. J Nucl Med 56(9):1458–1464. doi:10.2967/jnumed.115.158337
Thorek DL, Riedl CC, Grimm J (2014) Clinical Cerenkov luminescence imaging of (18)F-FDG. J Nucl Med 55(1):95–98. doi:10.2967/jnumed.113.127266
Liu H, Carpenter CM, Jiang H, Pratx G, Sun C, Buchin MP, Gambhir SS, Xing L, Cheng Z (2012) Intraoperative imaging of tumors using Cerenkov luminescence endoscopy: a feasibility experimental study. J Nucl Med 53(10):1579–1584. doi:10.2967/jnumed.111.098541
Spinelli AE, Boschi F (2012) Optimizing in vivo small animal Cerenkov luminescence imaging. J Biomed Opt 17(4):040506. doi:10.1117/1.jbo.17.4.040506
Hu Z, Qu Y, Wang K, Zhang X, Zha J, Song T, Bao C, Liu H, Wang Z, Wang J, Liu Z, Liu H, Tian J (2015) In vivo nanoparticle-mediated radiopharmaceutical-excited fluorescence molecular imaging. Nat Commun 6:7560. doi:10.1038/ncomms8560
Thorek DL, Ogirala A, Beattie BJ, Grimm J (2013) Quantitative imaging of disease signatures through radioactive decay signal conversion. Nat Med 19(10):1345–1350. doi:10.1038/nm.3323
Lewis MA, Kodibagkar VD, Oz OK, Mason RP (2010) On the potential for molecular imaging with Cerenkov luminescence. Opt Lett 35(23):3889–3891. doi:10.1364/ol.35.003889
Li C, Mitchell GS, Cherry SR (2010) Cerenkov luminescence tomography for small-animal imaging. Opt Lett 35(7):1109–1111. doi:10.1364/ol.35.001109
Hu Z, Liang J, Yang W, Fan W, Li C, Ma X, Chen X, Ma X, Li X, Qu X, Wang J, Cao F, Tian J (2010) Experimental Cerenkov luminescence tomography of the mouse model with SPECT imaging validation. Opt Express 18(24):24441–24450. doi:10.1364/oe.18.024441
Spinelli AE, Kuo C, Rice BW, Calandrino R, Marzola P, Sbarbati A, Boschi F (2011) Multispectral Cerenkov luminescence tomography for small animal optical imaging. Opt Express 19(13):12605–12618. doi:10.1364/oe.19.012605
Ma X, Yang W, Zhou S, Ma W, Hu Z, Liang J, Wang J (2012) Study of penetration depth and resolution of Cerenkov luminescence emitted from (18)F-FDG and (131)I. J Nucl Med 53:S1
Xu Y, Chang E, Liu H, Jiang H, Gambhir SS, Cheng Z (2012) Proof-of-concept study of monitoring cancer drug therapy with cerenkov luminescence imaging. J Nucl Med 53(2):312–317. doi:10.2967/jnumed.111.094623
Zhang X, Kuo C, Moore A, Ran C (2013) In vivo optical imaging of interscapular brown adipose tissue with (18)F-FDG via Cerenkov luminescence imaging. PLoS ONE 8(4):e62007. doi:10.1371/journal.pone.0062007
Hu H, Cao X, Kang F, Wang M, Lin Y, Liu M, Li S, Yao L, Liang J, Liang J, Nie Y, Chen X, Wang J, Wu K (2015) Feasibility study of novel endoscopic Cerenkov luminescence imaging system in detecting and quantifying gastrointestinal disease: first human results. Eur Radiol 25(6):1814–1822. doi:10.1007/s00330-014-3574-2
Robertson R, Germanos MS, Manfredi MG, Smith PG, Silva MD (2011) Multimodal imaging with (18)F-FDG PET and Cerenkov luminescence imaging after MLN4924 treatment in a human lymphoma xenograft model. J Nucl Med 52(11):1764–1769. doi:10.2967/jnumed.111.091710
Timmermand OV, Tran TA, Strand SE, Axelsson J (2015) Intratherapeutic biokinetic measurements, dosimetry parameter estimates, and monitoring of treatment efficacy using cerenkov luminescence imaging in preclinical radionuclide therapy. J Nucl Med 56(3):444–449. doi:10.2967/jnumed.114.148544
Cao X, Chen X, Kang F, Lin Y, Liu M, Hu H, Nie Y, Wu K, Wang J, Liang J, Tian J (2014) Performance evaluation of endoscopic Cerenkov luminescence imaging system: in vitro and pseudotumor studies. Biomed Opt Express 5(10):3660–3670. doi:10.1364/boe.5.003660
Natarajan A, Habte F, Liu H, Sathirachinda A, Hu X, Cheng Z, Nagamine CM, Gambhir SS (2013) Evaluation of 89Zr-rituximab tracer by Cerenkov luminescence imaging and correlation with PET in a humanized transgenic mouse model to image NHL. Mol Imaging Biol 15(4):468–475. doi:10.1007/s11307-013-0624-0
Hu Z, Yang W, Ma X, Ma W, Qu X, Liang J, Wang J, Tian J (2013) Cerenkov luminescence tomography of aminopeptidase N (APN/CD13) expression in mice bearing HT1080 tumors. Mol Imaging 12(3):173–181
Lohrmann C, Zhang H, Thorek DL, Desai P, Zanzonico PB, O’Donoghue J, Irwin CP, Reiner T, Grimm J, Weber WA (2015) Cerenkov luminescence imaging for radiation dose calculation of a (9)(0)Y-labeled gastrin-releasing peptide receptor antagonist. J Nucl Med 56(5):805–811. doi:10.2967/jnumed.114.149054
Fan D, Zhang X, Zhong L, Liu X, Sun Y, Zhao H, Jia B, Liu Z, Zhu Z, Shi J, Wang F (2015) (68)Ga-labeled 3PRGD2 for dual PET and Cerenkov luminescence imaging of orthotopic human glioblastoma. Bioconjug Chem 26(6):1054–1060. doi:10.1021/acs.bioconjchem.5b00169
Carpenter CM, Ma X, Liu H, Sun C, Pratx G, Wang J, Gambhir SS, Xing L, Cheng Z (2014) Cerenkov luminescence endoscopy: improved molecular sensitivity with beta–emitting radiotracers. J Nucl Med 55(11):1905–1909. doi:10.2967/jnumed.114.139105
Song T, Liu X, Qu Y, Liu H, Bao C, Leng C, Hu Z, Wang K, Tian J (2015) A novel endoscopic Cerenkov luminescence imaging system for intraoperative surgical navigation. Mol Imaging 14:443–449
Acknowledgments
The authors gratefully acknowledge grant funding from Innovate UK.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
David Tuch is an employee of and a shareholder in Lightpoint Medical Ltd. The other authors have no conflicts of interest to disclose.
Ethical approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008. Informed consent was obtained from all patients for being included in the study. All institutional and national guidelines for the care and use of laboratory animals were followed.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Grootendorst, M.R., Cariati, M., Kothari, A. et al. Cerenkov luminescence imaging (CLI) for image-guided cancer surgery. Clin Transl Imaging 4, 353–366 (2016). https://doi.org/10.1007/s40336-016-0183-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-016-0183-x