Abstract
Background
Targeted pharmacotherapy has been increasingly applied in cancer treatment due to its breakthroughs. However, the unmet needs of cancer patients are still significant, highlighting the urgency to investigate patient preferences. It is unclear how patients deliberate their choices between different aspects of targeted therapy, including cost, efficacy, and adverse events. Since discrete choice experiments (DCEs) have been widely applied to patient preference elicitation, we reviewed DCEs on targeted therapy for different cancers. We also synthesized evidence on the factors influencing patients’ choices and their willingness-to-pay (WTP) for survival when treated by targeted therapy.
Methods
We searched databases, including PubMed, EMBASE and MEDLINE, up to August 16, 2022, supplemented by a reference screening. The attributes from the selected studies were categorized into three groups: outcomes, costs, and process. We also calculated the relative importance of attributes and WTP for survival whenever possible. The purpose, respondents, explanation, findings, significance (PREFS) checklist was used to evaluate the quality of the included DCE studies.
Results
The review identified 34 eligible studies from 13 countries covering 14 cancers, such as breast, ovarian, kidney, prostate, and skin cancers. It also reveals a rising trend of DCEs on this topic, as most studies were published after 2018. We found that patients placed higher weights on the outcome (e.g., overall survival) and cost attributes than on process attributes. On average, patients were willing to pay $561 (95% confidence interval [CI]: $415–$758) and $716 (95% CI $524–$958) out-of-pocket for a 1-month increase in progression-free survival and overall survival, respectively. PREFS scores of the 34 studies ranged from 2 to 4, with a mean of 3.38 (SD: 0.65), suggesting a reasonable quality based on the checklist. However, most studies (n = 32, 94%) did not assess the impact of non-responses on the results.
Conclusions
This is the first systematic review focusing on patient preferences for targeted cancer therapy. We showcased novel approaches for evidence synthesis of DCE results, especially the attribute relative importance and WTP. The results may inform stakeholders about patient preferences toward targeted therapy and their WTP estimates. More studies with improved study design and quality are warranted to generate more robust evidence to assist decision making.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Targeted therapy is increasingly applied in cancer treatment, but unmet needs are significant. This review provides the first synthesized evidence on the patient preference for targeted therapy, with novel approaches developed for evidence synthesis of DCE results. |
Patients placed higher weights on the outcome (e.g., overall survival) and cost attributes than process attributes. On average, patients were willing to pay $561 and $716 out-of-pocket for a 1-month increase in progression-free survival and overall survival, respectively. Sample representativeness of the studies was rarely assessed. |
The results may inform policymakers, physicians, and other stakeholders about patients’ perceptions of novel therapies and their WTP for survival if treated by targeted cancer therapy. |
1 Introduction
Cancer is a significant public health problem and one of the leading causes of death worldwide [1]. Molecular aberrations play a causative role in many cancers [2], and understanding the molecular basis of cancer has ushered in the era of precision medicine [3]. The rapid evolution of precision medicine led to the development of a new type of pharmacotherapy called targeted therapy, which has been increasingly applied in clinical practice. By matching patients with drugs based on specific gene and protein biomarkers, targeted therapies minimize drug resistance, prolong survival, and avoid unwanted side effects [4]. Many targeted drugs have been approved as adjuvant therapy or even first-line options for recurrent or metastatic cancers [5]. In many cases, multiple targeted drugs are available for specific cancer or various stages of the disease, with different levels of efficacy, adverse events, and costs. For example, afatinib has a higher response rate in non-small cell lung cancer patients than gefitinib, but it may cause more adverse events [6]. Therefore, assuming that patients are presented with evidence-based treatment choices, choosing targeted drugs may be more complicated than cytotoxic chemotherapies because the choice largely depends on patients’ ability to trade-off between different factors, mainly cost, efficacy, and adverse events.
Patient preference information, especially synthesized preference evidence, could inform clinical decisions and facilitate shared decision-making to improve health outcomes [7]. Some regulatory agencies explicitly require incorporating patient preferences in clinical evaluation [8, 9]. The discrete choice experiment (DCE) is a commonly used approach for eliciting patient preferences based on random utility theory [10,11,12]. In a DCE, respondents are asked to choose a preferred therapeutic option between two or more alternatives that mimic the real-world choices, described by several attributes and associated levels [11, 13,14,15]. Through the analysis of respondents’ discrete choices, a DCE could estimate patients’ latent preferences (known as part-worth utilities) associated with the descriptive attributes and their willingness-to-pay (WTP) for changes to the attribute levels [13, 16]. To better understand patient preferences toward targeted therapy for cancers, there is a need to systematically review DCE studies on this topic.
Some previous reviews have summarized patient preferences for cancer treatments for specific cancers [17,18,19,20,21,22,23], and two studies synthesized evidence for multiple cancers [24, 25]. Despite these efforts, the published reviews either focused on treating a specific type of cancer or did not use the DCE results to derive patients’ WTP for cancer treatments, which would inform the clinical decisions and pharmaceutical regulations. Moreover, the broad coverage of cancer interventions (not only targeted therapy but also radiotherapy, surgery, chemotherapy, the management of cancer therapy adverse events, follow-up services, and support services) in some reviews may have limited the usefulness of their synthesized evidence. To date, no reviews have synthesized patient preferences specific to targeted therapy. Therefore, we aim to review DCEs on targeted therapy in a clearly defined and intrinsically comparable fashion and provide synthesized evidence on (i) the attributes and patient preferences for targeted therapy, (ii) the relative importance of the attributes, (iii) patients’ WTP for survival if treated by targeted therapy, and (iv) the quality of the DCE studies included in this review.
2 Methods
2.1 Literature Search
We designed the study as per the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [26]. Three widely used databases relevant to targeted therapy were searched, including PubMed, EMBASE, and MEDLINE, up to August 16, 2022. We developed search terms using a combination of the following keywords: DCE, patient preference, and cancer. We did not filter the search using terms such as targeted therapy and cancer treatment to broaden the search results. We supplemented the database search by examining the references of the included articles. We also compared with the past reviews to confirm that we did not miss any studies [24, 25]. The search strategy and results are presented in Supplementary Materials Section A.
Studies were included if they were: (i) peer-reviewed articles, (ii) written in English, (iii) DCEs eliciting patient preferences of medical treatments for cancers, and (iv) having targeted therapy as an option. In addition to DCEs, we included articles titled “conjoint analysis” if the article elicited patients’ part-worth utilities based on random utility theory, which implied the article was a DCE [10]. Studies were excluded if they did not elicit patient preferences for targeted therapy but other types of interventions for cancers (e.g., preventive care, pain management). Two authors (RR and PL) independently screened titles and abstracts, followed by screening the full text of the remaining articles to determine the final inclusion. Any disagreements between individual judgments were resolved by two authors (SJ and YG).
2.2 Information Extraction
An author (SJ) developed an initial version of the information extraction form based on a generic systematic review of DCEs [12] and two specific systematic reviews of DCEs on cancer treatments [24, 25]. The form was later revised and confirmed through discussions among all authors. Two authors (RR and PL) conducted the information extraction independently and cross-checked after completion. The lead author (SJ) examined all the retrieved information, which was discussed with a senior author (YG) when needed. Any disagreements were resolved through discussions involving the four authors.
We extracted the characteristics of the selected articles, including their location, publication year, target population, cancer type(s), administration method (e.g., online questionnaire, face-to-face interview), and sample size. We also extracted technical details on their experimental design and statistical analyses. In terms of the study results, we extracted the part-worth utilities (i.e., coefficients of the statistical models) of attribute levels, the relative importance of attributes if available, and the WTP values. All data generated or analyzed during this study are included in the article.
2.3 Evidence Derivation and Synthesis
To facilitate attribute comparison between studies, we followed the approach adopted by Bien et al [24] and categorized the attributes from the selected studies into three groups: outcome, cost, and process. The outcome attributes referred to either treatment efficacy or adverse events. The treatment efficacy was mainly demonstrated by progression-free survival (PFS) and overall survival (OS), which referred to an increase in median survival time or survival probability at a certain landmark time point. The process attributes included the route of administration, mode of administration, frequency of consultation, etc. After the categorization, we calculated the frequencies of the attribute groups.
We also calculated the relative importance of attributes if the authors did not report this information, following the range method recommended by the ISPOR Conjoint Analysis Good Research Practices Task Force [27]. We divided the range of each attribute (part-worth utility of the best level minus that of the worst level within the same attribute) by the sum of the ranges of all attributes and then multiplied the quotient by 100. Furthermore, we calculated the arithmetic average of the relative importance values of the same attribute in different studies. The average value indicated the overall importance of an attribute based on the included studies. By comparing the values, we assessed how important, on average, these attributes were to the patients.
We synthesized WTP values weighted by patient numbers and calculated the WTP values if the authors did not report them. If the study applied a multinomial logit model (MNL), we derived the WTP of an attribute level (non-cost type) by directly dividing the part-worth utility of the attribute level by the part-worth utility of the cost attribute. If the study applied advanced models such as the mixed logit model (MIXL), we used a simulation approach to derive the WTPs [28]. For each non-cost attribute level we were interested in, we drew a random sample 10,000 times according to the distributional information of the attribute level and the cost attribute. We then paired the sampled values and calculated the quotients to obtain the WTP for each pair. To prevent extreme values from distorting the mean estimates, we removed the largest 2% following Hensher et al [29]. If a study applied advanced models but did not report the standard deviation (SD) of the coefficients, we could not derive the simulated WTP (which requires both mean and SD estimates); instead, we calculated the WTP by simply dividing the coefficients, similar to the approach we adopted for the MNL. After calculating WTPs for individual studies, we converted the values to 2022 US dollars (US$) using Purchasing Power Parities for GDP calculated by the International Monetary Fund [30] and presented their average on a monthly basis weighted by the sample size of patients in each DCE. The weighted average implied, on average, the amount patients were willing to pay per month for a change in the non-cost attribute level.
2.4 Heterogeneity
We examined the analysis for unobserved and observed preference heterogeneity in the selected articles. The analysis for unobserved heterogeneity was by advanced models, including MIXL (also known as random-parameter logit model, RPL), Hierarchical Bayesian, latent class, and more advanced models such as generalized multinomial logit model. The observed heterogeneity was analyzed by exploring the interactions between patient characteristics (e.g., age, gender) and attributes. The interaction analysis implied specific preference patterns in some patient groups, which required attention from the stakeholders.
To detect the heterogeneity of patient preferences between cancers, we selected melanoma and prostate cancer as exemplars because they have different clinical characteristics, which may lead to different patient preferences. Melanoma is a severe form of invasive skin cancer with a high risk of death, while prostate cancer is usually a slow-growing non-cutaneous adenocarcinoma with indolent growth characteristics and a relatively favourable prognosis, commonly affecting older males. We selected melanoma and prostate cancer also because they had more studies included in this review than other cancer types, therefore the evidence synthesis for these two types of cancers would be more robust. We calculated the attribute relative importance for each cancer type and graphically illustrated the differences in results in a radar plot, with the results of all cancers as the background. The comparison indicated how cancer type influenced patient preferences in terms of the relative importance of the attribute.
2.5 Quality Assessment
We used the PREFS checklist (Purpose, Respondents, Explanation, Findings, Significance) to evaluate the quality of the included DCE studies [31]. Compared with other checklists, such as the ISPOR checklist [32], the ease of use, practicality, relevance, and wider acceptance made PREFS more frequently used for the quality assessment of DCEs [24, 33,34,35,36,37]. The PREFS checklist assesses the quality of a DCE from five aspects, including Purpose, Respondents, Explanation, Findings, and Significance. Each aspect has two clearly stated questions to assess the appropriateness of a study. The study receives one mark if deemed acceptable in one aspect and zero if not acceptable. The quality assessment was conducted by two authors (SJ and RR). A score of five indicates good quality, while a score lower than three indicates significant problems in the study. We calculated the score for each included study and their arithmetic mean, which implied an average quality of the studies included in our review.
3 Results
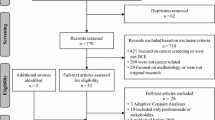
Figure 1 illustrates the process of study selection. The details of our search are in Supplementary Materials Section A. A total of 1133 studies were identified, from which 10 duplicates were removed. By screening the titles and abstracts, we excluded 1050 records. Through the full-text assessment, we further excluded 39 studies and then kept 34 studies for data extraction. No additional study was identified through the reference search.
3.1 Study Characteristics
While the included studies are from 13 countries, nearly half were conducted in the USA (n = 17, 50%) (Supplementary Table S1). Twenty-two studies were conducted after 2018, implying an increasing trend of using the DCE to elicit cancer patients’ preferences for medical treatments, including the targeted therapy (Fig. 2).
As indicated in Supplementary Table S1, the most frequently investigated cancer was breast cancer (n = 6, 18%), followed by melanoma (n = 5, 15%), renal cell carcinoma (n = 4, 12%), and prostate cancer (n = 4, 12%). Others included colorectal cancer, multiple myeloma, ovarian cancer, etc. The sample size ranged from 18 [38] to 444 [39] patients, with a median of 148. About 76% of studies (n = 26) had a sample size greater than 100 and 32% (n = 11) larger than 200 (Table 1).
Most studies reported the way in which the DCE was conducted. Twenty-seven studies (79%) conducted online surveys, and five (15%) conducted face-to-face interviews [39,40,41,42,43]. Four studies (12%) applied paper-based questionnaires [38, 44,45,46], and four (12%) used more than one administration method [38, 41, 44, 46]. The face-to-face approach was less frequently applied for preference elicitation than the online approach, probably because of the difficulties in the practice of the face-to-face approach. However, it is more likely to help respondents understand the survey questions correctly than online surveys [28]. One drawback of the online approach was the low response rate [47]. Five such studies (15%) reported a patient response rate of less than 30% [47,48,49,50,51].
3.2 Study Design
Most studies used literature searches and expert opinion to identify the attributes and associated levels of a DCE. About half of the studies (n = 16, 47%) used in-depth or semi-structured interviews. Only two studies (6%) involved patient focus groups in developing the attributes [41, 52]. We found that the attribute identification process was not sufficiently reported, which may have impacted the transparency and validity of some DCEs. Once attributes and levels were identified, most studies (n = 31, 91%) conducted a pilot study to assess and refine the DCE questionnaire. The number of patients involved in the pilot studies varied from 3 to 39 [53, 54].
About half (n = 17, 50%) of the studies applied the D-efficient or D-optimal design to generate choice sets, while the rest used other designs (e.g., orthogonal arrays) or did not introduce their study designs. The number of total DCE choice sets ranged from 18 [39] to 2250 [55]. When the total choice sets were beyond the cognitive capability of respondents, the blocking technique was used to reduce the number of choice tasks in one questionnaire [13]. Seven studies (21%) included opt-out or status quo options [43, 44, 53, 56,57,58,59].
There was no consensus among the included studies when identifying an appropriate sample size. Seven studies (21%) used the rule of thumb for minimum sample size estimation [42, 43, 46, 54, 60,61,62]. Six studies (18%) referred to other DCE studies to justify the sample size [50, 51, 62,63,64,65]. One study (3%) used the parametric approach for sample size calculation proposed by Rose and Bliemer [59]. Twenty-one studies (62%) did not explicitly report how the sample size was determined.
3.3 Study Quality
Table 1 provides a quality assessment of the included studies. PREFS scores of the 34 studies ranged from 2 to 4, with a mean of 3.38 (SD: 0.65). No study scored 5. Most studies scored 3 (n = 15, 44%) or 4 (n = 16, 47%), and three studies (9%) scored 2 [50, 56, 66]. Although most studies clearly stated the research purpose, some did not design the study well or provide a comprehensive analysis. For example, we found that most studies (n = 32, 94%) did not assess the impact of non-responders on their preferences. Sixteen studies (47%) excluded some responses without a clear rationale.
3.4 Attribute Frequency
Figure 3 illustrates how frequently different attributes (i.e., outcome, cost, or process) were used in the studies we reviewed (a complete list is provided in Supplementary Table S2). We found that the administration regimen was the most common attribute, which appeared in 25 studies (74%). The administration regimen included the frequency/route/mode of administration, treatment duration, and food restrictions.
Outcome-type attributes were also common, most referring to the risk of adverse events and treatment efficacy. For example, the risk of nausea/vomiting/diarrhea appeared in 15 studies (44%), the risk of fatigue/tiredness in 13 studies (38%), and the risk of skin disorder in 12 studies (35%). The attributes belonging to treatment efficacy, such as OS and PFS, appeared in most studies. For example, PFS was considered in 24 studies (71%) and OS in 11 studies (32%). No DCEs in this review considered health-related quality-of-life (HRQoL) as an outcome attribute.
Cost-type attributes were included in nine studies (26%). This type included out-of-pocket (OOP) cost of treatment (n = 8, 24%) and work loss due to treatment [45] (n = 1, 3%). All studies considering cost attributes indicated that patients preferred lower costs. Other studies did not include the cost in the DCE, likely because the authors wanted to focus on patients’ trade-offs between efficacy and adverse events.
3.5 Attribute Relative Importance
Figure 4 illustrates the relative importance of different types of attributes. We found that cancer patients perceived OS as the most critical attribute relative to other attributes. All efficacy attributes were considered more important than those concerning adverse events and treatment administration. Cost attributes were perceived as important as efficacy attributes. The least essential attributes were those regarding treatment processes, such as administration regimens.
Another perspective we examined was related to ways in which cancer patients perceive the attributes by ranking the proportion of one attribute as the most important among the selected DCEs. Figure 5 illustrates the proportions from this perspective. We found that cancer patients perceived OS as the most (or second most) important attribute in about 81% of studies that incorporated OS as a DCE attribute. PFS was perceived as the most (or second most) important attribute in about 58% of studies, and the cost was the most (or second most) important in about 55% of studies. In contrast, the administration regimen was perceived as most important in about 11% of studies, and other process-type attributes were not perceived as most important in any study.
The proportion of different types of attributes perceived as important in the selected DCE studies. Rank 1, perceived as the most important. Rank 2, perceived as the second most important. Rank 3, perceived as the third most important. Rank 4+, perceived as the fourth most important (or even lower). Y-axis represents the proportion of studies. Example interpretation: Overall survival (OS) was perceived as the most important attribute in 45% of studies that incorporated OS as a DCE attribute, the 2nd most important attribute in 36% of studies, the 3rd most important attribute in 0% of studies, and the 4th most important (or below) attribute in 18% of studies. PFS progression-free survival
3.6 Willingness to Pay
Table 2 provides an overview of patients’ WTP for improvements in survival. There were five studies (15%) examining patients’ WTP for PFS improvement [42, 43, 51, 57, 59]. Our calculation indicated that, on average, patients were willing to pay $561 (95% CI $415–$758) OOP per month for a 1-month increase in PFS. The largest number appeared in Gonzales et al, which found that patients were willing to pay $1039 with a 95% CI ranging from $755 to $1338 for a 1-month increase in PFS [51].
Four studies examined patients’ WTP for OS improvement [44, 51, 57, 58]. Our calculation indicated that the average WTP for a 1-month increase in OS was $716 (95% CI $524–$958) OOP. Although Stenehjem et al reported that the WTP was as low as $88 (95% CI $67–$110), Gonzales et al found that patients’ WTP was as high as $1638 (95% CI $1207–$2102) for a 1-month increase in OS [51].
3.7 Heterogeneity
Among the included studies, 11 (32%) used MIXL or RPL, 10 (29%) used Hierarchical Bayesian models, and 1 (3%) used the latent class model. The results indicated that most studies investigated unobserved preference heterogeneity.
A total of 16 studies investigated observed preference heterogeneity by analyzing the interactions between patients’ characteristics and attributes. Ten studies (29%) reported investigations of interactions between age and attributes [43, 45, 47, 48, 54, 60, 67,68,69,70] with mixed results. Two studies found that older patients placed higher weight on PFS than younger patients [47, 68]. Four studies reported that younger patients placed higher weight on PFS [54, 69] or OS [67, 70] than older patients. The rest found no significant interactions between age and preferences.
Four studies (12%) explored interactions between gender and preferences [43, 50, 67, 68]. One study reported that female patients were more averse to the shortest PFS (3 months of PFS) than male patients [50], implying that females had a stronger preference for longer PFS than males. Another study identified a significant interaction term between females and cost with a negative coefficient (− 0.075) [43], implying that females had a stronger preference for smaller costs than males. The rest found no significant interactions between gender and preferences [67, 68].
Based on the calculation of attribute relative importance, we found the results between melanoma and prostate cancer contrasted significantly. Figure 6 illustrates how the preferences of patients with melanoma and prostate cancer differ from each other. As indicated in the figure, patients with prostate cancer valued efficacy-type attributes the most, compared with patients with other types of attributes. In contrast, melanoma patients perceived cost as the most important attribute. The preferences toward PFS and OS revealed another difference between the two types of patients. Patients with prostate cancer were more concerned about PFS than OS, while melanoma patients cared about OS more than PFS.
When comparing the preferences of melanoma patients and all cancer patients, we found they had similar preference patterns, except that melanoma patients ranked cost as the most important factor. Patients with prostate cancer did not prioritize cost but valued PFS the most. The characteristics of different cancers might explain the heterogeneity observed by cancer type. Decision-makers might need to consider the preference patterns between different types of cancer patients.
4 Discussion
Although targeted pharmacotherapy has demonstrated promising outcomes in cancer treatment, patient preferences for this therapy remain unclear. Our study represents the first effort to systematically review the DCE literature on this topic and synthesize evidence on patient preferences for targeted pharmacotherapies. This review identified 34 eligible studies from 13 countries, covering 14 types of cancers, such as breast, ovarian, kidney, prostate, and skin cancers. The review reveals a rising trend of DCEs on this topic, as most studies were published after 2018, which warranted the necessity for evidence synthesis that has yet to be undertaken. In this review, we utilized the primary estimates to derive secondary results for evidence synthesis, such as the relative importance of attributes and patients’ WTP for survival. We explicitly focused on targeted cancer therapy, which may help to inform clinical decisions and pharmaceutical regulations on targeted therapy.
4.1 Attributes and Relative Importance
A large number of attributes were identified and categorized into three groups: outcome, process, and cost. We found that patients placed higher weights on the outcome (e.g., OS) and cost attributes than on the attributes concerning treatment processes (e.g., administration regimen) in their choices. In addition, OS was perceived as the most (or second most) important attribute in most DCEs. Considering that the DCEs had different levels of OS and were conducted among cancer patients in heterogeneous contexts, our results indicate that patients generally value OS the most among all the attributes.
Health economists have widely recognized that the HRQoL of patients is crucial for the health outcome assessment because it provides a comprehensive valuation of health interventions beyond clinical outcomes (e.g., PFS and OS) [71,72,73]. Although cancer patients were found to value outcome attributes the most in this review, no studies included in the review considered HRQoL as an attribute. One possible reason is that, although generic HRQoL instruments exist (e.g., EQ-5D and SF-6D), DCE practitioners may find them too generic to best capture the key outcomes of targeted therapy [74]. In the meantime, widely accepted cancer-specific HRQoL instruments are still lacking. Although some instruments have been developed [75, 76], it may take time for them to gain wide popularity among clinicians and DCE practitioners. Nevertheless, including HRQoL as an outcome attribute in future DCEs in this area is warranted. Researchers could use overall scores or qualitative descriptions of selected dimensions as potential attributes. Researchers could also use graphs (e.g., 0–100 scale) to help explain such attributes.
4.2 WTP for Survival
We found patients, on average, were willing to pay about $561 (95% CI $415–$758) from their own pockets (i.e., OOP cost) for a 1-month increase in PFS if they were treated by targeted therapy. The average WTP for a 1-month increase in OS was even higher, up to $716 (95% CI $524–$958). These imply mean WTPs of $6732 and $8592 for a 1-year increase in PFS and OS, respectively.
The level of WTP was associated with several factors. The first factor is the cancer type. As indicated in Table 2, the WTPs of patients with renal or colorectal cancers were higher than those with ovarian or breast cancers. According to cancer epidemiological statistics, the 5-year survival rate of breast and ovarian cancers are higher than that of renal and colorectal cancers [77]. A previous study found that patients with low-survival-rate cancers are willing to pay more than those with high-survival-rate cancers [78].
The second factor is the income level of patients. The economic theory suggests wealthier people have a greater WTP for goods and services. Therefore, a common finding in the health economics literature is that patients with higher incomes are willing to pay more for healthcare services than those with lower incomes [28, 78]. In the case of targeted therapy, the income level of cancer patients is likely to be positively associated with their WTP. However, the DCEs in the current review did not explore this relationship.
In addition, other characteristics of cancer patients, such as age and gender, may impact their WTPs. Less than half of DCEs (n = 16, 47%) in this review investigated interactions between patients’ characteristics and preferences. For example, some studies indicate that older patients place a higher weight on PFS than younger patients [47, 68], while other studies suggest the opposite [54, 67, 69]. Future DCE studies in this area should further explore such associations. Willingness-to-pay values may also be affected by the different attribute levels used to define the survival attributes [79].
Contingent valuation or model-based estimation may derive WTP values much larger than the synthesized WTP from DCEs in this review. For example, Olofsson and colleagues used a contingent method to measure the end-of-life premium in cancer and concluded that the mean value of a QALY was about €528,000 [80]. Diaby and colleagues used a Markov model to measure the mean value of a QALY for metastatic breast cancer patients and obtained values larger than $117,000 ($117,519.8–$185,210.7) per QALY [81]. The WTP values derived by different approaches might not be directly comparable. One explanation is that we derive WTP estimates from only seven DCEs. Most studies do not use OOP cost as an attribute, and thus we were unable to estimate the WTPs in these studies. Another explanation is that the non-DCE studies may have incorporated an end-of-life premium for cancer patients at advanced stages, future medical and nonmedical costs for patients after treatment, and the impact of cancers on their productivity [82, 83]. The third explanation is the presence of potential biases that occurred in implementing the seven DCEs, such as the selection bias [16, 84]. These issues need to be addressed in future research to reconcile the differences between different methods and produce more robust and reliable WTP estimates.
4.3 Non-Response Bias
The non-response bias remains a major concern in this literature since the preferences are elicited from cancer patients. Some patients may die soon after diagnosis, while others at advanced cancer stages may have difficulties participating in DCE studies. Non-response bias can impact the external validity of the DCEs as they may imply un- or under-representative samples. As indicated by our quality assessment (Table 1), no studies addressed the non-response problem or ascertained the extent of non-response bias to which it may lead to an unrepresentative sample. Future DCE studies may need to compare the responders and non-responders in terms of their characteristics that may impact preferences. Alternatively, one may refer to the characteristics of the target population to assess the sample representativeness. If the characteristics of the sample are not consistent with the target population, the practitioners may consider approaches to reweight their sample to match the key statistics of the population.
4.4 Opt-out Option
This review found that few DCEs provided opt-out or status quo options to respondents. Respondents were often forced to choose between two or three therapeutic alternatives. However, in the context of oncology, palliative care and withdrawal of treatment (that may cause extreme economic burden) are realistic options for patients. These options could be represented in choice tasks by opt-out or status quo options, which allow respondents not to choose the therapeutical alternatives in a DCE task [85]. This is important as the restricted choice tasks may bias the analysis results [86]. We therefore recommend the inclusion of the opt-out/status quo options in future DCEs to mimic the real-world context for cancer patients.
4.5 Other Issues
Most studies included in the review have not followed the general best practice in DCE methodology. Almost half of the studies included in the review did not claim the use of qualitative research to identify attributes, making the assessment of the reliability of the selected attributes challenging. Among those using a qualitative phase, the disclosure of details and results on how qualitative findings affected the DCE design was insufficient. Most studies did not report a pilot phase and how the sample size was calculated, likely because they had not been done. While patient recruitment is understandably challenging in this area, researchers should endeavour to follow the best practice for conducting DCE studies [11, 87].
4.6 Strengths and Limitations
This review provides the first synthesized evidence on patient preference for targeted therapy. The literature search process is comprehensive, with clearly defined inclusion and exclusion criteria. We applied rigorous quality control approaches to ensure correctness and preciseness in the literature screening and information retrieval process. For example, two junior authors independently screened the literature, extracted information from the selected studies and cross-checked the results. Two senior authors examined the retrieved information, and the evidence synthesis results. Any disagreements were resolved by discussion involving the four authors.
Although this review followed best practices, it has some limitations. First, we could not calculate the relative importance of attributes for each DCE included in the review as some studies did not report the estimates of preference weights. We could only approximate the weights from other sources, such as the graphs for these studies. Second, the WTP results reported in this review should be interpreted with caution as they are based on a small number of studies and thus have limited representativeness. It might be difficult to draw solid conclusions from the limited number of studies. Instead, the estimates provide a direction, and future research is needed. Third, the PREFS checklist used for the quality assessment may have limitations. It provides five questions for quality assessment and may miss important criteria, e.g., the validity and comprehensiveness of the attribute identification process and analysis of preference heterogeneity. Also, it does not provide sufficient tools to assess the biases in a DCE, such as selection bias and non-response bias. Considering the highly heterogeneous contexts where DCE studies are conducted, we call for establishing a specific checklist in oncology to help DCE researchers and practitioners with their study design and report, as well as future quality assessments in systematic reviews.
5 Conclusions
This review provides synthesized evidence on patient preferences regarding targeted cancer pharmacotherapies. The results on the attribute relative importance may inform policymakers, physicians, and other stakeholders about patients’ perceptions of this novel therapeutic option. The synthesized WTP for survival, although it included only a limited number of studies due to eligibility issues, may provide a direction for estimating patients’ monetary valuation of targeted therapy. We highlight that in order to achieve a better understanding and consensus among stakeholders surrounding targeted therapy, more DCEs or other stated preference studies are warranted for further evidence. To achieve robust evidence and facilitate evidence synthesis, a standardization of the DCE design and implementation is also needed. Future research could follow the recommendations on study design in this review to improve the internal and external validity and provide more robust results in this increasingly important field.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49.
Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372:793–5.
de Bono JS, Ashworth A. Translating cancer research into targeted therapeutics. Nature. 2010;467:543–9.
Martini M, Vecchione L, Siena S, Tejpar S, Bardelli A. Targeted therapies: how personal should we go? Nat Rev Clin Oncol. 2012;9(2):87–97.
Bedard PL, Hyman DM, Davids MS, Siu LL. Small molecules, big impact: 20 years of targeted therapy in oncology. Lancet (London, England). 2020;395:1078–88.
Park K, Tan E-H, O’Byrne K, Zhang L, Boyer M, Mok T, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016;17:577–89.
Bouvy JC, Cowie L, Lovett R, Morrison D, Livingstone H, Crabb N. Use of patient preference studies in HTA decision making: a NICE perspective. Patient Patient-Cent Outcomes Res. 2020;13(2):145–9.
FDA. Patient-Focused Drug Development. 2020. accessed on Aug 01 2022. https://www.fda.gov/drugs/developmentapprovalprocess/ucm579400.htm.
EMA. Patients and Consumers. 2020. accessed on Aug 01 2022. https://www.ema.europa.eu/en/partners-networks/patients-consumers.
Louviere JJ, Flynn TN, Carson RT. Discrete choice experiments are not conjoint analysis. J Choice Model. 2010;3(3):57–72.
Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making. Pharmacoeconomics. 2008;26(8):661–77.
Soekhai V, Bekker-Grob EWd, Ellis AR, Vass CM. Discrete choice experiments in health economics: past, present and future. Pharmacoeconomics. 2019;37(2):201–26.
Jiang S, Anis AH, Cromwell I, Mohammadi T, Schrader KA, Lucas J, et al. Health-care practitioners’ preferences for the return of secondary findings from next-generation sequencing: a discrete choice experiment. Genet Med. 2020;22(12):2011–2019.
Merlo G, Driel Mv, Hall L. Systematic review and validity assessment of methods used in discrete choice experiments of primary healthcare professionals. Health Econ Rev. 2020;10(1):39.
Liu P, Jiang S, Li S. A systematic review of measuring the preference for targeted therapy in cancer patients by discrete choice experiment. Chin J Cancer Prev Treat. 2021;28(4):318–22.
Lancsar E, Fiebig DG, Hole AR. Discrete choice experiments: a guide to model specification, estimation and software. Pharmacoeconomics. 2017;35(7):697–716.
Damm K, Vogel A, Prenzler A. Preferences of colorectal cancer patients for treatment and decision-making: a systematic literature review. Eur J Cancer Care. 2014;23:762–72.
Guerra RL, Castaneda L, de Albuquerque RCR, Ferreira CBT, Corrêa FM, Fernandes RRA, et al. Patient preferences for breast cancer treatment interventions: a systematic review of discrete choice experiments. Patient. 2019;12:559–69.
Livingstone A, Agarwal A, Stockler MR, Menzies AM, Howard K, Morton RL. Preferences for immunotherapy in melanoma: a systematic review. Ann Surg Oncol. 2020;27:571–84.
Neal D, Feit E, Etzkorn J. Patient preferences for the treatment of basal cell carcinoma: a mapping review of discrete choice experiments. Dermatol Surg. 2018;44:1041–9.
Schmidt K, Damm K, Prenzler A, Golpon H, Welte T. Preferences of lung cancer patients for treatment and decision-making: a systematic literature review. Eur J Cancer Care. 2016;25:580–91.
Showalter TN, Mishra MV, Bridges JF. Factors that influence patient preferences for prostate cancer management options: a systematic review. Patient Prefer Adherence. 2015;9:899–911.
Sugitani Y, Sugitani N, Ono S. Quantitative preferences for lung cancer treatment from the patients’ perspective: a systematic review. Patient. 2020;13:521–36.
Bien DR, Danner M, Vennedey V, Civello D, Evers SM, Hiligsmann M. Patients’ preferences for outcome, process and cost attributes in cancer treatment: a systematic review of discrete choice experiments. Patient Patient-Cent Outcomes Res. 2017;10(5):553–65.
Collacott H, Soekhai V, Thomas C, Brooks A, Brookes E, Lo R, et al. A systematic review of discrete choice experiments in oncology treatments. Patient. 2021;14(6):775–790.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;29(372): n71.
Hauber AB, González JM, Groothuis-Oudshoorn CG, Prior T, Marshall DA, Cunningham C, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR Conjoint Analysis Good Research Practices Task Force. Value Health. 2016;19:300–15.
Jiang S, Gu Y, Yang F, Wu T, Wang H, Cutler H, et al. Tertiary hospitals or community clinics? An enquiry into the factors affecting patients' choice for healthcare facilities in urban China. China Econ Rev. 2020;63:101538.
Hensher DA, Greene WHJT. The mixed logit model: the state of practice. 2003;30(2):133–76.
Shemilt I, Thomas J, Morciano M. A web-based tool for adjusting costs to a specific target currency and price year. Évid Policy J Res Debate Pract. 2010;6(1):51–9.
Joy SM, Little E, Maruthur NM, Purnell TS, Bridges JF. Patient preferences for the treatment of type 2 diabetes: a scoping review. Pharmacoeconomics. 2013;31:877–92.
Bridges JFP, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, et al. Conjoint analysis applications in health—a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value in Health. 2011;14(4):403–13.
Showalter TN, Mishra MV, Bridges JFP. Factors that influence patient preferences for prostate cancer management options: a systematic review. Patient Prefer Adher. 2015;9:899–911.
Whitty JA, Gonçalves ASO. A systematic review comparing the acceptability, validity and concordance of discrete choice experiments and best-worst scaling for eliciting preferences in healthcare. Patient Patient-Cent Outcomes Res. 2018;11(3):301–17.
Zhou M, Thayer WM, Bridges JFP. using latent class analysis to model preference heterogeneity in health: a systematic review. Pharmacoeconomics. 2018;36(2):175–87.
Tünneßen M, Hiligsmann M, Stock S, Vennedey V. Patients’ preferences for the treatment of anxiety and depressive disorders: a systematic review of discrete choice experiments. J Med Econ. 2020;23(6):1–11.
Lack A, Hiligsmann M, Bloem P, Tünneßen M, Hutubessy R. Parent, provider and vaccinee preferences for HPV vaccination: a systematic review of discrete choice experiments. Vaccine. 2020;38(46):7226–38.
Eriksson J, Landfeldt E, Ireland S, Jackson C, Wyatt E, Gaudig M. Stated preferences for relapsed or refractory mantle cell lymphoma treatments in Sweden and Germany. Future Oncol. 2020;16(13):859–68.
Park MH, Jo C, Bae EY, Lee EK. A Comparison of preferences of targeted therapy for metastatic renal cell carcinoma between the patient group and health care professional group in South Korea. Value Health. 2012;15:933–9.
Lee JY, Kim K, Lee YS, Kim HY, Nam EJ, Kim S, et al. Treatment preferences of advanced ovarian cancer patients for adding bevacizumab to first-line therapy. Gynecol Oncol. 2016;143:622–7.
Wilke T, Mueller S, Bauer S, Pitura S, Probst L, Ratsch BA, et al. Treatment of relapsed refractory multiple myeloma: which new PI-based combination treatments do patients prefer? Patient Prefer Adher. 2018;12:2387–96.
Nazari A, Lopez-Valcarcel BG, Najafi S. Preferences of patients with HR+ & HER2- breast cancer regarding hormonal and targeted therapies in the first line of their metastatic stage: a discrete choice experiment. Value Heal Regional Issues. 2021;25:7–14.
Wong XY, Lim AQJ, Shen Q, Chia JWK, Chew MH, Tan WS, et al. Patient preferences and predicted relative uptake for targeted therapies in metastatic colorectal cancer: a discrete choice experiment. Curr Med Res Opin. 2020;36(10):1–10.
Stenehjem DD, Au TH, Ngorsuraches S, Ma J, Bauer H, Wanishayakorn T, et al. Immunotargeted therapy in melanoma: patient, provider preferences, and willingness to pay at an academic cancer center. Melanoma Res. 2019;29(6):626–634.
Uemura H, Matsubara N, Kimura G, Yamaguchi A, Ledesma DA, DiBonaventura M, et al. Patient preferences for treatment of castration-resistant prostate cancer in Japan: a discrete-choice experiment. Bmc Urol. 2016;16(1):63.
Eliasson L, de Freitas HM, Dearden L, Calimlim B, Lloyd AJ. Patients’ preferences for the treatment of metastatic castrate-resistant prostate cancer: a discrete choice experiment. Clin Ther. 2017;39:723–37.
Mansfield C, Ndife B, Chen J, Gallaher K, Ghate S. Patient preferences for treatment of metastatic melanoma. Future Oncol. 2019;15(11):1255–68.
Hauber AB, Arellano J, Qian Y, Gonzalez JM, Posner JD, Mohamed AF, et al. Patient preferences for treatments to delay bone metastases. Prostate. 2014;74:1488–97.
Mohamed AF, Hauber AB, Neary MP. Patient benefit-risk preferences for targeted agents in the treatment of renal cell carcinoma. Pharmacoeconomics. 2011;29(11):977–88.
Wong MK, Mohamed AF, Hauber AB, Yang J-C, Liu Z, Rogerio J, et al. Patients rank toxicity against progression free survival in second-line treatment of advanced renal cell carcinoma. J Med Econ. 2012;15(6):1139–48.
Gonzalez JM, Doan J, Gebben DJ, Boeri M, Fishman M. Comparing the relative importance of attributes of metastatic renal cell carcinoma treatments to patients and physicians in the United States: a discrete-choice experiment. Pharmacoeconomics. 2018;36:973–86.
Auclair D, Mansfield C, Fiala MA, Chari A, Cole CE, Kaufman JL, et al. Preferences and priorities for relapsed multiple myeloma treatments among patients and caregivers in the United States. Patient Prefer Adher. 2022;16:573–85.
Beusterien K, Middleton M, Feng Wang P, Rao S, Kotapati S, Sabater J, et al. Patient and physician preferences for treating adjuvant melanoma: a discrete choice experiment. J Cancer Ther. 2017;8:37–50.
Omori Y, Enatsu S, Cai Z, Ishiguro H. Patients’ preferences for postmenopausal hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer treatments in Japan. Breast Cancer-tokyo. 2019;26(5):652–62.
Cromwell I, Smith LW, Hoek K, Hedden L, Coldman AJ, Cook D, et al. Cost‐effectiveness analysis of primary human papillomavirus testing in cervical cancer screening: Results from the HPV FOCAL Trial. Cancer Med. 2021;10(9):2996–3003.
Stellato D, Thabane M, Eichten C, Delea TE. Preferences of Canadian patients and physicians for adjuvant treatments for melanoma. Curr Oncol. 2019;26(6):755–65.
Havrilesky LJ, Lim S, Ehrisman JA, Lorenzo A, Secord AA, Yang JC, et al. Patient preferences for maintenance PARP inhibitor therapy in ovarian cancer treatment. Gynecol Oncol. 2020;156:561–7.
Fifer SJ, Ho K-A, Lybrand S, Axford LJ, Roach S. Alignment of preferences in the treatment of multiple myeloma—a discrete choice experiment of patient, carer, physician, and nurse preferences. BMC Cancer. 2020;20(1):546.
Ngorsuraches S, Thongkeaw K. Patients’ preferences and willingness-to-pay for postmenopausal hormone receptor-positive, HER2-negative advanced breast cancer treatments after failure of standard treatments. Springerplus. 2015;4(1):674.
Srinivas S, Mohamed AF, Appukkuttan S, Botteman M, Ng X, Joshi N, et al. Patient and caregiver benefit-risk preferences for nonmetastatic castration-resistant prostate cancer treatment. Cancer Med-us. 2020;9(18):6586–96.
Maculaitis MC, Liu X, Will O, Hanson M, McRoy L, Berk A, et al. Oncologist and patient preferences for attributes of CDK4/6 inhibitor regimens for the treatment of advanced/metastatic HR positive/HER2 negative breast cancer: discrete choice experiment and best-worst scaling. Patient Prefer Adher. 2020;14:2201–14.
Hauber AB, Gonzalez JM, Coombs J, Sirulnik A, Palacios D, Scherzer N. Patient preferences for reducing toxicities of treatments for gastrointestinal stromal tumor (GIST). Patient Prefer Adher. 2011;5:307–14.
de Freitas HM, Ito T, Hadi M, Al-Jassar G, Henry-Szatkowski M, Nafees B, et al. Patient preferences for metastatic hormone-sensitive prostate cancer treatments: a discrete choice experiment among men in three European countries. Adv Ther. 2019;36:318–32.
Bridges JFP, Cruz M, Pavilack M, Flood E, Janssen EM, Chehab N, et al. Patient preferences for attributes of tyrosine kinase inhibitor treatments for EGFR mutation-positive non-small-cell lung cancer. Future Oncol. 2019;15(34):3895–907.
Mohamed AF, González JM, Fairchild A. Patient benefit-risk tradeoffs for radioactive iodine-refractory differentiated thyroid cancer treatments. J Thyroid Res. 2015; p. 438235.
Beusterien K, Maculaitis MC, Hallissey B, Gaschler MM, Smith ML, Law EH. Patient, oncologist, and payer preferences for adjuvant endocrine therapy and CDK4/6 inhibitor regimens in early-stage breast cancer: a discrete choice experiment. Patient Prefer Adher. 2021;15:611–23.
Weilandt J, Diehl K, Schaarschmidt M, Kieker F, Sasama B, Pronk M, et al. Patient preferences in adjuvant and palliative treatment of advanced melanoma: a discrete choice experiment. Acta Dermato Venereol. 2020;100(6):adv00083-9.
González JM, Ogale S, Morlock R, Posner J, Hauber B, Sommer N, et al. Patient and physician preferences for anticancer drugs for the treatment of metastatic colorectal cancer: a discrete-choice experiment. Cancer Manag Res. 2017;9:149–58.
Le H, Ryan K, Wahlstrom SK, Maculaitis MC, Will O, Mulvihill E, et al. Oncologist and patient preferences for novel agents in first-line treatment for chronic lymphocytic leukemia: commonalities and disconnects. Patient Prefer Adher. 2021;15:99–110.
Ashaye A, Thomas C, Dalal M, Kota V, Krucien N, Sae-Hau M, et al. Patient preferences for frontline therapies for Philadelphia chromosome-positive acute lymphoblastic leukemia: a discrete choice experiment. Future Oncol. 2022;18(17):2075–85.
Yang F, Jiang S, He X-n, Li H-c, Wu H-y, Zhang T-t, et al. Do rural residents in china understand EQ-5D-5L as intended? Evidence from a qualitative study. Pharmacoecon Open. 2021;5(1):101–109.
Jiang S, Chen Z, Wu J, Zang X, Jiang Y. Addressing methodological and ethical issues in practicing health economic evaluation in China. Jo Glob Health. 2020; 72: 1–4.
Chen Z, Zhou L, Jiang S, Haddix A. Identifying options of best value: use of economic evaluation in public health. China Cdc Wkly. 2020;2(5):75–8.
Krahn M, Bremner KE, Tomlinson G, Ritvo P, Irvine J, Naglie G. Responsiveness of disease-specific and generic utility instruments in prostate cancer patients. Qual Life Res. 2006;16(3):509.
Blazeby JM, Hall E, Aaronson NK, Lloyd L, Waters R, Kelly JD, et al. Validation and reliability testing of the EORTC QLQ-NMIBC24 questionnaire module to assess patient-reported outcomes in non–muscle-invasive bladder cancer. Eur Urol. 2014;66(6):1148–56.
Wagner LI, Robinson D, Weiss M, Katz M, Greipp P, Fonseca R, et al. Content development for the functional assessment of cancer therapy-multiple myeloma (FACT-MM): use of qualitative and quantitative methods for scale construction. J Pain Symptom Manag. 2012;43(6):1094–104.
Allemani C, Matsuda T, Carlo VD, Harewood R, Matz M, Nikšić M, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. The Lancet. 2018;391(10125):1023–75.
Chaikumbung M. Democracy, culture and cancer patients’ willingness to pay for healthcare services: a meta-analysis. Inq J Heal Care Org Provis Financ. 2021;58:004695802110248.
Ryan M, Wordsworth S. Sensitivity of willingness to pay estimates to the level of attributes in discrete choice experiments. Scot J Polit Econ. 2000;47(5):504–24.
Olofsson S, Gerdtham UG, Hultkrantz L, Persson U. Measuring the end-of-life premium in cancer using individual ex ante willingness to pay. Eur J Health Econ. 2018;19(6):807–20.
Diaby V, Adunlin G, Ali AA, Zeichner SB, Lopes GdL, Kohn CG, et al. Cost-effectiveness analysis of 1st through 3rd line sequential targeted therapy in HER2-positive metastatic breast cancer in the United States. Breast Cancer Res Treat. 2016;160(1):187–96.
Jiang S, Wang Y, Zhou J, Jiang Y, Liu GG-E, Wu J. Incorporating future unrelated medical costs in cost-effectiveness analysis in China. Bmj Global Heal. 2021;6(10):e006655.
Jiang S, Wang Y, Si L, Zang X, Gu Y-Y, Jiang Y, et al. Incorporating productivity loss in health economic evaluations: a review of guidelines and practices worldwide for research agenda in China. Bmj Global Heal. 2022;7(8): e009777.
Johnson FR, Lancsar E, Marshall D, Kilambi V, Mühlbacher A, Regier DA, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR conjoint analysis experimental design good research practices task force. Value in Health. 2013;16(1):3–13.
Campbell D, Erdem S. Including Opt-Out Options in Discrete Choice Experiments: Issues to Consider. Patient. 2019;12(1):1–14.
Train KE. Discrete choice methods with simulation. Cambridge university press, 2009.
Johnson FR, Lancsar E, Marshall D, Kilambi V, Mühlbacher A, Regier DA, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value in Health. 2013;16(1):3–13.
Lee J-Y, Kim K, Lee YS, Kim HY, Nam EJ, Kim S, et al. Treatment preferences of advanced ovarian cancer patients for adding bevacizumab to first-line therapy. Gynecol Oncol. 2016;143(3):622–7.
Stellato D, Thabane M, Eichten C, Delea TE. Preferences of canadian patients and physicians for treatment of HR+/HER2− advanced breast cancer. Curr Oncol. 2021;28(1):491–508.
Stone RL, Cambron-Mellott MJ, Beusterien K, Maculaitis MC, Ritz S, Mulvihill E, et al. Patients’ and oncologists’ preferences for second-line maintenance PARP inhibitor therapy in epithelial ovarian cancer. Future Oncol. 2022;18(4):491–503.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Conflicts of interest/Competing interests
No authors have conflicts of interest to declare.
Authors’ contributions
Conceptualization: SJ; Methodology: SJ and YG; Literature search: SJ, RR, and VJ; Screening and data extraction: RR, PL, SJ, and YG; Article quality assessment: SJ and RR; Formal analysis and investigation: SJ and RR; Writing—original draft preparation: SJ and RR; Writing – review and editing: YG, VJ, PL, and SL; Funding acquisition: YG and SL; Resources: YG and SL; Supervision: YG.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Jiang, S., Ren, R., Gu, Y. et al. Patient Preferences in Targeted Pharmacotherapy for Cancers: A Systematic Review of Discrete Choice Experiments. PharmacoEconomics 41, 43–57 (2023). https://doi.org/10.1007/s40273-022-01198-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40273-022-01198-8