Abstract
Introduction
Compared with early stages (eBC) metastatic BC (mBC) is incurable. In mBC, aggressive treatment may increase the duration of survival but may also cause severe treatment side effects. A better understanding how patients with BC value different aspects of drug therapy might improve treatment effectiveness, satisfaction and adherence. This systematic review aims to identify and summarise studies evaluating patient preferences for drug therapy of BC and to compare preferences of patients with eBC and mBC.
Methods
The systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The electronic databases PubMed and Web of Science were searched on 22 June 2023. All studies published to this point were considered. Original studies reporting patient preferences on BC drug therapy determined by any type of choice experiment were eligible. A narrative synthesis of the effect measures presented as relative importance ratings, trade-offs (required benefit to make a therapy worthwhile) or monetary values of the treatment attributes was reported for each study. Risk of bias assessment for individual studies was performed using the checklist for observational studies from the STROBE Statement and the checklist from ‘Conducting Discrete Choice Experiments to Inform Healthcare Decision Making: A User’s Guide’. The study protocol was registered at the PROSPERO database (CRD42022377031).
Results
A total of 34 studies met the inclusion criteria were included in the analysis evaluating the preferences of patients with eBC (n = 18), mBC (n = 10) or any stage BC (n = 6) on, for example, chemotherapy, endocrine therapy, hormonal therapy or CKD4/6-inhibitors using different types of choice experiments. Regardless of the stage, most patients valued treatment effectiveness in terms of survival gains higher than potential adverse drug reactions (ADRs). Treatment cost, mode of administration, treatment regimen and monitoring aspects were considered as least important treatment attributes. In addition, preferences concerning 16 different types of ADRs were described, showing high heterogeneity within BC stages. Yet, comparable results across BC stages were observed.
Conclusions
Regardless of the stage, patients with BC consistently valued survival gains as the most important attribute and were willing to accept the risk of potential ADRs. Incorporating patient preferences in shared decision making may improve the effectiveness of interventions by enhancing adherence to drug therapy in patients suffering from BC.
Plain Language Summary
Preferences of patients with breast cancer for drug therapy play a crucial role in treatment efficacy, satisfaction and adherence. In this systematic review following the PRISMA guidelines, 34 studies were analysed to determine patient preferences at different stages of breast cancer, comparing early stage and metastatic disease. Regardless of stage, patients with breast cancer consistently prioritised survival benefit as the most important treatment feature. This universal emphasis on survival held true even in the face of potential side effects, with patients willing to accept the associated risks. Conversely, factors unrelated to efficacy, such as the cost of treatment, route of administration, characteristics of the treatment regimen and monitoring aspects, were considered less important in treatment decisions. The study revealed a nuanced landscape of patient preferences, with greater variation within breast cancer stages than between them. While survival remained an unwavering priority, the variability in expressed preferences emphasises the individual nature of patient perspectives. In conclusion, incorporating patient preferences, particularly those that emphasise the importance of survival, into shared decision-making processes is a critical factor in improving treatment adherence. This patient-centred approach is likely to improve the overall effectiveness of breast cancer treatment and highlights the need for tailored strategies that take into account the individual preferences of patients at different stages of the disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Regardless of their disease stage, patients with breast cancer place the highest value on treatments that improve their chances of survival, even if they are associated with potential side effects. |
Healthcare decision-makers should emphasise empathic communication and consider including discussions on benefits and risks of treatment in guidelines to increase patient involvement in decision making and, thereby, improve treatment adherence. |
1 Introduction
Discordance between patient and healthcare provider (e.g. physicians) preferences regarding treatment decision making is common and has been reported for different diseases, including cancer [1]. Such discrepancies can lead to failure of delivering a patient’s preferred treatment, also described as preference misdiagnosis [1, 2]. This misdiagnosis often remains unrecognised because healthcare providers mistakenly believe that their treatment choice is in line with patients’ preferences [1, 2]. Hence, a better understanding how patients value different aspects of drug therapy and aligning clinical practice guidelines with these preferences might improve treatment effectiveness by improving adoption of, satisfaction with and adherence to drug therapy [3, 4]. Assessing patient preferences is especially relevant when presented decision scenarios in which choosing from different treatment strategies must be weighted between a potential benefit and a possible harm that impacts quality of life [5, 6].
In general, two categories of methods to identify patient preferences exist: (1) methods using ranking, rating, (time-)trade-off or choice designs, often referred to as conjoint analysis (CA), or discrete-choice experiments (DCE); these designs can be used to measure patient preferences for various attributes of a treatment; and (2) methods using direct elicitation of monetary values of an intervention [e.g. willingness to pay (WTP)]. In CA or DCE, respondents are presented a set of hypothetical scenarios with competing multimodal alternatives of an intervention [7]. Each alternative is described in terms of attributes and attribute levels [7, 8]. Attributes and attribute levels are usually identified from the literature, qualitative research studies or focus group interviews with relevant respondents and experts and should be tested in pilot studies [8, 9]. In the DCE, respondents are then asked to choose their preferred alternative for each choice set. The respondents’ choices give information on attribute relevance or importance and the degree at which individuals are willing to trade between the characteristics of the choice sets. In WTP experiments, the aim is to estimate the monetary value to gain a benefit or avoid harm (e.g. treatment side effects) [7]. Assessing health states and their utilities can further be used to determine patient preferences by ranking different health states using visual analogue scales (VAS) or standard gamble (SG) [10,11,12].
Breast cancer (BC) is the most common cancer in women worldwide with 2.3 million new BC cases per year and a mortality rate of 6.9% [13]. Treatment of BC involves multiple health care professionals (e.g. physician and psychologist) as well as several disciplines of physicians (e.g. oncologist and gynaecologist). The advances in drug therapy for BC, the availability of numerous treatment alternatives and the increasing personalisation of drug therapy have made treatment selection a very complex process [14]. Treatment regimens for BC vary significantly regarding effectiveness, potential side effects, frequency and mode of administration [14]. It is useful for health care providers to understand how these differences may influence individual patient preferences. Severe side effects occur in about 45% of patients and include for example nausea/vomiting, diarrhoea, pain or breast skin irritation [15]. Involving patients with BC in treatment decision making has shown to improve their satisfaction, short and long-term well-being, as well as increasing their level of comfort with the decision made [16,17,18].
A metastatic stage of the disease is present in about 30% of all cases and is defined as breast cancer that has spread to other parts of the body [19,20,21]. It is incurable with a median survival time for patients between two and four years [19,20,21,22]. Due to the heterogeneity of mBC, often no standardised therapeutic recommendation can be made [14]. In general, the treatment focuses on extending patients’ survival, ensuring adequate symptom management, and promoting quality of life (QoL) [14, 20, 21]. The incurable nature and the potential side effects of available treatments highlight the importance of patient preferences in decision making regarding the desired outcomes. An aggressive treatment may maximize the duration of survival, but it may also be associated with severe side effects [20, 21].
Several systematic reviews have been published on patient preferences for BC treatment, focusing on specific therapies (e.g. locoregional treatment of eBC or adjuvant gonadotropin-releasing hormone analogues) or on patients with eBC only [23,24,25,26]. To the best of our knowledge, no systematic review so far compared the preferences of patients with eBC and mBC on drug therapy. This systematic review, therefore, aims to summarise studies evaluating patient preferences for drug therapy for eBC and mBC determined by DCE/CA or WTP analyses. Furthermore, we aim to compare the reported preferences of patients with eBC and mBC regarding different aspects of drug therapy, such as treatment effectiveness, adverse drug reactions (ADR), treatment cost and mode of administration.
2 Methods
The study protocol was registered at the PROSPERO database (CRD42022377031). The review process was compliant with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [27].
2.1 Eligibility Criteria
Original observational studies published in English including early stage to metastatic breast cancer patients (≥ 18 years), eliciting the preferences on drug therapy for BC (e.g. chemotherapy, endocrine therapy or hormonal therapy) and using any type of choice experiment were included. Regarding BC stage, studies including patients with any stage were only included if sensitivity analyses for BC stage were performed. Studies on children, animal or in vitro studies were excluded. Further exclusion criteria were clinical/interventional studies, cost-effectiveness or cost-utility analyses, letters to the editor, commentaries, ethics vignette, methodological studies, study protocols, reviews and meta-analyses, guidelines or white papers. With regard to tumour entities, studies that only included patients with ductal carcinoma in situ or inflammatory breast cancer were excluded. Lastly, studies investigating patient preferences regarding other treatment factors (i.e. surgery, radiotherapy, non-pharmacologic treatment, chemoprevention, screening, diagnosis, biopsy, surveillance or follow-up care, shared decision making and pharmacogenomic testing) were excluded.
2.2 Information Sources and Search
Relevant reports were identified by searching the electronic databases PubMed and Web of Science. The search in Web of Science was refined by excluding meeting abstracts. The search date was 22 June 2023. All studies published to this point were considered. The search strategy is displayed in Supplementary Table 1.
2.3 Study Selection
All retrieved records were uploaded into the Rayyan.ai software tool for systematic reviews [28] and in EndNote 20 (Thompson Reuters). Duplicate records were removed. Titles and abstracts of all retrieved records were screened in Rayyan.ai by two independent reviewers (L.B. and S.J.H.) to remove non-eligible articles. The second reviewer was blinded for the decisions of the first reviewer until completion of the screening process. Afterwards, disagreements between individual judgements were resolved by discussion.
2.4 Data extraction, Data Items and Synthesis of Results
Extracted data related to the (1) study characteristics: first author, year, country, study type, data source, breast cancer stage, sample size and inclusion/exclusion criteria; (2) choice experiment: type of choice experiment, type of choice, treatment scenarios, operationalisation (attributes, levels and choice alternatives), number of tasks per patient, experimental design and estimation method; and (3) outcome studied: patient preferences and influencing factors (if available). Two reviewers (L.B. and S.J.H.) extracted the data independently. If required, a third reviewer (J.P.R.) resolved any discrepancies. Missing information was documented within a data extraction form. Study investigators were not contacted for unreported data or additional details. All data were recorded in an Excel spreadsheet.
Due to the expected heterogeneity of the experiments and the descriptive measures of effects on patient preferences, the review analysis comprised a narrative synthesis of the studies’ uncombined results.
2.5 Effect Measures
Results for patient preferences from the individual studies were extracted according to the type of choice experiment and the applied treatment scenarios. DCEs and SGs resulted in preference weights assigned to the different attributes and levels representing the relative importance ratings of attributes [29]. Patient preferences elicited in (time-)trade-off experiments were presented as sufficient benefit (in %) or increase in survival time (in weeks/months/years) to make a therapy worthwhile and to accept a potential harm, respectively. Results of WTP experiments were reported as monetary value (in the respective currency) that patients were willing to pay for a specific benefit (e.g. survival rate) or to avoid a specific harm/ADR.
2.6 Risk of Bias Assessment for Individual Studies
First, the checklist for observational studies from the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement was used to assess risk of bias [30]. Second, the ‘Checklist of factors to consider in undertaking and assessing the quality of a discrete choice experiment’ from ‘Conducting Discrete Choice Experiments to Inform Healthcare Decision Making: A User’s Guide’ was applied [8]. The assessed domains were concept of the choice process, attribute selection, level selection, experimental design, questionnaire design, piloting, population/study perspective, sample and sample size, data collection, coding of data, validity and interpretation. A response to the initial question for each domain was coded as ‘not reported’ when insufficient data were described to permit a judgment. Two reviewers (L.B. and S.J.H.) performed the risk of bias assessment independently. If required, a third reviewer (J.P.R.) resolved any discrepancies.
3 Results
3.1 Study Selection
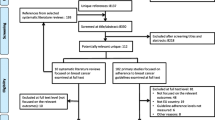
From the database search, 642 records were retrieved, from which 144 duplicates were removed. 455 records were excluded based on title and abstract. Nineteen further records were identified in searching reference list if included publications, and 28 records were excluded based on full-text screening. Excluded studies and reasons for exclusion are displayed in Supplementary Table 2. Hence, 34 studies were included in the analysis [19, 31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63]. Figure 1 shows the flow chart of study selection according to the PRISMA statement.
3.2 Study Characteristics
The characteristics of the included studies are described in Supplementary Table 3. The studies were conducted in a variety of countries covering different health care systems: Australia [35, 37, 38, 55, 61], Belgium [54], Canada [42, 45, 58, 60], France [54, 59], Germany [53, 54, 57, 62], Ireland [59], Italy [48, 54], Japan [40, 50, 52], Lebanon [54], Peru [54], Poland [54, 59], Portugal [54], Serbia [54], Slovenia [54], South Africa [54], South Korea [51], Spain [59], Switzerland [54], Taiwan [34], Thailand, the Netherlands [39, 41, 54], Turkey [54], the UK [36, 54] and the USA [19, 31,32,33, 43, 44, 46, 47, 56, 63]. Sample sizes ranged from 35 to 452 respondents included in the data analyses. Regarding the BC stage, 18 studies included patients with eBC (0–III) [32, 33, 35,36,37,38,39,40,41, 44, 45, 47, 48, 54, 55, 58, 61, 62]; ten studies investigated the preferences of advanced and/or patients with mBC [19, 31, 34, 43, 46, 51, 53, 56, 57, 60]. In six studies, patients with any BC stage were included [42, 49, 50, 52, 59, 63].
In most studies, eligible patients were recruited at outpatient departments of local hospitals (n = 20) [34, 35, 38,39,40,41,42, 44, 45, 47,48,49,50,51, 54, 55, 57, 58, 61]. In 12 studies, participants were recruited via newspaper advertisements, social media outreach, BC support forums, physician outreach, screening of records of BC programs or databases (etc.) and contacted by telephone or e-mail [19, 31, 32, 43, 46, 52, 53, 56, 59, 60, 62, 63]. In one study, subjects participating in a trial were approached [36]. Furthermore, one study did not report the way of study recruitment [33]. All but one of the studies [41] were cross-sectional. To capture patient preferences, participants either filled out questionnaires (n = 19 studies) [19, 31,32,33, 36, 40, 42, 43, 45, 46, 48, 52,53,54, 56, 57, 59, 60, 63] or were interviewed (n = 14 studies) [34, 35, 37,38,39, 41, 47, 49,50,51, 55, 58, 61, 62]. In one study, both modes of administration were used [44].
Preferences for the following drug therapies for BC were evaluated: chemotherapy including (neo-)adjuvant chemotherapy [19, 32, 35, 37,38,39,40,41,42,43,44, 47, 50, 51, 53,54,55,56,57,58, 62], (adjuvant) endocrine therapy [33, 36, 48, 50, 61], (adjuvant) hormonal therapy [39, 49, 60], (adjuvant) therapy with CKD-4/6 inhibitors [33, 45, 46, 60], relevant treatment options for patients with HR+/HER2- advanced or mBC [49, 59]. In four studies, the drug treatment for BC was not specified [31, 34, 52, 63].
3.3 Characteristics of DCEs
The characteristics of the choice experiments used in the studies are displayed in Supplementary Table 4. A total of 15 studies performed DCEs [19, 31,32,33, 43, 46, 49, 52, 53, 56, 57, 59, 60, 62, 63], 17 studies used (time-)trade-off tasks [34,35,36,37,38,39,40,41, 44, 45, 47, 48, 50, 54, 55, 58, 61], 2 studies included WTP experiments [43, 51] and 1 study performed an SG [42]. The number of tasks per patient varied between one and 39 in the different studies. In most studies (n = 20) respondents were faced with choosing between two alternatives [19, 31, 33,34,35,36,37,38,39, 42, 45, 46, 48, 49, 53, 55, 57, 60, 62, 63]. In eight studies three alternatives were given [32, 40, 41, 43, 52, 56, 58, 59]. These mostly consisted of two treatment alternatives and one opt-out option. One study included four alternatives [47]; in five studies, the number of alternatives was not reported [44, 50, 51, 54, 61].
In the DCEs, respondents were asked to choose between different hypothetical treatment scenarios for different eBC or mBC treatments (see study characteristics). The number of included attributes varied from 2 to 13. The corresponding levels were categorical or continuous.
In the time-to-trade-off (TTO) tasks, survival TTO and survival rate trade-off tasks in hypothetical treatment scenarios were presented to the respondents, based on increasing a given length of survival time or increasing the probability of surviving a given length of time, respectively. In the trade-off tasks, patients had to decide either to choose a treatment or not, under varying conditions, including treatment efficiency, potential side effects, dosing regimens and treatment cost. One study focused on the mode of administration in letting respondents decide on oral versus intravenous application [40].
3.4 Risk of Bias in Individual Studies
The risk of bias in the individual studies is shown in Supplementary Table 5. The main limitations of the included studies regarding the STROBE Statement were how sample size was reported; only 11 of the included studies described a dedicated sample size calculation [42, 46, 49, 52,53,54, 56, 58,59,60,61]. Furthermore, efforts to address risk of bias were seldom discussed (n.r. in 20 studies [19, 34, 36,37,38, 40,41,42,43,44, 47, 48, 50, 52, 53, 55, 56, 60,61,62]). Only one study reported how missing data were addressed [52]. Additionally, the study flow (number of individuals screened, asked to participate, included in the study, etc.) was not reported in nine studies [32, 33, 43, 48, 49, 51, 56, 59, 62]. Only three studies provided a study flow diagram [52, 54, 60]. Regarding the checklist by Lancsar and Louviere, the main limitation of all studies was missing information on methodological aspects of various quality domains comprising: type of design used (n.r. in 6 of 14 applicable studies [31,32,33, 39, 56, 60]), generation of profiles (n.r. in 10 of 15 applicable studies [19, 31,32,33, 39, 49, 56, 57, 59, 60]), properties of the design (n.r. in 6 of 14 applicable studies [32, 33, 46, 56, 57, 59]), efficiency of the design (n.r. in 11 of 14 applicable studies [32,33,34, 42, 43, 46, 49, 56, 57, 60, 63]), random allocation of choice sets (n.r. in 8 of 21 applicable studies [19, 31,32,33, 43, 46, 56, 59, 63]), definition of implausible designs (n.r. in any of 17 applicable studies [19, 31,32,33,34, 42, 43, 45, 46, 49, 52, 53, 56, 57, 59, 60, 63]) and coding of data (n.r. in 28 of 34 applicable studies [19, 32, 34,35,36,37,38,39,40,41,42, 44,45,46,47,48, 50,51,52,53,54,55,56,57,58, 61,62,63]).
3.5 Patient Preferences Comparing mBC and eBC
3.5.1 Treatment Effectiveness
The preferences results are presented in Supplementary Table 6. A total of 14 studies on eBC, 7 studies on mBC and 4 studies including patients with any BC stage considered treatment effectiveness in their DCEs. The remaining nine studies did not include treatment effectiveness in their DCEs. Regarding chemotherapy, most studies on eBC (n = 10), mBC (n = 6) and on any stage BC (n = 5) reported that respondents considered treatment effectiveness, i.e. overall (OS) or progression-free survival (PFS) as the most important attribute (compared to ADRs, treatment cost and mode of administration). Specifically, women judged small benefits (i.e., one extra year in life expectancy or 3–5% in survival rate) sufficient to make (adjuvant) chemotherapy worthwhile [19, 31, 33,34,35, 37,38,39, 41, 42, 44, 49, 50, 52, 53, 55, 56, 60,61,62]. For eBC, Jansen et al. (2001) found that in patients already receiving chemotherapy, more than one-third would accept chemotherapy even if there was no evidence base for additional clinical benefit [41]. Comparing eBC and mBC, contrasting results were reported. Three studies found that patients with earlier stage BC placed higher importance on PFS than patients with more advanced BC stages [42, 49, 57]. The latter valued quality of life and the avoidance of side effects higher. On the contrary, Omori et al. (2019) showed that women with recurrent or mBC had the strongest preference for PFS compared to women with eBC [52].
Comparing different chemotherapy regimens (taxane and anthracycline) in eBC, Ballinger et al. (2017) showed that the most preferred regimen did not have the highest benefit in terms of risk reduction of BC recurrence, yet this regimen did not include the potential side effect of congestive heart failure [32]. By comparing different BC treatment approaches, Niikura et al. (2011) found that more women with mBC would accept endocrine therapy compared with chemotherapy or antibody therapy (trastuzumab) [50]. Jansen et al. (2001) reported that about one-third of patients with mBC who were already receiving treatment other than chemotherapy (i.e. hormonal therapy), would refuse chemotherapy, regardless of the potential benefit [41].
McQuellon et al. (1995) interviewed women with eBC on their preferences on treatment for mBC [47]. They reported that after 6 months of treatment subjects were inclined to shift their preference for accepting treatment. Comparing regimens, only a small number of patients considered a 1-month increase in survival sufficient to make a chemotherapy regimen worthwhile. Contrarily, almost all patients would accept an endocrine therapy for a 1-week increase in life expectancy. However, no difference in acceptance was observed between chemotherapy and endocrine therapy when the hypothetical survival gain for both treatments was set at 5 years. For patients with eBC, two studies found that, in comparison with chemotherapy, higher benefits were needed to make (adjuvant) hormonal therapy worthwhile [39, 48]. However, almost all women reported that they would agree to continue the treatment (beyond the standard of 5 years) if this proved to be beneficial for survival [48]. Regarding (adjuvant) endocrine therapy in eBC, one study found that only a third of the respondents considered small survival gains to be sufficient, while the majority needed much greater benefits to make the treatment worthwhile [36]. Comparably, Stamuli et al. (2022) observed that for patients with hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2−) advanced or mBC PFS was of moderate importance compared to pain and functional well-being [59].
Only one study reported preferences of patients with eBC on cyclin-dependent kinase (CKD) 4/6 inhibitors as additional treatment [45]. If having a high risk for BC recurrence, almost all women were more willing to accept additional treatment with CKD 4/6 inhibitors. If women were at a lower risk, only half would accept the additional treatment.
3.5.2 Adverse Drug Reactions (ADRs)
For both eBC and mBC, 16 different types of ADRs were considered in the different studies. In general, large heterogeneity regarding the importance of the included ADRs was observed within eBC and mBC stages, respectively. Yet comparable results across BC stages were observed. In addition, more studies including patients with mBC or with any BC stage examined preferences in ADRs compared with studies including patients with eBC. Six studies on eBC, ten studies on mBC and five studies including any BC stage considered patient preferences on ADRs in their DCEs. In Table 1, the attribute importance of ADRs in the different studies is displayed.
Nausea and vomiting were considered in nine studies. One study reported that it was ranked as less important for patients with eBC compared with other ADRs [33]. However, two studies described that nausea was among the most important ADRs [42, 62]. In patients with mBC, it was rated as one of the most important treatment attributes in four studies [31, 34, 42, 43, 53]. Contrastingly, two studies reported for mBC that nausea and vomiting was among the least important ADRs [19, 60].
Diarrhoea was included in nine studies. Four studies showed a high importance for diarrhoea or a high WTP to avoid diarrhoea (US$3894) in patients with eBC and mBC, respectively [33, 42, 43]. Omori et al. (2019) included incidence of diarrhoea, frequency of loose stools or severity of diarrhoea and duration of diarrhoea as sole ARD [52]. After PFS, duration of diarrhoea was the most important attribute, followed by frequency of stools or severity of diarrhoea and incidence of diarrhoea. Another four studies investigating patients with mBC indicated a low-to-moderate importance of diarrhoea [19, 34, 46, 53]. Comparably, in the study by Thill et al. (2016), diarrhoea was ranked as the least important ADR by respondents with eBC [62].
Alopecia (hair loss) was included in eight studies and predominantly considered as low to moderately important by patients with BC, regardless of the disease stage [31, 33, 42, 53, 62]. Likewise, women were willing to pay the second least to reduce alopecia (US$1853) [43]. Yet, in two studies it was ranked as most important issue by patients with mBC [19, 57].
Eight studies investigated the importance of fatigue on treatment choice. In two studies, patients with eBC and mBC considered fatigue as most important ADR [19, 62]. For CKD 4/6 inhibitors, Lipton et al. (2020) reported that the willingness to accept the additional treatment for eBC was lower if respondents were informed that the drug could cause fatigue [45]. Further studies showed a moderate [43, 53] to (very) low importance of fatigue or WTP to avoid fatigue (US$2652), respectively [34, 42, 57], regardless of the BC stage of the patients.
Neuropathy or hand–foot syndrome as ADRs were considered in eight studies. Ballinger et al. (2017) compared different chemotherapy (taxane and anthracycline) regimens for eBC [32]. Among respondents, regimens including the risk of peripheral neuropathy had a higher acceptance than regimens including the risk of congestive heart failure. For mBC, three studies described a moderate importance for the risk of peripheral neuropathy [19, 34, 57]. Likewise, Lalla et al. (2014) reported that women had a moderate-to-low willingness to pay (US$2764) to avoid this ADR [43]. Kuchuk et al. (2013) showed a difference between sensory and motor neuropathy, with the former being moderately and the latter being the least important ADR, regardless of the BC stage [42]. On the contrary, Amin et al. (2022) stated that women with HER2− mBC assigned the second highest importance to avoiding neuropathy [31].
Eight studies investigated the importance of neutropenia on treatment choice. Beusterien et al. (2021) found that patients with eBC considered neutropenia as most important ADR [33]. In studies on mBC, most respondents rated neutropenia as low-to-moderate importance [19, 31, 34, 49, 59]. However, in two further studies it was ranked as one of the most important attributes [46, 57]. Spaich et al. (2018) reported that it was ranked even higher than PFS [57]. Immune-related side effects or infections were considered in only one study on eBC documenting the lowest importance for this ADR [62]. In four studies on mBC, two reported moderate importance [31, 53] and two reported high importance of immune-related side effects or infections [43, 60].
Mucositis, stomatitis or dry mucosa were considered in six studies including patients with mBC or any stage BC. Consistently all respondents rated these ADRs as less or least important [19, 34, 42, 49, 53]. Myalgia, arthralgia or unspecified pain were evaluated in six studies. Women with mBC assigned a low-to-moderate importance, or a low willingness to pay (US$1458) to avoid these ADRs [19, 34, 43]. Contrarily, Stamuli et al. (2022) reported a moderately high WTP to avoid pain (15,138€), regardless of the BC stage [59]. Furthermore, physical, emotional and/or social functioning were included in four studies. Two studies found that QoL, physical functioning and the ability to participate in social life or being a burden for relatives were rated among the most important attributes by respondents, regardless of the BC stage [53, 63]. Similarly, Stamuli et al. (2022) showed that women were willing to pay the largest amount of money per year to avoid impairment in functional well-being (17,288€) [59].
Several ADRs were included in one or two studies only. Chou et al. (2020) stated that respondents with mBC rated thrombocytopenia as second worse and anemia as second most acceptable ADR [34]. For cardiac dysfunction or heart failure, Maculaitis et al. (2020) reported that women with HR+/HER2− mBC assigned the highest importance to cardiac dysfunction when rating the attributes of CDK 4/6 inhibitor regimens [46]. Similarly, for chemotherapy in eBC, Ballinger et al. (2017) found that women rated regimens that included the risk of congestive heart failure as least favourable [32]. For mBC, Stellato et al. (2021) showed that hot flushes were among the ADRs ranked less important [60]. Additionally, Thill et al. (2016) reported that weight gain was considered as second least important ADR for eBC [62].
Two studies investigated on the effect of fertility reduction on preferences of patients with eBC regarding chemotherapy [54, 58]. In both studies, only few women would refuse chemotherapy, if it would affect their fertility. Furthermore, Srikanthan et al. (2019) found that more women with stage III tumors would accept chemotherapy and its consequences on fertility compared with women with stage II tumors [58].
Smith et al. (2014) did not consider individual ADRs but compared paclitaxel and capecitabine regimens for mBC. They found that with regard to toxicity, the severity, duration and type of side effect had only modest effects on the choice to accept a treatment [56].
3.5.3 Treatment Cost
For eBC, none of the included studies considered treatment costs or women’s WTP for treatment attributes. For mBC, one study including women receiving palliative chemotherapy reported that the average WTP per month for a hypothetical treatment to return from the current state to the pre-cancer state was US$7555 (range US$87 to US$52,123) [51]. However, three studies including any BC stage considered (out-of-pocket) costs as treatment attribute with contrasting results. Stamuli et al. (2022) reported that out-of-pocket costs had only a moderate importance in treatment choice [59]. Contrastingly, in two further studies costs were the (second) most important attribute [49, 63]. Yet, Williams et al. (2021) did not include treatment efficacy [63].
3.5.4 Mode of Administration, Regimen Attributes and Monitoring
Regarding the mode of administration, most women preferred oral over intravenous chemotherapy, regardless of the stage of BC [31, 40, 57]. Moreover, Amin et al. (2022) and Spaich et al. (2018) found that in patients with mBC in the case of intravenous chemotherapy, shorter infusion times and longer cycles without premedication were favuored [31, 57]. Furthermore, Ishitobi et al. (2013) found that, in women with eBC, the preference depended whether they previously had received oral or intravenous chemotherapy [40]. Only half of the women who previously received intravenous chemotherapy preferred an oral treatment. Nazari et al. (2021) reported that women with HR+/HER2− any stage BC had no preference regarding the administration of hormonal and targeted treatments (monthly intramuscular injection versus daily oral administration) [49].
Further treatment attributes were for example dosing schedule and the necessity of ECG monitoring. In one study both were ranked as least important attributes [33]. Likewise for mBC, Stellato et al. (2022) found that healthcare services required for follow-up and monitoring were less relevant than treatment efficacy and ADRs [60]. Eventually, Simes et al. (2001) investigated factors associated with stronger preferences for adjuvant treatment of eBC and identified not receiving radiotherapy as part of the initial treatment, full-dose chemotherapy, having better social support and having others at home dependent on their support [55]. In general, these types of treatment attributes were ranked as less or least relevant by the respondents, regardless of the BC stage [19, 33, 52, 57, 60].
4 Discussion
4.1 Patient Preferences
The aim of this systematic review was to identify and summarise studies evaluating patient preferences regarding treatment effectiveness, ADR, treatment cost and mode of administration for drug therapy of eBC and mBC determined by DCE/CA or WTP analyses. We included 34 studies in the analysis. In most studies, treatment effectiveness in terms of survival gains was considered as the most important attribute and respondents considered small benefits on survival sufficient to accept treatment. We did not find substantial differences comparing patients’ preferences in eBC and mBC. Moreover, this finding was consistent for different types of BC treatment. In studies including patients of any stage, sensitivity analyses showed contrasting results. Patients either valued PFS more and were, therefore, more likely to choose a treatment, or valued potential ADRs more and were, therefore, less likely to choose a treatment [42, 49, 50, 52, 59]. For clinical practice, these findings implicate that the BC stage may not be of relevance for the assessment of patient preferences regarding treatment. Therefore, the choices of patients need to be considered individually as part of shared decision making. Incorporating these individual preferences in shared decision making may possibly improve the effectiveness of interventions by enhancing adherence to BC drug therapy regardless of the disease stage [3, 4].
Only few studies reported that other factors (i.e. QoL, physical agility/mobility and specific ADRs) were more important than treatment effectiveness [53, 57]. Our findings are consistent with the reviews of Hamelinck et al. (2014) and Guerra et al. (2019) [23, 24]. Both concluded that outcome attributes, such as efficacy and adverse events were considered more important than process (e.g. mode of administration) or cost-related attributes [23, 24]. Additionally, Guerra et al. (2019) found that patients with advanced disease stages valued greater benefit and are willing to trade the risk of potential side effects for survival gains [23].
Most patients considered small to moderate benefits sufficient to accept treatment and were willing to trade the risk of potential side effects for even negligible survival gains. Potential reasons for this finding could be on one hand that receiving chemotherapy might provide some sense of control and, therefore, might help with the feeling of helplessness that accompanies the diagnosis of BC [64]. On the other hand, irrational beliefs about the benefits of therapy can lead to a decision in favour of a treatment that brings minor to negligible survival gains [41, 65]. Additionally, the fear of regretting not having opted for treatment might also contribute to such a decision [41, 65]. Duric et al. (2005a) also suggested that the availability of better supportive care may result in better toleration of chemotherapy [35]. These observations may also apply to patients with mBC valuing benefit over the potential ADRs, despite the incurable nature of the disease and the impaired QoL. Two previous reviews reinforced that patients with cancer approaching the end of life had higher expectations of treatment benefit and accepted greater treatment-related risk increases, as well as were overestimating their life expectancy and probabilities of cure when compared with patients with earlier disease stages and with physician estimates [66, 67]. Jansen et al. (2001) discussed the potential implications of these findings for clinical practice and concluded that more research is needed into the reasons patients give for their choices [41]. Patients may make choices that appear irrational based on the available evidence for treatment effectiveness. Hence, physicians need to strike a balance between respecting patients’ choices and considering whether those choices may be constrained by the principles of healthcare systems (i.e. constraints on reimboursement of therapies by health insurances) [41]. Physicians must, therefore, consider the balance between normative and patient-related choices in empathic physician–patient communication.
Regarding ADRs, in general, for both eBC and mBC, different ADRs were considered in the different studies. In addition, more studies including patients with mBC examined preferences in ADRs compared to studies including patients with eBC. The most frequently considered ADRs were nausea/ vomiting, diarrhoea, alopecia, fatigue, neuropathy and neutropenia. These also includes the most frequently occurring ADRs for chemotherapy in BC in general [15, 68]. Partially, huge differences in the results for the importance of the included ADRs were described showing high heterogeneity within the BC stages. Hence, it is hard to generate an overall ADR importance rating. However, across BC stages, no differences were observed in preferences for the most frequently included ADRs were. Considering the effect of fertility reduction on patients with eBC preferences for chemotherapy, two studies reported that only few women would refuse chemotherapy if it would affect their fertility [54, 58]. In particular, older women and women who already had children or do not want children had a more positive attitude towards chemotherapy [54]. A systematic review on fertility-related concerns, needs and preferences of younger women with BC regarding adjuvant systemic therapy summarised that fertility- and menopause-related concerns were frequently inadequately addressed in treatment decision making [69].
Treatment cost was seldom included as treatment attribute. Three studies including any BC stage considered (out-of-pocket) costs as treatment attribute with contrasting results, being either moderately, or even highly, important to the respondents [49, 59, 63]. However, the importance of treatment cost may be depending on the type of health care system or to be more specific whether the patients live in a country where cancer therapy is reimbursed by health insurances. Studies considering the mode of administration showed that most women preferred oral over intravenous chemotherapy, regardless of the BC stage [31, 40, 57]. Ishitobi et al. (2013) described that the individual preference was associated with the mode of administration the patients had received previously [40]. Other treatment related factors such as the necessity for monitoring or the dosing regimen were ranked as least important attributes, regardless of the BC stage [19, 33, 52, 57, 60]. However, we focused on health-related treatment effects rather than on structural feasibility or process-related aspects of treatment, such as monitoring of the drug.
Choice experiments identify and include attributes related to treatments and their outcomes evaluating their relative utility and consequent importance on individual choices by exploring trade-offs between them [70]. Two previous systematic reviews found that the mean number of DCE studies rose from three per year in 1990–2000 to 60 per year in 2013–2017 [71, 72]. The majority evaluated health outcomes or trade-offs between these outcomes and factors related to patient experience. Most common attributes covered aspects of time, risk, healthcare and cost. The increase of DCE application allowed to include 34 studies into this review.
4.2 Risk of Bias Assessment for Individual Studies
No standard method exists for quantifying the risk of bias in studies on patient preferences. However, checklists have been developed to provide guidance on good research practices for DCEs. The International Society for Pharmacoeconomics and Outcomes Research (ISPOR) checklist was proposed for the development, analysis and publication of conjoint analyses in health, while the Purpose, Respondents, Explanation, Findings, and Significance (PREFS) checklist may not cover in sufficient detail the relevant aspects of assessing quality and bias in studies investigating patient preferences [7, 73]. To assess the risk of bias of studies included in this review, we used the checklist developed by Lancsar and Louviere [8]. It suggests key factors to be considered when assessing the quality of DCEs and was used as the basis for the aforementioned lists. To cover general aspects of the quality of the included studies, the STROBE statement checklist for cross-sectional studies was used [30]. In general, all studies reasonably described the conceptualisation of the choice process including attribute and level selection as well as concerning the STROBE statement background information, general methodology, patient selection, recruitment methods and subgroup or sensitivity analysis. Yet, most studies failed to provide detailed methodology related to several aspects of experimental design. As the methodology of DCEs is particularly important for the interpretation of study results, special attention should be given to these aspects in the design of future studies. Previous systematic reviews have already pointed out the need for a clearer methodological description to allow better quality assessment and results interpretation [23, 71, 72, 74].
4.3 Strengths and Limitations
To our knowledge, this is the first systematic review to identify and summarise studies evaluating patient preferences regarding drug therapy in mBC compared with eBC. We were able to include an adequate number of studies to answer our research question. Additionally, we focused on studies including patients with BC and excluded those inquiring healthy individuals imagining they would suffer from BC about their preferences. Moreover, in the included studies, only patients with mBC were asked about their preferences on mBC therapy and, likewise, patients with eBC on their preferences on eBC. This may provide a more realistic view as patients could actually face the decisions presented in the choice experiments. However, the studies seldom specified the timepoint of the experiment in the patients’ course of a drug therapy. Only one longitudinal study evaluated patient preferences of patients with eBC collected at three different timepoints [41]. Of note, they found no change in preferences over time, which indicates that the individual patient preference is a stable construct inherent to a person´s identity. Further longitudinal studies using choice experiments to elicit patient preferences of patients with BC on drug therapy are needed to confirm or disprove this finding. The same applies for preferences regarding ADRs. It will be important to further investigate the effects of severity of BC-associated ADRs on patient preferences. Furthermore, we recommend that future studies on patient preferences should be conducted in patients before the actual treatment decision has been made. By repeating the measurement after the treatment decision has been made, the potential impact of ‘reconciliation with the treatment decision’ can be determined in other patient groups and with other treatments.
Potential influencing factors on patient preferences evaluated in the included studies (e.g. sociodemographics and disease-related factors) were not considered explicitly in this review. Thus, further reviews should focus on summarising these factors.
Studies evaluating preferences of health care professions or stakeholders were not included, considering the expected heterogeneity of stated preferences between different perspectives in health care. Three studies highlighted differences between preferences of patients, their partners, physicians and payers [33, 38, 46]. Moreover, we excluded studies investigating other types of BC treatment (i.e. surgery, radiotherapy and non-pharmacologic treatment), since the reasons for preferences for different drug therapies (efficacy, ADRs, etc.) are not comparable to preferences regarding different types of surgery. Additionally, Hamelinck et al. (2014) already described patient preferences of patients with eBC for breast conserving surgery versus mastectomy [24].
Another limitation of this review is that we did not perform sensitivity analyses considering the year of publication of the included studies. Changing drug therapy and ADR management may have an impact on the generalisability of the results of older studies for the current situation of patients with BC.
4.4 General Strengths and Limitations of the DCEs
DCEs have the general strength of measuring patients’ relative importance towards various medication attributes such as benefits and side-effects. They assume that people trade-off different medication attributes to maximise their utility. This is particularly important for adherence research, as previous studies indicated that patients make trade-offs between effectiveness and side effects when deciding whether or not to take a medication. Furthermore, DCEs allow to compare and quantify the relative importance of factors that influence medication adherence. This can inform policy decisions by prioritising interventions that target the most important factors to improve patient’s medication adherence [75].
General limitations of all kinds of choice experiments are the hypothetical nature of the presented treatment scenarios. The hypothetical treatments may not reflect all aspects of a treatment or other factors that might influence patient preferences. Furthermore, the descriptions of included attributes may not represent the actual experience of the respondents. DCEs are intended to simulate possible clinical decisions, but do not have the same clinical, financial or emotional consequences of actual decisions. Hence, differences can arise between stated and actual choices [19, 31,32,33,34, 42, 45, 46, 49, 52, 57]. This so-called potential hypothetical bias may be limited by performing extensive piloting of the included attributes [8].
5 Conclusions
Regardless of the stage of disease, patients with BC valued survival gains as the most important attribute and were willing to accept the risk of potential side effects. Hence, a sensitive physician-patient communication is needed in treatment decision making, especially in patients with advanced stages of disease. It may further be important to include the communication of benefits and risks of drug therapies in the respective treatment Guidelines. Eventually, incorporating patient preferences in shared decision making may possibly improve the effectiveness of interventions by enhancing adherence to BC drug therapy, regardless of the disease stage.
Abbreviations
- ADR:
-
Adverse drug reaction
- BC:
-
Breast cancer
- CA:
-
Conjoint analysis
- DCE:
-
Discrete-choice experiment
- DFS:
-
Disease-free survival
- eBC:
-
Early breast cancer
- HER2:
-
Human epidermal growth factor receptor 2
- HR:
-
Hormonal receptor
- mBC:
-
Metastatic breast cancer
- PFS:
-
Progression free survival
- QoL:
-
Quality of life
- SG:
-
Standard gamble
- TTO:
-
Time-trade-off
- WTP:
-
Willingness-to-pay
References
Harrison M, Milbers K, Hudson M, Bansback N. Do patients and health care providers have discordant preferences about which aspects of treatments matter most? Evidence from a systematic review of discrete choice experiments. BMJ Open. 2017;7(5): e014719.
Mulley AG, Trimble C, Elwyn G. Stop the silent misdiagnosis: patients’ preferences matter. BMJ. 2012;345: e6572.
Krahn M, Naglie G. The next step in guideline development: incorporating patient preferences. JAMA. 2008;300(4):436–8.
Owens DK. Spine update. Patient preferences and the development of practice guidelines. Spine. 1998;23(9):1073–9.
Politi MC, Studts JL, Hayslip JW. Shared decision making in oncology practice: what do oncologists need to know? Oncologist. 2012;17(1):91–100.
Kane HL, Halpern MT, Squiers LB, Treiman KA, McCormack LA. Implementing and evaluating shared decision making in oncology practice. CA Cancer J Clin. 2014;64(6):377–88.
Bridges JF, Hauber AB, Marshall D, Lloyd A, Prosser LA, Regier DA, Johnson FR, Mauskopf J. Conjoint analysis applications in health–a checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value Health. 2011;14(4):403–13.
Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making: a user’s guide. Pharmacoeconomics. 2008;26(8):661–77.
Coast J, Horrocks S. Developing attributes and levels for discrete choice experiments using qualitative methods. J Health Serv Res Policy. 2007;12(1):25–30.
Torrance GW. Measurement of health state utilities for economic appraisal: a review. J Health Econ. 1986;5(1):1–30.
Kaplan RM, Feeny D, Revicki DA. Methods for assessing relative importance in preference based outcome measures. Qual Life Res. 1993;2(6):467–75.
Gafni A. The standard gamble method: what is being measured and how it is interpreted. Health Serv Res. 1994;29(2):207–24.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49.
Leitlinienprogramm Onkologie (Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF): S3-Leitlinie Früherkennung, Diagnose, Therapie und Nachsorge des Mammakarzinoms. In., vol. Version 4.3; 2020.
Friese CR, Harrison JM, Janz NK, Jagsi R, Morrow M, Li Y, Hamilton AS, Ward KC, Kurian AW, Katz SJ, et al. Treatment-associated toxicities reported by patients with early-stage invasive breast cancer. Cancer. 2017;123(11):1925–34.
Janz NK, Wren PA, Copeland LA, Lowery JC, Goldfarb SL, Wilkins EG. Patient–physician concordance: preferences, perceptions, and factors influencing the breast cancer surgical decision. J Clin Oncol. 2004;22(15):3091–8.
Andersen MR, Sweet E, Hager S, Gaul M, Dowd F, Standish LJ. Use of integrative oncology, involvement in decision-making, and breast cancer survivor health-related quality of life in the first 5 years postdiagnosis. Integr Cancer Ther. 2018;17(3):636–45.
Brown R, Butow P, Wilson-Genderson M, Bernhard J, Ribi K, Juraskova I. Meeting the decision-making preferences of patients with breast cancer in oncology consultations: impact on decision-related outcomes. J Clin Oncol. 2012;30(8):857–62.
daCosta DM, Copher R, Basurto E, Faria C, Lorenzo R. Patient preferences and treatment adherence among women diagnosed with metastatic breast cancer. Am Health Drug Benefits. 2014;7(7):386–96.
Cardoso F, Harbeck N, Fallowfield L, Kyriakides S, Senkus E. Locally recurrent or metastatic breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annal Oncol. 2012;23(Suppl 7):vii11–9.
Hattori M, Iwata H. Advances in treatment and care in metastatic breast cancer (MBC): are there MBC patients who are curable? Chin Clin Oncol. 2018;7(3):23.
Chung CT, Carlson RW. Goals and objectives in the management of metastatic breast cancer. Oncologist. 2003;8(6):514–20.
Guerra RL, Castaneda L, de Albuquerque RCR, Ferreira CBT, Corrêa FM, Fernandes RRA, de Almeida LM. Patient preferences for breast cancer treatment interventions: a systematic review of discrete choice experiments. Patient. 2019;12(6):559–69.
Hamelinck VC, Bastiaannet E, Pieterse AH, Jannink I, van de Velde CJ, Liefers GJ, Stiggelbout AM. Patients’ preferences for surgical and adjuvant systemic treatment in early breast cancer: a systematic review. Cancer Treat Rev. 2014;40(8):1005–18.
Minami CA, King TA, Mittendorf EA. Patient preferences for locoregional therapy in early-stage breast cancer. Breast Cancer Res Treat. 2020;183(2):291–309.
Sukumar JS, Quiroga D, Kassem M, Grimm M, Shinde NV, Appiah L, Palettas M, Stephens J, Gatti-Mays ME, Pariser A, et al. Patient preferences and adherence to adjuvant GnRH analogs among premenopausal women with hormone receptor positive breast cancer. Breast Cancer Res Treat. 2021;190(2):183–8.
Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372: n160.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):210.
Hauber AB, González JM, Groothuis-Oudshoorn CGM, Prior T, Marshall DA, Cunningham C, Ijzerman MJ, Bridges JFP. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR conjoint analysis good research practices task force. Value Health. 2016;19(4):300–15.
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–9.
Amin S, Tolaney SM, Cambron-Mellott MJ, Beusterien K, Maculaitis MC, Mulvihill E, Shinde R, McLaurin K. Benefit-risk trade-offs in treatment choice in advanced HER2 negative breast cancer: patient and oncologist perspectives. Future Oncol. 2022;18(16):1927–41.
Ballinger TJ, Kassem N, Shen F, Jiang GL, Smith ML, Railey E, Howell J, White CB, Schneider BP. Discerning the clinical relevance of biomarkers in early stage breast cancer. Breast Cancer Res Treat. 2017;164(1):89–97.
Beusterien K, Maculaitis MC, Hallissey B, Gaschler MM, Smith ML, Law EH. Patient, oncologist, and payer preferences for adjuvant endocrine therapy and CDK4/6 inhibitor regimens in early-stage breast cancer: a discrete choice experiment. Patient Prefer Adherence. 2021;15:611–23.
Chou TC, Chiang SC, Ko Y. Health state utilities for metastatic breast cancer in Taiwan. Breast. 2020;51:57–64.
Duric V, Stockler M, Heritier S, Boyle F, Beith J, Sullivan A, Wilcken N, Coates A, Simes R, Duric V, et al. Patients’ preferences for adjuvant chemotherapy in early breast cancer: what makes AC and CMF worthwhile now? Ann Oncol. 2005;16(11):1786–94.
Duric V, Fallowfield L, Saunders C, Houghton J, Coates A, Stockler M, Duric V, Fallowfield L, Saunders C, Houghton J, et al. Patients’ preferences for adjuvant endocrine therapy in early breast cancer: what makes it worthwhile? Br J Cancer. 2005;93(12):1319–23.
Duric VM, Butow PN, Sharpe L, Boyle F, Beith J, Wilcken NRC, Heritier S, Coates AS, Simes RJ, Stockler MR. Psychosocial factors and patients’ preferences for adjuvant chemotherapy in early breast cancer. Psychosoc Oncol. 2007;16(1):48–59.
Duric V, Butow P, Sharpe L, Heritier S, Boyle F, Beith J, Wilcken N, Coatest A, Simes R, Stockler M, et al. Comparing patients’ and their partners’ preferences for adjuvant chemotherapy in early breast cancer. Patient Educ Couns. 2008;72(2):239–45.
Hamelinck VC, Bastiaannet E, Pieterse AH, de Glas NA, Portielje JE, Merkus JW, den Hoed ID, van de Velde CJ, Liefers GJ, Stiggelbout AM. A prospective comparison of younger and older patients’ preferences for adjuvant chemotherapy and hormonal therapy in early breast cancer. Clin Breast Cancer. 2016;16(5):379–88.
Ishitobi M, Shibuya K, Komoike Y, Koyama H, Inaji H. Preferences for oral versus intravenous adjuvant chemotherapy among early breast cancer patients. Patient Prefer Adherence. 2013;7:1201–6.
Jansen SJ, Kievit J, Nooij MA, de Haes JC, Overpelt IM, van Slooten H, Maartense E, Stiggelbout AM. Patients’ preferences for adjuvant chemotherapy in early-stage breast cancer: is treatment worthwhile? Br J Cancer. 2001;84(12):1577–85.
Kuchuk I, Bouganim N, Beusterien K, Grinspan J, Vandermeer L, Gertler S, Dent SF, Song X, Segal R, Mazzarello S, et al. Preference weights for chemotherapy side effects from the perspective of women with breast cancer. Br Cancer Res Treat. 2013;142(1):101–7.
Lalla D, Carlton R, Santos E, Bramley T, D’Souza A. Willingness to pay to avoid metastatic breast cancer treatment side effects: results from a conjoint analysis. Springerplus. 2014;3:7.
Lindley C, Vasa S, Sawyer WT, Winer EP. Quality of life and preferences for treatment following systemic adjuvant therapy for early-stage breast cancer. J Clin Oncol. 1998;16(4):1380–7.
Lipton NJ, Jesin J, Warner E, Cao X, Kiss A, Desautels D, Jerzak KJ. Willingness of women with early estrogen receptor-positive breast cancer to take adjuvant CDK4/6 inhibitors. Curr Oncol. 2020;27(3):127–34.
Maculaitis MC, Liu X, Will O, Hanson M, McRoy L, Berk A, Crastnopol M. Oncologist and patient preferences for attributes of CDK4/6 inhibitor regimens for the treatment of advanced/metastatic HR positive/HER2 negative breast cancer: discrete choice experiment and best-worst scaling. Patient Prefer Adherence. 2020;14:2201–14.
McQuellon RP, Muss HB, Hoffman SL, Russell G, Craven B, Yellen SB. Patient preferences for treatment of metastatic breast-cancer—a study of women with early-stage breast-cancer. J Clin Oncol. 1995;13(4):858–68.
Montagna E, Pagan E, Bagnardi V, Colleoni M, Cancello G, Munzone E, Dellapasqua S, Bianco N, Campenni G, Iorfida M, et al. Evaluation of endocrine therapy and patients preferences in early breast cancer: results of Elena study. Breast Cancer Res Treat. 2020;184(3):783–95.
Nazari A, Lopez-Valcarcel BG, Najafi S. Preferences of patients with HR+ and HER2- breast cancer regarding hormonal and targeted therapies in the first line of their metastatic stage: a discrete choice experiment. Value Health Region Issues. 2021;25:7–14.
Niikura N, Kimura M, Iwamoto T, Hayashi N, Shintoku J, Saito Y, Suzuki Y, Tokuda Y. Women prefer adjuvant endocrine therapy to chemotherapy for breast cancer treatment. Breast Cancer. 2013;20(1):67–74.
Oh DY, Crawford B, Kim SB, Chung HC, McDonald J, Lee SY, Ko SK, Ro J. Evaluation of the willingness-to-pay for cancer treatment in Korean metastatic breast cancer patients: a multicenter, cross-sectional study. Asia Pac J Clin oncol. 2012;8(3):282–91.
Omori Y, Enatsu S, Cai ZH, Ishiguro H. Patients’ preferences for postmenopausal hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer treatments in Japan. Breast Cancer. 2019;26(5):652–62.
Reinisch M, Marschner N, Otto T, Korfel A, Stoffregen C, Wockel A. Patient Preferences: results of a German adaptive choice-based conjoint analysis (Market Research Study sponsored by Eli Lilly and Company) in patients on palliative treatment for advanced breast cancer. Breast Care. 2021;16(5):491–9.
Senkus E, Gomez H, Dirix L, Jerusalem G, Murray E, Van Tienhoven G, Westenberg AH, Bottomley A, Rapion J, Bogaerts J, et al. Attitudes of young patients with breast cancer toward fertility loss related to adjuvant systemic therapies. EORTC study 10002 BIG 3–98. Psychosoc Oncol. 2014;23(2):173–82.
Simes RJ, Coates AS. Patient preferences for adjuvant chemotherapy of early breast cancer: how much benefit is needed? J Natl Cancer Inst Monogr. 2001;30:146–52.
Smith ML, White CB, Railey E, Sledge GW Jr. Examining and predicting drug preferences of patients with metastatic breast cancer: using conjoint analysis to examine attributes of paclitaxel and capecitabine. Breast Cancer Res Treat. 2014;145(1):83–9.
Spaich S, Kinder J, Hetjens S, Fuxius S, Gerhardt A, Sutterlin M, Spaich S, Kinder J, Hetjens S, Fuxius S, et al. Patient preferences regarding chemotherapy in metastatic breast cancer-a conjoint analysis for common taxanes. Front Oncol. 2018. https://doi.org/10.3389/fonc.2018.00535.
Srikanthan A, Amir E, Gupta A, Baxter N, Kennedy ED. Assisting with decision-making: how standardized information impacts breast cancer patient decisions regarding fertility trade-offs and chemotherapy. J Adolesc Young Adult oncol. 2019;8(6):660–7.
Stamuli E, Corry S, Ross D, Konstantopoulou T, Stamuli E, Corry S, Ross D, Konstantopoulou T. Patient preferences for breast cancer treatments: a discrete choice experiment in France, Ireland, Poland, Spain. Future Oncol. 2022;18(9):1115–32.
Stellato D, Thabane M, Eichten C, Delea TE. Preferences of Canadian patients and physicians for treatment of HR+/HER2-advanced breast cancer. Curr Oncol. 2021;28(1):491–508.
Thewes B, Meiser B, Duric VM, Stockler MR, Taylor A, Stuart-Harris R, Links M, Wilcken N, McLachlan SA, Phillips KA, et al. What survival benefits do premenopausal patients with early breast cancer need to make endocrine therapy worthwhile? Lancet Oncol. 2005;6(8):581–8.
Thill M, Pisa G, Isbary G, Thill M, Pisa G, Isbary G. Targets for neoadjuvant therapy—the preferences of patients with early breast cancer. Geburtshilfe Frauenheilkd. 2016;76(5):551–6.
Williams CP, Gallagher KD, Deehr K, Aswani MS, Azuero A, Daniel CL, Ford EW, Ingram SA, Balch AJ, Rocque GB. Quantifying treatment preferences and their association with financial toxicity in women with breast cancer. Cancer. 2021;127(3):449–57.
Levine MN, Gafni A, Markham B, MacFarlane D. A bedside decision instrument to elicit a patient’s preference concerning adjuvant chemotherapy for breast cancer. Ann Intern Med. 1992;117(1):53–8.
Palda VA, LlewellynThomas HA, Mackenzie RG, Pritchard KI, Naylor CD. Breast cancer patients attitudes about rationing postlumpectomy radiation therapy: applicability of trade-off methods to policy-making. J Clin Oncol. 1997;15(10):3192–200.
Zafar SY, Alexander SC, Weinfurt KP, Schulman KA, Abernethy AP. Decision making and quality of life in the treatment of cancer: a review. Support Care Cancer. 2009;17(2):117–27.
Back AL, Anderson WG, Bunch L, Marr LA, Wallace JA, Yang HB, Arnold RM. Communication about cancer near the end of life. Cancer. 2008;113(S7):1897–910.
Brustkrebs – Chemotherapie. https://www.krebsgesellschaft.de/onko-internetportal/basis-informationen-krebs/krebsarten/brustkrebs/therapie/chemotherapie.html
Peate M, Meiser B, Hickey M, Friedlander M. The fertility-related concerns, needs and preferences of younger women with breast cancer: a systematic review. Breast Cancer Res Treat. 2009;116(2):215–23.
Louviere JJ, Hensher DA, Swait JD. Stated choice methods: analysis and applications. Cambridge: Cambridge University Press; 2000.
Marshall D, Bridges JF, Hauber B, Cameron R, Donnalley L, Fyie K, Johnson FR. Conjoint analysis applications in health—how are studies being designed and reported?: An update on current practice in the published literature between 2005 and 2008. Patient. 2010;3(4):249–56.
Soekhai V, de Bekker-Grob EW, Ellis AR, Vass CM. Discrete choice experiments in health economics: past, present and future. Pharmacoeconomics. 2019;37(2):201–26.
Joy SM, Little E, Maruthur NM, Purnell TS, Bridges JF. Patient preferences for the treatment of type 2 diabetes: a scoping review. Pharmacoeconomics. 2013;31(10):877–92.
Clark MD, Determann D, Petrou S, Moro D, de Bekker-Grob EW. Discrete choice experiments in health economics: a review of the literature. Pharmacoeconomics. 2014;32(9):883–902.
Khan MU, Brien J-A, Aslani P. The use of discrete choice experiments in adherence research: a new solution to an old problem. Res Soc Admin Pharm. 2020;16(10):1487–92.
digiOnko [https://www.digionko-bayern.de/]
Acknowledgements
We thank Anna Horn (Institute for Clinical Epidemiology and Biometry, Würzburg) for the critical revision of the manuscript. Furthermore, we would like to thank the reviewers of The Patient–Patient-Centered Outcomes Research for their insightful comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was supported as a part of the project BRE-BY-MED ‘Breast Cancer Care in Bavaria for Patients with Metastatic Disease’ (DRKS00026601), which is integrated in the mantle project digiOnko ‘Integrative Concept for personalised precision medicine in prevention, screening, therapy and recurrence prevention on the example of breast cancer’ [76], funded by the Bavarian State Ministry for Health and Care (project grant number PBN-MGP-2010-0004-DigiOnko).
Competing Interests
L.B. and S.J.H. report no conflict of interest. St.S. received speaker honoraria as well as reimbursement of travel costs from AstraZeneca, Bayer, Boehringer-Ingelheim, Sanofi, Servier; received financial support for carrying out clinical studies from AstraZeneca, Bayer, Boehringer-Ingelheim, Novartis, NovoNordisk, Pfizer, Sanofi; received personal payments for Data Safety Monitoring Board or Advisory Board activities from AstraZeneca, Bayer, Boehringer-Ingelheim, NovoNordisk, Sanofi, Servier; unpaid Board Membership in the Writing Group National Guideline Heart Failure Guideline Germany, ESC-HFA Committee of the National Heart Failure Societies, ESC-HFA Patient Website: Heartfailurematters.org, outside the submitted work. P.U.H. reports research grants from the German Ministry of Research and Education, Federal Joint Committee (G-BA) within the Innovationfond, European Union, German Parkinson Society, University Hospital Würzburg, German Heart Foundation, German Research Foundation, Bavarian State, German Cancer Aid, Charité – Universitätsmedizin Berlin (within Mondafis; supported by an unrestricted research grant to the Charité from Bayer), University Göttingen (within FIND-AF randomized; supported by an unrestricted research grant to the University Göttingen from Boehringer-Ingelheim), University Hospital Heidelberg (within RASUNOA-prime; supported by an unrestricted research grant to the University Hospital Heidelberg from Bayer, BMS, Boehringer-Ingelheim, Daiichi Sankyo); participation on DSMB in publicly funded studies (by German Research Foundation, German Ministry of Research, Foundations), outside the submitted work. AW reports research grants from the Federal Joint Committee (G-BA) within the Innovationfond, outside the submitted work, consulting fees received from AstraZeneca, Lilly, Novartis, Pfizer, Roche, MSD, Pierre Fabre, Seagen, Exact Sciences, Gilead, Dajichi Sanko; speaker honoraria received from Aurikamed and Onkowissen; support for attending meetings and/or travel received from Pfizer, Dajichi Sanko; leadership or fiduciary role in AGO, S3-Guideline Breast Cancer, German Society of Breast Cancer (BGGF). J.P.R. reports research grants from the German Ministry of Research and Education, Bavarian State (ministry for science and the arts), Federal Joint Committee (G-BA) within the Innovationfond, German Center for Lung Research; payment for expert testimony from the German Ministry of Health (BMG); payment for EBM Training Lecture by the Landesaerztekammer Hessen, outside the submitted work.
Ethics Approval
Not applicable.
Consent for Publication
Not applicable.
Consent to Participate
Not applicable.
Availability of Data and Materials
Data sharing is not applicable to this article as no datasets were generated during the current study.
Code Availability
Not applicable.
Author Contributions
All authors contributed to the conception and design of the work. L.B. and S.J.H. contributed to material preparation, data collection and analysis. The first draft of the manuscript was written by L.B. and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Brandstetter, L.S., Jírů-Hillmann, S., Störk, S. et al. Differences in Preferences for Drug Therapy Between Patients with Metastatic Versus Early-Stage Breast Cancer: A Systematic Literature Review. Patient 17, 349–362 (2024). https://doi.org/10.1007/s40271-024-00679-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40271-024-00679-6