Abstract
Loop diuretics (LDs) represent the cornerstone treatment for relieving pulmonary congestion in patients with heart failure (HF). Their benefit is well-recognized in the short term because of their ability to eliminate fluid retention. However, long term, they could adversely influence prognosis due to activation of the neurohumoral mechanism, particularly in older, frail patients. Moreover, the advent of new drugs capable of improving outcomes and reducing pulmonary and systemic congestion signs in HF emphasizes the possibility of a progressive reduction and discontinuation of LD treatment. Nevertheless, few studies were aimed at investigating the safety of LDs withdrawal in older patients with chronic stable HF. This current review aims to approach current evidence regarding the safety and effectiveness of LDs discontinuation in patients with chronic stable HF, and is based on the material obtained via the PubMed and Scopus databases from January 2000 to November 2022. Our search yielded five relevant studies, including two randomized controlled trials. All participants presented stable HF at the time of study enrolment. Apart from one study, all the investigations were conducted in patients with HF with reduced ejection fraction. The most common outcomes examined were the need for diuretic resumption or the event of death and rehospitalization after diuretic withdrawal. As a whole, although based on a few investigations with a low grade of evidence, diuretic therapy discontinuation might be a safe strategy that deserves consideration for patients with stable HF. However, extensive investigations in older adults, accounting for frailty status, are warranted to confirm these data in this peculiar class of patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The advent of new drugs such as sodium-glucose co-transporter-2 inhibitors and angiotensin receptor neprilysin inhibitors opens the possibility of re-evaluating the long-term diuretic approach of stable heart failure (HF). Nevertheless, a shared consensus regarding the optimal time and modality of dose reduction or withdrawal is still missing. |
In older patients affected by HF, loop diuretics may have potentially negative effects that can adversely influence prognosis in the mid- or long-term, such as dehydration, electrolyte imbalances, falls, and neurohumoral hyperactivation. |
Diuretic therapy discontinuation, guided also by the screening of frailty for older adults, might be a safe strategy that deserves consideration for patients with stable chronic HF. |
1 Introduction
The advent of new drugs able to positively influence the volume homeostasis in patients with heart failure (HF), such as sodium-glucose co-transporter 2 (SGLT-2) inhibitors and angiotensin receptor neprilysin inhibitors (ARNIs), opens the possibility of re-evaluating the long-term diuretic approach of HF in the outpatient setting and primary care. Recent studies have suggested several schemes for HF medication sequential introduction [1, 2]. However, although loop diuretics (LDs) are one of the most commonly prescribed drugs in HF patients, there has yet to be a shared consensus regarding the time and the modality of dose reduction or withdrawal in stable HF. Diuretic therapy in HF patients is often as much an art as a science [3]. The practice of downtitrating LDs in stable HF patients has already been endorsed in the 2016 European Society of Cardiology (ESC) guidelines [4]; however, only a few data are available on this process. Indeed, LDs such as furosemide, bumetanide, and torsemide remain the cornerstone of decongestion therapy regardless of the HF subtype [5]. LDs work by inhibiting the sodium-potassium-chloride cotransporter (NKCC2) at the luminal side of the renal tubules in the thick ascending limb of Henle’s loop, which accounts for approximately 25% of the total renal sodium reabsorption. The primary pharmacodynamic effect of LDs is induction of natriuresis, chloruresis and kaliuresis. LDs also impair the kidneys’ urinary concentration capacity dependent on NKCC2, stimulating water loss and producing hypo- to isotonic urine [6]. The increased urine production results in a decrease in blood volume and blood pressure.
Furosemide is the most common diuretic drug utilized in hospitals and primary care, and it is well-appreciated by physicians for its handling and safety; yet LDs are often overused and their adverse events are underestimated. In older patients, LDs can be useful in managing symptoms of HF but they may also have potentially negative effects. For example, older adults are more likely to experience adverse effects such as dehydration, electrolyte imbalances, and falls [7, 8]. Furthermore, it is important to consider that LDs possess the potential to detrimentally impact cardiac preload when utilized inappropriately or excessively. Specifically in older individuals, this reduction in preload has been associated with a subsequent decrease in stroke volume and cardiac output, thus potentially influencing overall cardiac function [9].In support of this notion, a recent study [10] revealed the adverse effects of LDs in stable HF with preserved ejection fraction (HFpEF) on exercise intolerance, as assessed by peak oxygen consumption (VO2peak). This finding suggests that the withdrawal of LDs in patients with elevated left ventricular stiffness could potentially yield beneficial outcomes by augmenting left ventricular diastolic filling and improving orthostatic tolerance. Therefore, it is important to monitor older patients closely while they are taking LDs and adjust the dosage as needed to prevent adverse effects. In this regard, post-discharge therapeutic re-evaluation is crucial in older patients, typically those with HF, since polypharmacy is inevitable and often unavoidable [11]. It is unclear how to apply traditional principles of pharmacotherapy deprescribing to older adults with HF, who often coexist with both HF and non-HF medications. Notably, timely discontinuation of LDs is an interesting target of drug de-prescribing in the elderly with HF. Indeed, LDs cause renin-angiotensin-aldosterone system (RAAS) activation, which may lead to increased morbidity and mortality despite short-term symptomatic improvement. Since chronic use of LDs has been associated with poor outcomes, several consensus statements advocate reducing its utilization in the case of decongestion and gradually reducing it in the case of stabilization [12]. The current review aims to approach current evidence regarding the safety and effectiveness of furosemide discontinuation in older adults with stable HF.
2 Methods
The PubMed and Scopus databases were employed. We searched for articles between January 2000 and November 2022 using the key terms ‘loop diuretics’ AND ‘withdrawal’ OR ‘furosemide’ OR ‘loop diuretics’ AND ‘discontinuation’ OR ‘loop diuretics reduction’ using the age filter ‘> 65 years old’. The entire article was read if the abstract indicated that the article potentially met the inclusion criteria. Articles were included in the review according to the following inclusion criteria: English language, year of publication from 2000, and reporting withdrawal of diuretics in the studies. Articles were excluded according to title, abstract, or full text for irrelevance to the topic. Further exclusion criteria were article reviews and editorial comments. Two independent reviewers (CC and TM) performed the literature search and data extraction. In the case of a disagreement between the two reviewers, the input of a third more senior reviewer (DG) was requested.
2.1 Type of Studies
Randomized controlled trials (RCTs), cohort studies, cross-sectional studies, and case-control studies were included. If a published study had more than one publication, then the most recent publication or the publication with the complete dataset was selected.
2.2 Type of Participants
Chronic stable heart failure patients.
2.3 Exposure Factors
The factors related to adverse outcomes included withdrawal of LDs in patients with chronic stable HF, irrespective of ejection fraction subtypes.
2.4 Outcomes
The outcomes reported were HF-related hospitalization, acute HF relapse, all-cause mortality, and diuretic reuse.
3 Results
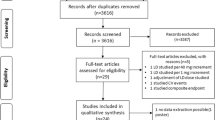
A total of 243 publications were identified through multiple search engines, with 102 being excluded due to duplications. After examining the titles and abstracts, 120 articles were excluded, and after reviewing the full texts of the remaining 21 articles, 5 relevant studies were included in this review (Table 1). These studies included 594 participants and the sample size ranged from 26 to 190. Two studies were RCTs, two were retrospective observational studies, and one was a longitudinal study. The studies were conducted in Brazil, Japan, Italy, Spain, and The Netherlands. The mean age of patients included in the studies was 64.2 years (range 59–75), with a majority of male patients in four of the five studies; the remaining study reported a majority of females [13]. Apart from one study, all investigations were carried out in patients with HF with reduced ejection fraction (HFrEF). The main characteristics of the included studies are presented in Table 1. The range of follow-up was 3–24 months.
3.1 Study Objectives
The selected studies investigated the effects of LDs withdrawal in patients with chronic stabilized systolic HF, each with specific objectives. Galve et al. [14] aimed to determine the clinical changes induced by withdrawing LDs and examined the associated modifications in biochemical and neurohumoral parameters. Oshima et al. [15] focused on the endpoint of 1-year mortality or HF readmission, while the study conducted by Romano et al. [3] aimed to evaluate the need for diuretic resumption or the occurrence of death and rehospitalization after diuretic withdrawal. Furthermore, the authors sought to identify clinical, biochemical, and echocardiographic parameters associated with diuretic withdrawal.
The ReBIC-1 trial had two co-primary endpoints. The first was to assess dyspnea using a visual analog scale (VAS), while the second endpoint was to determine the proportion of patients maintained without LDs during a 90-day follow-up. Additionally, the authors evaluated the composite clinical endpoints of HF-related death, hospitalization, or emergency room visits during the follow-up period [16].
In the study by van Kraaij et al. [13], the primary endpoint was to determine the requirement for restarting or increasing furosemide therapy during the 3-month follow-up period. The authors also explored whether hormonal changes were associated with alterations in clinical parameters such as HF symptoms, blood pressure, or heart rate.
3.2 Dyspnea and Heart Failure (HF) Recurrence Following Loop Diuretic (LD) Discontinuation
In the study conducted by Galve et al. [14], it was demonstrated that diuretic withdrawal (DW) was well tolerated by 65% of patients without a decline in exercise capacity or deterioration of their New York Heart Association functional class during the 3-month follow-up period. Similarly, Oshima et al. [15] found that the 1-year event rate, encompassing death or HF admission, did not differ significantly between patients receiving standard doses of diuretics and those receiving low doses or no LDs at all.
Romano et al. [3] observed that no patients required resumption of diuretic therapy, and there were no occurrences of cardiovascular death or rehospitalization due to acutely decompensated HF following withdrawal of LDs. Furthermore, the ReBIC trial found no significant difference in patients’ assessment of dyspnea between furosemide withdrawal and continuous administration. Additionally, the incidence of HF-related events, including hospitalizations, emergency room visits, and deaths, was similar between patients without LDs and those receiving standard doses of LDs [16]. In another study [13], it was reported that 10.5% of patients in the withdrawal group and 10% in the continuation group experienced an episode of recurrent congestive HF.
3.3 Effects of LD Discontinuation on Renal Function
In one study, renal function parameters (i.e., urea and creatinine) significantly improved at 3 months in DW patients [14]. Romano et al. found a worsening of renal function tests in patients taking LDs compared with their counterparts [3]. On the other hand, no difference in creatinine and blood urea nitrogen (BUN) were found in another study [16].
3.4 Effect on the Neuro-ormonal Function
Two of the selected studies evaluated neuro-ormonal status in LD and DW patients. Both studies reported a significant decrease in plasma renin activity of DW patients compared with the LDs group. Galve et al. did not find meaningful changes in aldosterone, arginine–vasopressin, endothelin-1 and norepinephrine across the groups [14]. In the study by van Kraaij et al. [13], the average decrease in aldosterone levels was not significant between the two groups.
4 Discussion
This is the first report, based on epidemiological data, to study whether discontinuation of LDs is safe and feasible in stable HF older patients. A total of five articles including 594 participants were included in this review and the results suggested that the evidence regarding the safety and effectiveness of LDs discontinuation still needs to be improved. The mean age of the studies selected was 64.2 years, and none of the investigations dealt with the oldest-old patients or accounted for frailty status. Except for one study, all papers had a 3-month follow-up after hospitalization for acute HF. In all the selected studies, most HF patients with LDs discontinued did not experience a relapse of HF, worsening dyspnea, or increased mortality, highlighting the possibility of a safe withdrawal in stable HF patients.
Heart failure is an increasing pathology and its prevalence increases with age. It is estimated that four of five patients with HF are over 65 years of age, and thus an holistic, multidisciplinary assessment of HF patients is warranted. Nevertheless, in the present review, only a small study attempted to evaluate the effect of LD discontinuation in the oldest patient. Given that older adults are prone to polypharmacy and drug–drug interactions, deprescribing is a cornerstone of geriatric medicine. In the last two decades, the debate on the appropriate timing for LDs discontinuation has been lively among different specialists. International guidelines do not provide straightforward recommendations on how to deal with diuretic adjustments in stable HF outpatients. The 2022 American College of Cardiology Foundation/American Heart Association (ACCF/AHA) Guideline for the Management of Heart Failure states that few patients with HF can maintain their target weight without using diuretics; however, the basis for such a statement is unclear [17]. It appears correct to attempt to discontinue LDs when stability is achieved. In contrast, the apprehension of an HF relapse hinders the physician’s decision to reduce diuretics.
Recently, new treatments modulating neurohormonal activation and volume status, such as SGLT-2 inhibitors and ARNIs, highlight the possibility of an effective reduction of LDs in stable HF. This aspect is particularly true for patients with chronic HF and ventricular dysfunction, for whom, according to the latest guidelines [17], a wide range of medications can be used. Interestingly, in the PARADIGM-HF trial [18], HFrEF patients receiving sacubitril-valsartan reduced diuretic doses; however, the same benefit was not reported in patients with HFpEF treated with sacubitril/valsartan. Observational data suggested that HF patients managed chronically without an LD agent generally have a good prognosis [19, 20].
4.1 Effect of LDs in Renin–Angiotensin–Aldosterone System Activation and its Implication in HF Prognosis
LDs increase neurohumoral activation (renin–angiotensin–aldosterone, sympathoadrenal, and other systems) in HF. Patients chronically treated with LDs will consistently show increased plasma renin activity and angiotensin II and aldosterone concentrations [13, 14]. LDs are also associated with sympathetic activation, shown by increases in plasma noradrenaline, heart rate, blood pressure, and vascular resistance. This sympathetic activation is associated with acute deterioration of ventricular pump function. Despite the widespread use of diuretic agents in chronic heart failure and their beneficial influence in the short-term, they cause neurohumoral hyperactivation and adversely influence prognosis in the mid- or long-term [14, 21]. Testani et al. demonstrated a direct association between high-dose LDs and a surrogate marker for renal neurohormonal activation and BUN with survival in HF. More in-depth, patients with an elevated BUN level showed lower survival than their counterparts receiving LDs with a normal BUN level. These data suggest a role for neurohormonal activation in LDs-associated mortality [22].
4.2 Stable HF Definition and the Importance of Volume Status Assessment
One of the key points of successful LD withdrawal is HF stability. Regarding the articles reviewed, we found heterogeneous methods to define stable HF. Most studies suggested a period of at least 3 months free from New York Heart Association (NYHA) class I–II HF symptoms before attempting LD withdrawal. This is a crucial point for the physician’s decision making regarding when to initiate the LD de-escalation; however, it underlies the need for common standardization on stable HF and successful decongestion definition. Clearly, patients at risk for congestion would benefit from maintenance therapy with LDs [22].
As diuretics are mainly used to relieve excessive volume, the ESC guidelines for the diagnosis and treatment of acute and chronic HF recommended detecting true volume overload. The gold standard for diagnosing congestion in HF is cardiac catheterization with direct measurement of right atrial pressure and pulmonary capillary wedge pressure (PCWP). However, the invasive nature of this technique limits its routine use in clinical practice. In patients with a history of HF or cardiac disease, the combination of signs and symptoms of congestion, an indicative chest x-ray, and measurement of elevated natriuretic peptides allows for a diagnosis of congestion [20]. The advent of portable ultrasound devices has recently highlighted the high diagnostic accuracy of a lung ultrasound, and feasibility for detecting pulmonary congestion [23, 24], and its usefulness in elderly patients with dyspnea [25]. In contrast, jugular venous distension, orthopnea, bendopnea, and pulmonary edema are less specific as they reflect increased cardiac filling pressures but not necessarily volume overload [6].
4.3 Withdrawal of LDs: Pros
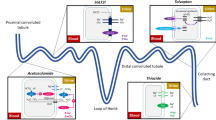
When administering LDs in older adults, clinicians should be mindful of the physiologic decrease in renal function with age and the more frequent renal impairment in the elderly receiving diuretics for HF management. Electrolyte abnormalities occur more frequently in patients with HF and pre-existing renal impairments, representing an important potential adverse effect in the geriatric population (Fig. 1).
Indeed, LD use is the most common reason for hypokalemia. In addition, other clinically important effects of LDs include a significant increase in magnesium and fractional calcium excretion. Hypercalciuria, produced dose-dependent by LDs, increases the bone fracture rate, representing a negative adverse effect, especially for the elderly [26]. Considering this, LD treatment of any HF patient should always be at the lowest effective dose. Several studies demonstrated that the dose of diuretics could be safely lowered in most patients presenting with stable chronic HF [27, 28]. A recent investigation suggested that the lowest achievable diuretic dose to provide effective decongestion may be favored over higher doses in chronic HF, even if exercise and health-related quality of life (HRQoL) are considered [29].
Moreover, activation of RAAS is of pathophysiologic and prognostic importance in HF. By activating the RAAS, diuretics may re-inforce fluid retention and peripheral vasoconstriction. Most of the studies included in this review found beneficial effects of LD removal in terms of decreasing plasma renin activity (PRA) levels and improved renal function and glucose metabolism. In all studies, no differences were found regarding adverse events following diuretic discontinuation in stable HF patients. Regarding the neurohumoral determinations, according to the data reported by Galve et al., the discontinuation of diuretic therapy was associated with a decrease in plasma renin activity at 3 months and no change in aldosterone, vasopressin, endothelin-1, and norepinephrine. van Kraaij et al. concluded that successful withdrawal of LDs was correlated with a decrease in PRA and aldosterone levels in most patients. A decline in PRA was associated with decrements in systolic and diastolic blood pressure. According to the data by van Kraaij et al., norepinephrine levels also tended to decrease after 3 months from LD discontinuation, which might signify a decline in the sympathetic nervous system [13]. These results proved that successful withdrawal was associated with reduced neurohormonal activation and, consequently, with improved morbidity and mortality risks. According to the findings reported by Romano et al. [3], indicators such as NT-pro-BNP levels and echocardiographic parameters can help identify patients with the safest profile for diuretic withdrawal. Given these premises, promoting the reduction or discontinuation of furosemide seems pivotal and reasonable for HF patients. LD withdrawal might reduce the inconvenient adverse effects of polypharmacy and simplify HF therapy, allowing physicians to titrate the dose of inhibitors of the renin-angiotensin system, as both classes of drugs may be associated with azotemia [16].
4.4 Withdrawal of Furosemide: Cons
Although all the studies included in this review demonstrated the safety of LD discontinuation, it must be acknowledged that most studies were carried out in HFrEF cohorts. Since diastolic dysfunction is prevalent among people aged ≥60 years [30], the benefits of diuretic discontinuation in older adults may be questionable, considering that in the selected studies, only one study determined clinical benefit in elderly HF patients without left ventricular systolic dysfunction [13]. Moreover, most studies showed a male majority, highlighting the underrepresentation of the female population, who tendentially develop a later presentation of HF and express an increasing prevalence of HFpEF.
Most importantly, none of the studies showed a standard algorithm for LD downtitration and subsequent withdrawal in stable HF. Indeed, a standardized diuretic treatment algorithm for LD downtitration may be extremely challenging. No such algorithm can ever meet the treatment needs of all patients, particularly elderly patients [26]. Older patients overstep the stability and instability phases, driven mainly by their frailty, a common condition in older adults that has recently been identified as an independent risk factor for long‐term mortality and hospital readmission in the elderly with HF [30, 31]. Hence, the so-called ‘robust’ patient is unlikely to have an adverse event following acute HF. In contrast, in a frail patient, even a minor stressor may beget discomfort and increase the risk of acute decompensated HF. Therefore, an evaluation of frailty status could help physicians to determine the timing of LD reduction and ambulatory re-evaluation. In the reviewed papers, no mention of frailty was made and the few oldest-old patients (aged ≥85 years) were included. This aspect is particularly crucial since, as Romano et al. [3] evaluated, increasing age emerged as an independent risk factor of LD reuse after withdrawal. Only one study with a shallow sample size attempted to evaluate the effect in older patients with HF [13]. Currently, there are several approaches for the assessment of frailty; however, many of these measures are not integrated into routine care for all patients since they are time‐consuming and of specialist expertise.
4.5 Proposed Algorithm for LD Discontinuation in Very Old Patients with HF
The management of HF patients necessitates careful consideration of various factors such as frailty, congestion, and appropriate drug treatment; however, it is noteworthy that this particular area of research lacks comprehensive exploration and empirical evidence. Consequently, in the absence of established evidence, it becomes imperative to explore alternative strategies based on previous studies. In light of this knowledge gap, we propose a suggested approach that duly recognizes the intricate nature of frailty in tailoring diuretic therapy. Nonetheless, it is crucial to emphasize the ongoing requirement for further investigation to solidify the role of frailty in guiding treatment decisions and to establish more robust guidelines in this domain.
Recently, the Clinical Frailty Scale (CFS) emerged as a powerful tool for screening frailty that offers a quick head-to-toe assessment of the patient and covers their physical functioning and dependence as well as comorbidities [31]. The CFS could be used to detect which patients, among the oldest old, could or could not benefit from diuretic discontinuation.
Prefrail and frail phenotypes (CFS ≥ 4) present an increased risk of presenting multiple episodes of acute decompensated heart failure (ADHF); thus, LD withdrawal should be careful and with a stricter follow-up. Based on the findings of our review, following a period from 1 to 3 months of successful decongestion and clinical stability in NYHA class I–II following an ADHF, according to frailty status and burden of comorbidities, an LD downtitration could be attempted. The LD dose should be reduced from 25 to 50% of the starting dose following a complete clinician evaluation, including blood examinations, lung ultrasound, and physical examinations. In the case of a non-frail patient, to confirm the prescription, LD downtitration could follow a brief re-evaluation at a 1-week comprehensive of BNP levels and electrolytes; an ambulatory visit at 3 months. Regarding older frail patients, after the downtitration attempt, the ambulatory visit should be assessed at least monthly for 3 months, being frail individuals most at risk for adverse events and HF relapse. Weight changes may be an easy and practical metric to monitor accumulation volume overload. Importantly, the individual diuretic’s need significantly changes over time. For this reason, repeated evaluation of volume status is crucial to reassess the need for LDs and to consider the downtitration or interruption of diuretic therapy.
5 Conclusions
This review revealed that diuretic therapy discontinuation might be a safe strategy that deserves consideration for patients with stable HF; however, the few included studies mostly evaluated people aged < 75 years with reduced ejection fraction. Older adults have an increased risk of acute decompensated HF, thus discouraging clinicians from diuretic deprescribing. The CFS could be used to screen frailty in elderly patients with stable HF to guide physician decisions regarding LD discontinuation. More RCTs and real-world data are warranted to detect the real benefit and long-term neuroendocrine effect of LD discontinuation in HF patients.
References
Rosano GMC, Allen LA, Abdin A, Lindenfeld J, O’Meara E, Lam CSP, et al. Drug layering in heart failure: phenotype-guided initiation. JACC Heart Fail. 2021;9(11):775–83.
Stolfo D, Sinagra G, Savarese G. Evidence-based therapy in older patients with heart failure with reduced ejection fraction. Card Fail Rev. 2022;8: e16.
Romano G, Vitale G, Bellavia D, Agnese V, Clemenza F. Is diuretic withdrawal safe in patients with heart failure and reduced ejection fraction? A retrospective analysis of our outpatient cohort. Eur J Intern Med. 2017;42:e11–3.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution. Eur J Heart Fail. 2016;18(8):891–975.
McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contributio. Eur Heart J. 2021;42(36):3599–726.
Verbrugge FH. Editor’s choice-diuretic resistance in acute heart failure. Eur Hear J Acute Cardiovasc Care. 2018;7(4):379–89.
Damman K, Ng Kam Chuen MJ, MacFadyen RJ, Lip GYH, Gaze D, Collinson PO, et al. Volume status and diuretic therapy in systolic heart failure and the detection of early abnormalities in renal and tubular function. J Am Coll Cardiol. 2011;57(22):2233–41.
Miura M, Sugimura K, Sakata Y, Miyata S, Tadaki S, Yamauchi T, et al. Prognostic impact of loop diuretics in patients with chronic heart failure—effects of addition of renin–angiotensin–aldosterone system inhibitors and β-blockers. Circ J. 2016;80(6):1396–403.
Nagano R, Masuyama T, Lee JM, Yamamoto K, Naito J, Mano T, et al. Prediction of the changes in cardiac output in association with preload reduction therapy in patients with hypertensive heart failure. Cardiovasc drugs Ther. 1997;11(1):49–56.
Montero D, Haider T. Relationship of loop diuretic use with exercise intolerance in heart failure with preserved ejection fraction. Eur Hear J Cardiovasc Pharmacother. 2018;4(3):138–41.
Sukumar S, Orkaby AR, Schwartz JB, Marcum Z, Januzzi JL, Vaduganathan M, et al. Polypharmacy in older heart failure patients: a multidisciplinary approach. Curr Heart Fail Rep. 2022;19(5):290–302.
Kociol RD, McNulty SE, Hernandez AF, Lee KL, Redfield MM, Tracy RP, et al. Markers of decongestion, dyspnea relief, and clinical outcomes among patients hospitalized with acute heart failure. Circ Hear Fail. 2013;6(2):240–5.
van Kraaij DJW, Jansen RWMM, Sweep FCGJ, Hoefnagels WHL. Neurohormonal effects of furosemide withdrawal in elderly heart failure patients with normal systolic function. Eur J Heart Fail. 2003;5(1):47–53.
Galve E, Mallol A, Catalan R, Palet J, Méndez S, Nieto E, et al. Clinical and neurohumoral consequences of diuretic withdrawal in patients with chronic, stabilized heart failure and systolic dysfunction. Eur J Heart Fail. 2005;7(5):892–8.
Oshima K, Kohsaka S, Koide K, Yoshikawa T. Reducing the dose of diuretics for heart failure patients: how low can it go? Cardiology. 2009;114(2):89.
Rohde LE, Rover MM, Neto JAF, Danzmann LC, Bertoldi EG, Simoes MV, et al. Short-term diuretic withdrawal in stable outpatients with mild heart failure and no fluid retention receiving optimal therapy: a double-blind, multicentre, randomized trial. Eur Heart J. 2019;40(44):3605–12.
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2022;145(18):e895-1032.
McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin–neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004.
Pellicori P, Cleland JGF, Zhang J, Kallvikbacka-Bennett A, Urbinati A, Shah P, et al. Cardiac dysfunction, congestion and loop diuretics: their relationship to prognosis in heart failure. Cardiovasc Drugs Ther. 2016;30(6):599–609.
Mullens W, Damman K, Harjola VP, Mebazaa A, Brunner-La Rocca HP, Martens P, et al. The use of diuretics in heart failure with congestion—a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21(2):137–55.
Van Zwieten PA. Neuroendocrine effects of diuretics in heart failure. Heart. 1994;72(2 Suppl):51–3.
Testani JM, Cappola TP, Brensinger CM, Shannon RP, Kimmel SE. Interaction between loop diuretic-associated mortality and blood urea nitrogen concentration in chronic heart failure. J Am Coll Cardiol. 2011;58(4):375–82.
Iwakura K, Onishi T. A practical guide to the lung ultrasound for the assessment of congestive heart failure. J Echocardiogr. 2021;19(4):195–204.
Picano E, Scali MC, Ciampi Q, Lichtenstein D. Lung ultrasound for the cardiologist. JACC Cardiovasc Imaging. 2018;11(11):1692–705.
Linsalata G, Okoye C, Antognoli R, Guarino D, Ravenna V, Orsitto E, et al. Pneumonia Lung Ultrasound Score (PLUS): a new tool for detecting pneumonia in the oldest patients. J Am Geriatr Soc. 2020;68(12):2855–62.
Sica DA, Gehr TWB, Frishman WH. Use of diuretics in the treatment of heart failure in the elderly. Clin Geriatr Med. 2007;23(1):107–21.
Kapelios CJ, Kaldara E, Ntalianis A, Nana E, Pantsios C, Repasos E, et al. Lowering furosemide dose in stable chronic heart failure patients with reduced ejection fraction is not accompanied by decompensation: a randomized study. Int J Cardiol. 2014;177(2):690–2.
Martens P, Verbrugge FH, Boonen L, Nijst P, Dupont M, Mullens W. Value of routine investigations to predict loop diuretic down-titration success in stable heart failure. Int J Cardiol. 2018;250:171–5.
Fudim M, O’Connor CM, Mulder H, Coles A, Bhatt AS, Ambrosy AP, et al. Loop diuretic adjustments in patients with chronic heart failure: insights from HF-ACTION. Am Heart J. 2018;205:133–41.
Kundi H, Wadhera RK, Strom JB, Valsdottir LR, Shen C, Kazi DS, et al. Association of frailty with 30-day outcomes for acute myocardial infarction, heart failure, and pneumonia among elderly adults. JAMA Cardiol. 2019;4(11):1084–91.
Sze S, Pellicori P, Zhang J, Weston J, Clark AL. Which frailty tool best predicts morbidity and mortality in ambulatory patients with heart failure? A prospective study. Eur Hear J Qual Care Clin Outcomes. https://doi.org/10.1093/ehjqcco/qcac073. (Epub 17 Nov 2022).
Acknowledgements
A preliminary version of this manuscript is available from the Research Square preprint server at https://www.researchsquare.com/article/rs-2704684/v1.
Funding
Open access funding provided by Karolinska Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This research received no external funding.
Conflict of interest
Chukwuma Okoye, Tessa Mazzarone, Cristina Cargiolli, and Daniela Guarino declare they have no conflict of interest to disclose.
Ethics approval
This review did not require Ethics Committee approval.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Availability of data and material
Not applicable.
Author contributions
All authors wrote the review and corrected the text. CO and TM supervised the writing of the review and corrected the final version of the article. All authors contributed to the article and approved the submitted version.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Okoye, C., Mazzarone, T., Cargiolli, C. et al. Discontinuation of Loop Diuretics in Older Patients with Chronic Stable Heart Failure: A Narrative Review. Drugs Aging 40, 981–990 (2023). https://doi.org/10.1007/s40266-023-01061-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40266-023-01061-1