Abstract
Cannabidiol (CBD) is a major active component of the Cannabis plant, which, unlike tetrahydrocannabinol (THC), is devoid of euphoria-inducing properties. During the last 10 years, there has been increasing interest in the use of CBD-enriched products for the treatment of epilepsy. In 2018, an oil-based highly purified liquid formulation of CBD (Epidiolex) derived from Cannabis sativa was approved by the US Food and Drug Administration for the treatment of seizures associated with Dravet syndrome (DS) and Lennox-Gastaut syndrome (LGS). The mechanisms underlying the antiseizure effects of CBD are unclear but may involve, among others, antagonism of G protein-coupled receptor 55 (GPR55), desensitization of transient receptor potential of vanilloid type 1 (TRPV1) channels, and inhibition of adenosine reuptake. CBD has complex and variable pharmacokinetics, with a prominent first-pass effect and a low oral bioavailability that increases fourfold when CBD is taken with a high-fat/high-calorie meal. In four randomized, double-blind, parallel-group, adjunctive-therapy trials, CBD given at doses of 10 and 20 mg/kg/day administered in two divided administrations was found to be superior to placebo in reducing the frequency of drop seizures in patients with LGS and convulsive seizures in patients with DS. Preliminary results from a recently completed controlled trial indicate that efficacy also extends to the treatment of seizures associated with the tuberous sclerosis complex. The most common adverse events that differentiated CBD from placebo in controlled trials included somnolence/sedation, decreased appetite, increases in transaminases, and diarrhea, behavioral changes, skin rashes, fatigue, and sleep disturbances. About one-half of the patients included in the DS and LGS trials were receiving concomitant therapy with clobazam, and in these patients a CBD-induced increase in serum levels of the active metabolite norclobazam may have contributed to improved seizure outcomes and to precipitation of some adverse effects, particularly somnolence.

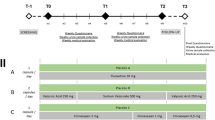
Reproduced from Taylor et al. (2018) [69], with permission
Similar content being viewed by others
References
Russo EB. Cannabis and epilepsy: an ancient treatment returns to the fore. Epilepsy Behav. 2017;70(Pt B):292–7.
Friedman D, Sirven JI. Historical perspective on the medical use of cannabis for epilepsy: ancient times to the 1980s. Epilepsy Behav. 2017;70(Pt B):298–301.
O’Shaughnessy WB. On the preparations of the Indian hemp, or Gunjah (Cannabis indica): their effects on the animal system in health, and their utility in the treatment of tetanus and other convulsive diseases. Prov Med J Retrosp Med Sci. 1843;5:363–9.
Gowers W. Epilepsy and other chronic convulsive disorders. London: Churchill; 1881.
Reynolds JR. On some of the therapeutical uses of Indian hemp. Arch Med. 1868;2:154–60.
McMeens RR. Cannabis indica in convulsions. West Lancet 1856:327–31.
Carlini EA, Leite JR, Tannhauser M, Berardi AC. Letter: Cannabidiol and Cannabis sativa extract protect mice and rats against convulsive agents. J Pharm Pharmacol. 1973;25(8):664–5.
Consroe P, Wolkin A. Cannabidiol–antiepileptic drug comparisons and interactions in experimentally induced seizures in rats. J Pharmacol Exp Ther. 1977;201(1):26–32.
Izquierdo I, Orsingher OA, Berardi AC. Effect of cannabidiol and of other cannabis sativa compounds on hippocampal seizure discharges. Psychopharmacologia. 1973;28(1):95–102.
Karler R, Cely W, Turkanis SA. The anticonvulsant activity of cannabidiol and cannabinol. Life Sci. 1973;13(11):1527–31.
Leite JR, Carlini EA, Lander N, Mechoulam R. Anticonvulsant effects of the (−) and (+)isomers of cannabidiol and their dimethylheptyl homologs. Pharmacology. 1982;24(3):141–6.
Perucca E. Cannabinoids in the treatment of epilepsy: hard evidence at last? J Epilepsy Res. 2017;7(2):61–76.
Maa E, Figi P. The case for medical marijuana in epilepsy. Epilepsia. 2014;55(6):783–6.
Epidiolex. Full Prescribing Information. https://www.epidiolex.com/sites/default/files/EPIDIOLEX_Full_Prescribing_Information.pdf. Accessed 30 Jan 2019.
GWPharmaceuticals. Press release: GW Pharmaceuticals reports positive Phase 3 pivotal trial results for EPIDIOLEX (cannabidiol) oral solution in patients with seizures associated with tuberous sclerosis complex. Carlsbad, USA, May 6, 2019 http://ir.gwpharm.com/news-releases/news-release-details/gw-pharmaceuticals-reports-positive-phase-3-pivotal-trial. Accessed 25 June 2019.
Consroe P, Benedito MA, Leite JR, Carlini EA, Mechoulam R. Effects of cannabidiol on behavioral seizures caused by convulsant drugs or current in mice. Eur J Pharmacol. 1982;83(3–4):293–8.
Klein BD, Jacobson CA, Metcalf CS, Smith MD, Wilcox KS, Hampson AJ, et al. Evaluation of cannabidiol in animal seizure models by the Epilepsy Therapy Screening Program (ETSP). Neurochem Res. 2017;42(7):1939–48.
Patra PH, Barker-Haliski M, White HS, Whalley BJ, Glyn S, Sandhu H, et al. Cannabidiol reduces seizures and associated behavioral comorbidities in a range of animal seizure and epilepsy models. Epilepsia. 2019;60(2):303–14.
Jones NA, Hill AJ, Smith I, Bevan SA, Williams CM, Whalley BJ, et al. Cannabidiol displays antiepileptiform and antiseizure properties in vitro and in vivo. J Pharmacol Exp Ther. 2010;332(2):569–77.
Karler R, Turkanis SA. Cannabis and epilepsy. Adv Biosci. 1978;22–23:619–41.
Karler R, Turkanis SA. The cannabinoids as potential antiepileptics. J Clin Pharmacol. 1981;21(S1):437S–48S.
Gobira PH, Vilela LR, Goncalves BD, Santos RP, de Oliveira AC, Vieira LB, et al. Cannabidiol, a Cannabis sativa constituent, inhibits cocaine-induced seizures in mice: possible role of the mTOR pathway and reduction in glutamate release. Neurotoxicology. 2015;50:116–21.
Do Val-da Silva RA, Peixoto-Santos JE, Kandratavicius L, De Ross JB, Esteves I, De Martinis BS, et al. Protective effects of cannabidiol against seizures and neuronal death in a rat model of mesial temporal lobe epilepsy. Front Pharmacol. 2017;8:131.
Jones NA, Glyn SE, Akiyama S, Hill TD, Hill AJ, Weston SE, et al. Cannabidiol exerts anti-convulsant effects in animal models of temporal lobe and partial seizures. Seizure. 2012;21(5):344–52.
Chiu P, Olsen DM, Borys HK, Karler R, Turkanis SA. The influence of cannabidiol and delta 9-tetrahydrocannabinol on cobalt epilepsy in rats. Epilepsia. 1979;20(4):365–75.
Turkanis SA, Smiley KA, Borys HK, Olsen DM, Karler R. An electrophysiological analysis of the anticonvulsant action of cannabidiol on limbic seizures in conscious rats. Epilepsia. 1979;20(4):351–63.
Rosenberg EC, Patra PH, Whalley BJ. Therapeutic effects of cannabinoids in animal models of seizures, epilepsy, epileptogenesis, and epilepsy-related neuroprotection. Epilepsy Behav. 2017;70(Pt B):319–27.
Kaplan JS, Stella N, Catterall WA, Westenbroek RE. Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. Proc Natl Acad Sci USA. 2017;114(42):11229–34.
Bialer M, Johannessen SI, Koepp MJ, Levy RH, Perucca E, Tomson T, et al. Progress report on new antiepileptic drugs: A summary of the Fourteenth Eilat Conference on New Antiepileptic Drugs and Devices (EILAT XIV). II. Drugs in more advanced clinical development. Epilepsia. 2018;59(10):1842-66.
Reddy DS, Golub VM. The pharmacological basis of cannabis therapy for epilepsy. J Pharmacol Exp Ther. 2016;357(1):45–55.
Katona I. Cannabis and endocannabinoid signaling in epilepsy. Handb Exp Pharmacol. 2015;231:285–316.
Blair RE, Deshpande LS, DeLorenzo RJ. Cannabinoids: is there a potential treatment role in epilepsy? Expert Opin Pharmacother. 2015;16(13):1911–4.
Di Marzo V, De Petrocellis L, Bisogno T. The biosynthesis, fate and pharmacological properties of endocannabinoids. Handb Exp Pharmacol. 2005;168:147–85.
Friedman D, French JA, Maccarrone M. Safety, efficacy, and mechanisms of action of cannabinoids in neurological disorders. Lancet Neurol. 2019;18(5):504–12.
Lupica CRHY, Devinsky O, Hoffman AF. Cannabinoids as hippocampal network administrators. Neuropharmacology. 2017;124:25–37.
Soltesz I, Alger BE, Kano M, Lee SH, Lovinger DM, Ohno-Shosaku T, et al. Weeding out bad waves: towards selective cannabinoid circuit control in epilepsy. Nat Rev Neurosci. 2015;16(5):264–77.
Ledgerwood CJ, Greenwood SM, Brett RR, Pratt JA, Bushell TJ. Cannabidiol inhibits synaptic transmission in rat hippocampal cultures and slices via multiple receptor pathways. Br J Pharmacol. 2011;162(1):286–94.
Hofmann ME, Frazier CJ. Marijuana, endocannabinoids, and epilepsy: potential and challenges for improved therapeutic intervention. Exp Neurol. 2013;244:43–50.
Goffin K, Van Paesschen W, Van Laere K. In vivo activation of endocannabinoid system in temporal lobe epilepsy with hippocampal sclerosis. Brain. 2011;134(Pt 4):1033–40.
Ludanyi A, Eross L, Czirjak S, Vajda J, Halasz P, Watanabe M, et al. Downregulation of the CB1 cannabinoid receptor and related molecular elements of the endocannabinoid system in epileptic human hippocampus. J Neurosci. 2008;28(12):2976–90.
Magloczky Z, Toth K, Karlocai R, Nagy S, Eross L, Czirjak S, et al. Dynamic changes of CB1-receptor expression in hippocampi of epileptic mice and humans. Epilepsia. 2010;51(Suppl 3):115–20.
Romigi A, Bari M, Placidi F, Marciani MG, Malaponti M, Torelli F, et al. Cerebrospinal fluid levels of the endocannabinoid anandamide are reduced in patients with untreated newly diagnosed temporal lobe epilepsy. Epilepsia. 2010;51(5):768–72.
Gaston TE, Friedman D. Pharmacology of cannabinoids in the treatment of epilepsy. Epilepsy Behav. 2017;70(Pt B):313–8.
Nichol K, Stott C, Jones N, Bazelot M, Whalley BJ. The proposed multimodal mechanism of action of cannabidiol in epilepsy: Modulation of intracellular calcium and adenosine-mediated signaling. [abstract] American Epilepsy Society Annual Meeting, New Orleans, LA, November 30–December 4, 2018. https://www.aesnet.org/meetings_events/annual_meeting_abstracts/view/554059. Accessed 12 Feb 2019.
Laprairie RB, Bagher AM, Kelly ME, Denovan-Wright EM. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br J Pharmacol. 2015;172(20):4790–805.
Tham M, Yilmaz O, Alaverdashvili M, Kelly MEM, Denovan-Wright EM, Laprairie RB. Allosteric and orthosteric pharmacology of cannabidiol and cannabidiol-dimethylheptyl at the type 1 and type 2 cannabinoid receptors. Br J Pharmacol. 2019;176(10):1455–69.
Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol. 2007;150(5):613–23.
Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134(4):845–52.
Carrier EJ, Auchampach JA, Hillard CJ. Inhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppression. Proc Natl Acad Sci USA. 2006;103(20):7895–900.
De Petrocellis L, Ligresti A, Moriello AS, Allara M, Bisogno T, Petrosino S, et al. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol. 2011;163(7):1479–94.
Gaston TE, Szaflarski JP. Cannabis for the treatment of epilepsy: an update. Curr Neurol Neurosci Rep. 2018;18(11):73.
Aso E, Fernandez-Duenas V, Lopez-Cano M, Taura J, Watanabe M, Ferrer I, et al. Adenosine A2A-cannabinoid CB1 receptor heteromers in the hippocampus: cannabidiol blunts Δ(9)-tetrahydrocannabinol-induced cognitive impairment. Mol Neurobiol. 2019. [Epub ahead of print].
Devinsky O, Cilio MR, Cross H, Fernandez-Ruiz J, French J, Hill C, et al. Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia. 2014;55(6):791–802.
Hill AJ, Jones NA, Smith I, Hill CL, Williams CM, Stephens GJ, et al. Voltage-gated sodium (NaV) channel blockade by plant cannabinoids does not confer anticonvulsant effects per se. Neurosci Lett. 2014;566:269–74.
Iannotti FA, Hill CL, Leo A, Alhusaini A, Soubrane C, Mazzarella E, et al. Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: potential for the treatment of neuronal hyperexcitability. ACS Chem Neurosci. 2014;5(11):1131–41.
Ibeas Bih C, Chen T, Nunn AV, Bazelot M, Dallas M, Whalley BJ. Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics. 2015;12(4):699–730.
Leo A, Russo E, Elia M. Cannabidiol and epilepsy: Rationale and therapeutic potential. Pharmacol Res. 2016;107:85–92.
McPartland JM, Duncan M, Di Marzo V, Pertwee RG. Are cannabidiol and Δ(9)-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br J Pharmacol. 2015;172(3):737–53.
Peres FF, Lima AC, Hallak JEC, Crippa JA, Silva RH, Abilio VC. Cannabidiol as a promising strategy to treat and prevent movement disorders? Front Pharmacol. 2018;9:482.
Rong C, Lee Y, Carmona NE, Cha DS, Ragguett RM, Rosenblat JD, et al. Cannabidiol in medical marijuana: research vistas and potential opportunities. Pharmacol Res. 2017;121:213–8.
Thompson CH, Kearney JA. Cannabidiol mellows out resurgent sodium current. Epilepsy Curr. 2016;16(6):399–401.
Bouron A. Phyto and endocannabinoids exert complex actions on calcium and zinc signaling in mouse cortical neurons. Biochem Pharmacol. 2018;152:244–51.
Muller C, Morales P, Reggio PH. Cannabinoid ligands targeting TRP channels. Front Mol Neurosci. 2019;11:487.
Vilela LR, Lima IV, Kunsch EB, Pinto HPP, de Miranda AS, Vieira ELM, et al. Anticonvulsant effect of cannabidiol in the pentylenetetrazole model: pharmacological mechanisms, electroencephalographic profile, and brain cytokine levels. Epilepsy Behav. 2017;75:29–35.
Pandolfo P, Silveirinha V, dos Santos-Rodrigues A, Venance L, Ledent C, Takahashi RN, et al. Cannabinoids inhibit the synaptic uptake of adenosine and dopamine in the rat and mouse striatum. Eur J Pharmacol. 2011;655(1–3):38–45.
Ghovanloo MR, Shuart NG, Mezeyova J, Dean RA, Ruben PC, Goodchild SJ. Inhibitory effects of cannabidiol on voltage-dependent sodium currents. J Biol Chem. 2018;293(43):16546–58.
Watkins AR. Cannabinoid interactions with ion channels and receptors. Channels (Austin). 2019;13(1):162–7.
Millar SA, Stone NL, Yates AS, O’Sullivan SE. A systematic review on the pharmacokinetics of cannabidiol in humans. Front Pharmacol. 2018;9:1365.
Taylor L, Gidal B, Blakey G, Tayo B, Morrison G. A phase I, randomized, double-blind, placebo-controlled, single ascending dose, multiple dose, and food effect trial of the safety, tolerability and pharmacokinetics of highly purified cannabidiol in healthy subjects. CNS Drugs. 2018;32(11):1053–67.
Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. 2003;42(4):327–60.
Birnbaum AK, Karanam A, Marino SE, Barkley CM, Remmel RP, Roslawski M, et al. Food effect on pharmacokinetics of cannabidiol oral capsules in adult patients with refractory epilepsy. Epilepsia. 2019. [Epub ahead of print].
Jiang R, Yamaori S, Takeda S, Yamamoto I, Watanabe K. Identification of cytochrome P450 enzymes responsible for metabolism of cannabidiol by human liver microsomes. Life Sci. 2011;89(5–6):165–70.
Mazur A, Lichti CF, Prather PL, Zielinska AK, Bratton SM, Gallus-Zawada A, et al. Characterization of human hepatic and extrahepatic UDP-glucuronosyltransferase enzymes involved in the metabolism of classic cannabinoids. Drug Metab Dispos. 2009;37(7):1496–504.
Zendulka O, Dovrtelova G, Noskova K, Turjap M, Sulcova A, Hanus L, et al. Cannabinoids and cytochrome P450 interactions. Curr Drug Metab. 2016;17(3):206–26.
Morrison G, Crockett J, Blakey G, Sommerville K. A Phase 1, open-label, pharmacokinetic trial to investigate possible drug-drug interactions between clobazam, stiripentol, or valproate and cannabidiol in healthy subjects. Clin Pharmacol Drug Dev. 2019. [Epub ahead of print].
Whalley BJ, Stott C, Gray RA, N.A. J. The human metabolite of cannabidiol, 7-hydroxy-cannabidiol, but not 7-carboxy-cannabidiol, is anticonvulsant in the maximal electroshock threshold test (MEST) in mouse [abstract]. American Epilepsy Society Annual Meeting, December 1-5, 2017, Washington, DC. https://www.aesnet.org/meetings_events/annual_meeting_abstracts/view/381222. Accessed 09 Feb 2019.
Devinsky O, Patel AD, Thiele EA, Wong MH, Appleton R, Harden CL, et al. Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology. 2018;90(14):e1204–11.
Wheless JW, Dlugos D, Miller I, Oh DA, Parikh N, Phillips S, et al. Pharmacokinetics and tolerability of multiple doses of pharmaceutical-grade synthetic cannabidiol in pediatric patients with treatment-resistant epilepsy. CNS Drugs. 2019;33(6):593–604.
Taylor L, Crockett J, Tayo B, Morrison G. A phase 1, open-label, parallel-group, single-dose trial of the pharmacokinetics and safety of cannabidiol (CBD) in subjects with mild to severe hepatic impairment. J Clin Pharmacol. 2019. [Epub ahead of print].
Stott C, White L, Wright S, Wilbraham D, Guy G. A phase I, open-label, randomized, crossover study in three parallel groups to evaluate the effect of rifampicin, ketoconazole, and omeprazole on the pharmacokinetics of THC/CBD oromucosal spray in healthy volunteers. Springerplus. 2013;2(1):236.
Sativex. Orosomucosal Spray, Summary of Product Characteristics, 28 August 2018 revision. https://www.medicines.org.uk/emc/product/602/smpc#DOCREVISION. Accessed 11 Feb 2019.
Arellano AL, Papaseit E, Romaguera A, Torrens M, Farre M. Neuropsychiatric and general interactions of natural and synthetic cannabinoids with drugs of abuse and medicines. CNS Neurol Disord Drug Targets. 2017;16(5):554–66.
Jiang R, Yamaori S, Okamoto Y, Yamamoto I, Watanabe K. Cannabidiol is a potent inhibitor of the catalytic activity of cytochrome P450 2C19. Drug Metab Pharmacokinet. 2013;28(4):332–8.
Stout SM, Cimino NM. Exogenous cannabinoids as substrates, inhibitors, and inducers of human drug metabolizing enzymes: a systematic review. Drug Metab Rev. 2014;46(1):86–95.
Yamaori S, Okamoto Y, Yamamoto I, Watanabe K. Cannabidiol, a major phytocannabinoid, as a potent atypical inhibitor for CYP2D6. Drug Metab Dispos. 2011;39(11):2049–56.
Yamaori S, Ebisawa J, Okushima Y, Yamamoto I, Watanabe K. Potent inhibition of human cytochrome P450 3A isoforms by cannabidiol: role of phenolic hydroxyl groups in the resorcinol moiety. Life Sci. 2011;88(15–16):730–6.
Morrison G, Taylor L, Crockett J, Critchley D, Tayo B. A Phase 1 investigation into the potential effects of cannabidiol on CYP3A4-mediated drug-drug interactions in healthy volunteers [abstract]. American Epilepsy Society Annual Meeting, New Orleans, LA, November 30–December 4, 2018. https://www.aesnet.org/meetings_events/annual_meeting_abstracts/view/500033. Accessed 09 Feb 2019.
Devinsky O, Patel AD, Cross JH, Villanueva V, Wirrell EC, Privitera M, et al. Effect of cannabidiol on drop seizures in the Lennox-Gastaut syndrome. N Engl J Med. 2018;378(20):1888–97.
Friedman D, Devinsky O. Cannabinoids in the treatment of epilepsy. N Engl J Med. 2015;373(11):1048–58.
Friedman D, Cilio MR, Tilton N, Sullivan J, Hedlund J, Rosenberg E, et al. The effect of epidiolex (cannabidiol) on serum levels of concomitant anti-epileptic drugs in children and young adults with treatment-resistant epilepsy in an expanded access program [abstract]. American Epilepsy Society 2014 annual meeting. Seattle, WA, December 5–9, 2014. https://www.aesnet.org/annual_meeting/abstract_search#/sortDate_na_dt/DESC/0/epidiolex/?Year=2014. Accessed 09 Feb 2019.
Gaston TE, Bebin EM, Cutter GR, Liu Y, Szaflarski JP. Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia. 2017;58(9):1586–92.
Geffrey AL, Pollack SF, Bruno PL, Thiele EA. Drug–drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia. 2015;56(8):1246–51.
Giraud C, Treluyer JM, Rey E, Chiron C, Vincent J, Pons G, et al. In vitro and in vivo inhibitory effect of stiripentol on clobazam metabolism. Drug Metab Dispos. 2006;34(4):608–11.
Diacomit. Summary of Product Characteristics, revised 8 January 2014, European Medicines Agency. https://www.ema.europa.eu/en/documents/product-information/diacomit-epar-product-information_en.pdf. Accessed 06 Mar 2019.
Klotz KA, Hirsch M, Heers M, Schulze-Bonhage A, Jacobs J. Effects of cannabidiol on brivaracetam plasma levels. Epilepsia. 2019;60(7):e74–7.
Tayo B, Ben-Menachem E, Gunning B, Arenas Cabrera CM, Crockett J, Taylor L, et al. Exploration of the potential for plasma protein binding displacement and drug–drug interactions of valproate in combination with cannabidiol [abstract]. American Epilepsy Society Annual Meeting, New Orleans, LA, November 30–December 4, 2018. https://www.aesnet.org/meetings_events/annual_meeting_abstracts/view/540271. Accessed 09 Feb 2019.
Grayson L, Vines B, Nichol K, Szaflarski JP. An interaction between warfarin and cannabidiol, a case report. Epilepsy Behav Case Rep. 2018;9:10–1.
Al Saabi A, Allorge D, Sauvage FL, Tournel G, Gaulier JM, Marquet P, et al. Involvement of UDP-glucuronosyltransferases UGT1A9 and UGT2B7 in ethanol glucuronidation, and interactions with common drugs of abuse. Drug Metab Dispos. 2013;41(3):568–74.
Englund A, Morrison PD, Nottage J, Hague D, Kane F, Bonaccorso S, et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J Psychopharmacol. 2013;27(1):19–27.
Niesink RJ, van Laar MW. Does cannabidiol protect against adverse psychological effects of THC? Front Psychiatry. 2013;4:130.
Petitet F, Jeantaud B, Reibaud M, Imperato A, Dubroeucq MC. Complex pharmacology of natural cannabinoids: evidence for partial agonist activity of Δ9-tetrahydrocannabinol and antagonist activity of cannabidiol on rat brain cannabinoid receptors. Life Sci. 1998;63(1):1–6.
Todd SM, Arnold JC. Neural correlates of interactions between cannabidiol and Δ(9)-tetrahydrocannabinol in mice: implications for medical cannabis. Br J Pharmacol. 2016;173(1):53–65.
Todd SM, Zhou C, Clarke DJ, Chohan TW, Bahceci D, Arnold JC. Interactions between cannabidiol and Δ(9)-THC following acute and repeated dosing: Rebound hyperactivity, sensorimotor gating and epigenetic and neuroadaptive changes in the mesolimbic pathway. Eur Neuropsychopharmacol. 2017;27(2):132–45.
Devinsky O, Cross JH, Laux L, Marsh E, Miller I, Nabbout R, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376(21):2011–20.
Devinsky O, Marsh E, Friedman D, Thiele E, Laux L, Sullivan J, et al. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. 2016;15(3):270–8.
Ahn Y, Drummond-Main C, Kiroski I, Rho JM. Cannabidiol impairs mitochondrial function independent of CB1 and GPR55 receptors [abstract]. American Epilepsy Society Annual Meeting, New Orleans, LA, November 30–December 4, 2018. https://www.aesnet.org/meetings_events/annual_meeting_abstracts/view/500201. Accessed 10 Feb 2019.
Ahn Y, Drummond-Main C, Yee NC, Kim DY, Rho JM. Effects of cannabidiol on mitochondrial bioenergetics and homeostasis [abstract]. American Epilepsy Society Annual Meeting, New Orleans, LA, November 30–December 4, 2018. https://www.aesnet.org/meetings_events/annual_meeting_abstracts/view/344542. Accessed 10 Feb 2019.
Stockings E, Zagic D, Campbell G, Weier M, Hall WD, Nielsen S, et al. Evidence for cannabis and cannabinoids for epilepsy: a systematic review of controlled and observational evidence. J Neurol Neurosurg Psychiatry. 2018;89(7):741–53.
McCoy B, Wang L, Zak M, Al-Mehmadi S, Kabir N, Alhadid K, et al. A prospective open-label trial of a CBD/THC cannabis oil in dravet syndrome. Ann Clin Transl Neurol. 2018;5(9):1077–88.
Hess EJ, Moody KA, Geffrey AL, Pollack SF, Skirvin LA, Bruno PL, et al. Cannabidiol as a new treatment for drug-resistant epilepsy in tuberous sclerosis complex. Epilepsia. 2016;57(10):1617–24.
Kaplan EH, Offermann EA, Sievers JW, Comi AM. Cannabidiol treatment for refractory seizures in Sturge–Weber syndrome. Pediatr Neurol. 2017;71(18–23):e2.
Gofshteyn JS, Wilfong A, Devinsky O, Bluvstein J, Charuta J, Ciliberto MA, et al. Cannabidiol as a potential treatment for Febrile Infection-Related Epilepsy Syndrome (FIRES) in the acute and chronic phases. J Child Neurol. 2017;32(1):35–40.
Saade D, Joshi C. Pure cannabidiol in the treatment of malignant migrating partial seizures in infancy: a case report. Pediatr Neurol. 2015;52(5):544–7.
Dale T, Downs J, Olson H, Bergin AM, Smith S, Leonard H. Cannabis for refractory epilepsy in children: a review focusing on CDKL5 Deficiency Disorder. Epilepsy Res. 2019;151:31–9.
Devinsky O, Verducci C, Thiele EA, Laux LC, Patel AD, Filloux F, et al. Open-label use of highly purified CBD (Epidiolex®) in patients with CDKL5 deficiency disorder and Aicardi, Dup15q, and Doose syndromes. Epilepsy Behav. 2018;86:131–7.
Elliott J, DeJean D, Clifford T, Coyle D, Potter BK, Skidmore B, et al. Cannabis-based products for pediatric epilepsy: a systematic review. Epilepsia. 2019;60(1):6–19.
Pamplona FA, da Silva LR, Coan AC. Potential clinical benefits of CBD-rich cannabis extracts over purified CBD in treatment-resistant epilepsy: observational data meta-analysis. Front Neurol. 2018;9:759.
Pamplona FA, da Silva LR, Coan AC. Corrigendum: Potential clinical benefits of CBD-rich Cannabis extracts over purified CBD in treatment-resistant epilepsy: observational data meta-analysis. Front Neurol. 2018;9:1050.
White CM. A review of human studies assessing cannabidiol’s (CBD) therapeutic actions and potential. J Clin Pharmacol. 2019. [Epub ahead of print].
FDA. United States of America, 2016. Warning letters and test results for cannabidiol-related products. https://www.fda.gov/NewsEvents/PublicHealthFocus/ucm484109.htm. Accessed 17 Feb 2019.
Bettiol A, Lombardi N, Crescioli G, Maggini V, Gallo E, Mugelli A, et al. Galenic preparations of therapeutic Cannabis sativa differ in cannabinoids concentration: a quantitative analysis of variability and possible clinical implications. Front Pharmacol. 2019;9:1543.
Bonn-Miller MO, Loflin MJE, Thomas BF, Marcu JP, Hyke T, Vandrey R. Labeling accuracy of Cannabidiol extracts sold online. JAMA. 2017;318(17):1708–9.
Carcieri C, Tomasello C, Simiele M, De Nicolo A, Avataneo V, Canzoneri L, et al. Cannabinoids concentration variability in cannabis olive oil galenic preparations. J Pharm Pharmacol. 2018;70(1):143–9.
Dryburgh LM, Bolan NS, Grof CPL, Galettis P, Schneider J, Lucas CJ, et al. Cannabis contaminants: sources, distribution, human toxicity and pharmacologic effects. Br J Clin Pharmacol. 2018;84(11):2468–76.
Pavlovic R, Nenna G, Calvi L, Panseri S, Borgonovo G, Giupponi L, et al. Quality traits of “Cannabidiol Oils”: Cannabinoids content, terpene fingerprint and oxidation stability of European commercially available preparations. Molecules. 2018;23(5).
Vandrey R, Raber JC, Raber ME, Douglass B, Miller C, Bonn-Miller MO. Cannabinoid dose and label accuracy in edible medical cannabis products. JAMA. 2015;313(24):2491–3.
Pacifici R, Marchei E, Salvatore F, Guandalini L, Busardo FP, Pichini S. Evaluation of cannabinoids concentration and stability in standardized preparations of cannabis tea and cannabis oil by ultra-high performance liquid chromatography tandem mass spectrometry. Clin Chem Lab Med. 2017;55(10):1555–63.
Szaflarski JP, Bebin EM, Comi AM, Patel AD, Joshi C, Checketts D, et al. Long-term safety and treatment effects of cannabidiol in children and adults with treatment-resistant epilepsies: expanded access program results. Epilepsia. 2018;59(8):1540–8.
Szaflarski JP, Bebin EM, Cutter G, DeWolfe J, Dure LS, Gaston TE, et al. Cannabidiol improves frequency and severity of seizures and reduces adverse events in an open-label add-on prospective study. Epilepsy Behav. 2018;87:131–6.
Gaston T, Szaflarski M, Hansen B, Grayson L, Bebin EM, Szaflarski J. Improvement in quality of life ratings after one year of treatment with pharmaceutical formulation of cannabidiol (CBD). Epilepsia. 2017;58:S155.
Rosenberg EC, Louik J, Conway E, Devinsky O, Friedman D. Quality of Life in Childhood Epilepsy in pediatric patients enrolled in a prospective, open-label clinical study with cannabidiol. Epilepsia. 2017;58(8):e96–100.
Thiele EA, Marsh ED, French JA, Mazurkiewicz-Beldzinska M, Benbadis SR, Joshi C, et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10125):1085–96.
Cunha JM, Carlini EA, Pereira AE, Ramos OL, Pimentel C, Gagliardi R, et al. Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology. 1980;21(3):175–85.
Mechoulam R, Carlini EA. Toward drugs derived from cannabis. Naturwissenschaften. 1978;65(4):174–9.
Trembly B, Sherman M. Double-blind clinical study of cannabidiol as a secondary anticonvulsant. Marijuana ‘90 International Conference on Cannabis and Cannabinoids; July 8–11, 1990; Kolympari, Crete (cited by Devinsky et al. 2014).
Ames FR, Cridland S. Anticonvulsant effect of cannabidiol. S Afr Med J. 1986;69(1):14.
Gloss D, Vickrey B. Cannabinoids for epilepsy. Cochrane Database Syst Rev. 2014(3):CD009270.
Koppel BS, Brust JC, Fife T, Bronstein J, Youssof S, Gronseth G, et al. Systematic review: efficacy and safety of medical marijuana in selected neurologic disorders: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82(17):1556–63.
Miller I, Perry MS, Saneto RP, Scheffer I, Gunning B, Sanchez-Carpintero R, et al. Cannabidiol (CBD; 10 and 20 mg/kg/day) significantly reduces convulsive seizure frequency in children and adolescents with Dravet syndrome (DS): results of a dose-ranging, multi-center, randomized, double-blind, placebo-controlled trial (GWPCARE2). [abstract]. Annual Meeting of the American Academy of Neurology, Philadelphia, May 4–10, 2019. http://file:///E:/2019-emerging-science-schedule-and-abstracts-with-disclosures.pdf. Accessed 13 May 2019.
Thiele E, Marsh E, Mazurkiewicz-Beldzinska M, Halford JJ, Gunning B, Devinsky O, et al. Cannabidiol in patients with Lennox-Gastaut syndrome: Interim analysis of an open-label extension study. Epilepsia. 2019;60(3):419–28.
Devinsky O, Nabbout R, Miller I, Laux L, Zolnowska M, Wright S, et al. Long-term cannabidiol treatment in patients with Dravet syndrome: an open-label extension trial. Epilepsia. 2019;60(2):294–302.
Thiele EA, Devinsky O, Checketts D, Knappertz V. Cannabidiol treatment responder analysis in patients with Lennox-Gastaut syndrome on and off clobazam [abstract]. American Epilepsy Society Annual Meeting, New Orleans, LA, November 30–December 4, 2018. https://www.aesnet.org/meetings_events/annual_meeting_abstracts/view/381224. Accessed 04 Mar 2019.
Lattanzi S, Brigo F, Trinka E, Zaccara G, Cagnetti C, Del Giovane C, et al. Efficacy and safety of cannabidiol in epilepsy: a systematic review and meta-analysis. Drugs. 2018;78(17):1791–804.
Sands TT, Rahdari S, Oldham MS, Caminha Nunes E, Tilton N, Cilio MR. Long-term safety, tolerability, and efficacy of cannabidiol in children with refractory epilepsy: results from an expanded access program in the US. CNS Drugs. 2019;33(1):47–60.
Babalonis S, Haney M, Malcolm RJ, Lofwall MR, Votaw VR, Sparenborg S, et al. Oral cannabidiol does not produce a signal for abuse liability in frequent marijuana smokers. Drug Alcohol Depend. 2017;172:9–13.
Schoedel KA, Szeto I, Setnik B, Sellers EM, Levy-Cooperman N, Mills C, et al. Abuse potential assessment of cannabidiol (CBD) in recreational polydrug users: a randomized, double-blind, controlled trial. Epilepsy Behav. 2018;88:162–71.
Schonhofen P, Bristot IJ, Crippa JA, Hallak JEC, Zuardi AW, Parsons RB, et al. Cannabinoid-based therapies and brain development: potential harmful effect of early modulation of the endocannabinoid system. CNS Drugs. 2018;32(8):697–712.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This work was not supported by any funding source.
Conflict of interest
Valentina Franco received consultancy fees from GW Pharma. Emilio Perucca received speaker and/or consultancy fees from Amicus Therapeutics, Biogen, Eisai, GW Pharma, Sanofi, Sun Pharma, Takeda, UCB Pharma and Xenon Pharma.
Additional information
Note Added in Proof
On 26 July 2019, the EMA Committee for Medicinal Products for Human Use (CHMP) has adopted a positive opinion recommending marketing authorisation of GW CBD (Epidyolex™) for use as adjunctive therapy of seizures associated with LGS or DS, in conjunction with clobazam, for patients 2 years of age and older. The European Commission is expected to make a final decision on the marketing authorisation application approximately two months after the CHMP's positive opinion. (http://ir.gwpharm.com/node/10891/pdf. Accessed 28 July 2019).
Rights and permissions
About this article
Cite this article
Franco, V., Perucca, E. Pharmacological and Therapeutic Properties of Cannabidiol for Epilepsy. Drugs 79, 1435–1454 (2019). https://doi.org/10.1007/s40265-019-01171-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-019-01171-4