Abstract
Cannabidiol (CBD) has been studied for substance use disorders treatment due to its anxiolytic effects, for sleep, appetite, reduction of craving, and maintenance of abstinence. The study aims to assess CBD’s feasibility, safety/tolerability, and preliminary efficacy compared to pharmacological treatment as usual for reducing crack use in people with crack use disorder (CUD) and investigate other parameters: adverse events, physical health symptoms, and craving. A double-blind, randomized clinical trial (RCT) with two treatment arms (CBD and control group) was conducted. Ninety participants were randomized and 73 were allocated: 37 control group and 36 CBD group for a 10-week treatment, comparing CBD (600 mg) with three drugs (fluoxetine, valproic acid, and clonazepam). The per-protocol analysis of participants who did not deviate from the study protocol compared the control and CBD treatment groups. Thirty-four completed at least half of the study and 25 finished. Participants attended weekly meetings for the study procedures (e.g., to receive the medication and provide urine for toxicological tests). Inter-group differences were performed with the Mann–Whitney test, the Wilcoxon test for differences intra-group, and Pearson’s Chi-square test or Fisher’s exact test to compare inter-group demographic data. The significance level was 5%. A “veracity index” (VI) was created as counterevidence (questionnaire data vs. the toxicological test result). Medications were considered safe/tolerable. The CBD group presented significantly fewer adverse events compared to the control group [e.g., dizziness (p = 0.001), memory impairment (p = 0.043)], which performed better in the reduction of clinical and psychiatric complaints (p = 0.008). In the intra-group analyses, the CBD group performed better in more parameters than the control group [e.g., reducing crack use (p = 0.016; T0 to T1)]. Data questionnaires were reliable regarding the use/non-use of crack (VI = 0.787). CBD is a safe/tolerable product. The CBD group manifested fewer adverse events than the control group, which had better clinical and psychiatric complaints results. There are some advantages for the CBD group in the intra-group analysis. Drug use self-report methodologies can be reliable. Trial registration details: This study is registered with Universal Trial Number (UTN) code: U1111-1234-0806. Available at https://ensaiosclinicos.gov.br/rg/RBR-4stgs8 (Effect of cannabidiol in the treatment of crack dependents)
Similar content being viewed by others
Substance use disorders (SUD), including smoked crack/cocaine—crack use disorder (CUD)—pose a considerable challenge and have been the focus of scientific research in public health. According to Fischer, Blanken, et al. (2015) and the United Nations Office on Drugs and Crime (UNODC, 2020), CUD is more common in the Americas, with an estimated 2.8 million people between 15 and 64 years of age using cocaine in South America. According to the report, Brazil contributes heavily to the cocaine market, with a large portion of consumption occurring in the form of crack. However, it is difficult to accurately estimate use, as those who use the drug are generally part of socially marginalized groups not included in household surveys (Amundsen & Reid, 2014).
The socio-demographic profile of people with CUD in Brazil is characterized by high vulnerability, making engaging them in treatment challenging. They are often young (30% 18 to 24 years of age), male (78.7%), non-White (79%), with low socioeconomic status (87% do not have regular work), and low schooling (55% only have a primary school education). Additionally, most were people who used polydrugs (92% also used tobacco, and 83.8% used alcohol) and were not in treatment but wanted to be (78%; Bastos & Bertoni, 2014).
Regarding pharmacological treatment for CUD and its derivatives, several agents have been investigated in-depth to improve responses to treatment, especially by reducing cravings and minimizing withdrawal symptoms. However, no specific pharmacological therapy with established efficacy is approved by regulatory authorities (Chan et al., 2019; Rodrigues et al., 2020). The interest in pharmacological interventions for treating CUD has produced an increasing number of scientific studies in the last 10 years (Carvalho et al., 2016; Fischer, Kuganesan, et al., 2015; Ronsley et al., 2020). More recently, there has been an interesting hypothesis about the potential treatment for CUD with glutamatergic agents, such as ketamine. These agents could inhibit NMDA receptors in the GABAergic terminal of inhibitory interneurons, increasing glutamate release. Glutamate activates AMPA receptors in lateral habenula neurons, directly affecting downstream mechanisms, and promoting synaptic plasticity induced by BDNF, mTOR, GSK-3, and others. It improves cognitive processes, memory, and learning, reducing symptoms of major depression (Zanos & Gould, 2018; Serafini et al., 2013; Zanos &). The antidepressant effects of glutamatergic derivatives, particularly ketamine, are relevant. However, the effects of this treatment on other mental health parameters and substance use disorders are inconclusive, although promising (Walsh et al., 2021).
The therapeutic potential of Cannabis spp. has been known for many years. This considerable medicinal potential is due to the large number of chemical substances discovered in the plant, particularly the phytocannabinoids, along with the discovery and understanding of the critical role that the endocannabinoid system plays in the human organism (Mechoulam & Parker, 2013; Morgan et al., 2013). The clinical uses of these compounds, especially cannabidiol (CBD), have been useful for many objectives, including preventing relapse in SUD (Cristino et al., 2020; Rodrigues et al., 2020). CBD has been studied more frequently in recent years due to its anxiolytic (Skelley et al., 2020), antipsychotic (Englund et al., 2013), and anticonvulsant (Lazarini-Lopes et al., 2020) effects as well as its potential benefit for sleep (Shannon et al., 2019) and appetite problems (Roberts et al., 2019). Rodrigues et al. (2020) conducted a systematic review to determine the therapeutic potential of CBD for cocaine use disorder in animal models, reporting a reduction in the use of cocaine or the maintenance of abstinence, hepatic protection/reduction in hepatotoxicity, neural proliferation, and anxiolytic effects.
CBD may interact with multiple targets in the central nervous system. It binds to both CB1 and CB2 receptors at orthosteric sites at certain concentrations, exhibiting antagonist/inverse agonist properties (An et al., 2020; Pertwee, 2008). CBD has been observed to attenuate THC effects mediated through CB1 receptors. Blocking CB1 could potentially inhibit reward-seeking responses. (Spanagel, 2020). For example, rodents receiving CBD in the nucleus accumbens inhibited amphetamine-induced dopaminergic activity in the ventral tegmental area (VTA) (Renard et al., 2016). CBD also reduced self-administration of low doses of cocaine, and this effect was mediated by multiple receptor mechanisms, including CB2, TRPV1, and 5HT1A (Galaj et al., 2020). Thus, CBD may interfere with behaviors associated with compulsive crack/cocaine use in individuals with CUD.
In this sense, studies investigating the potential therapeutic benefits of cannabinoids in treating stimulant use disorders, especially CUD in the context of the Americas, appear warranted and are urgently needed (Fischer, Blanken, et al., 2015).
Clinical trials with long-term follow-up using cannabis compounds on humans with CUD are scarce. The literature offers studies on the intentional use of smoked cannabis as a harm-reduction strategy to minimize the unpleasant effects of cravings and withdrawal from crack (Gonçalves & Nappo, 2015; Pereira & Wurfel, 2011; Socías et al., 2017). However, recently published clinical studies on CBD did not identify reductions in withdrawal-related cravings (Meneses-Gaya et al., 2020; Mongeau-Pérusse et al., 2021).
Therefore, the present study aims to assess CBD’s feasibility, safety/tolerability, and preliminary efficacy compared to pharmacological treatment as usual for reducing crack use in people with CUD and investigate other parameters—adverse events, physical health symptoms, and craving—based on data from a double-blind, randomized clinical trial (RCT).
Methods
Study Design
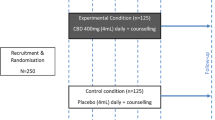
This is a double-blind RCT with two treatment arms and parallel allocation (1:1) where participants were randomized for 10 weeks of treatment in two groups (CBD and control).
Participants and Recruitment
The sample was recruited (i) based on the records of individuals who reported CUD and were undergoing treatment at a Psycho-Social Care Center (CAPS-AD; a community-based drug treatment), (ii) through broad divulgation in social media, (iii) as well as the distribution of flyers at hospitals/health units and public spaces (bus stop, public square) in Brasília, Federal District, Brazil.
The inclusion criteria were men and women 18 to 65 years old with reported crack use at least 20 times in the previous 30 days, regular crack use for at least 1 year (even with interruptions), and those who had the desire/intention to treat. The exclusion criteria were the presence of clinical (e.g., heart, liver, or neurological) or mental (e.g., schizophrenia or bipolar) disorders, as well the use of other medications that may constitute a potential risk of drug interaction or source of bias to the study results. Many people with CUD are expected to also use other substances, such as alcohol, tobacco, and marijuana. Since this study was focused on the feasibility, safety/tolerability, and preliminary efficacy under realistic conditions (outpatient care, without strict control of abstinence), participants with polydrug use were not excluded, considering that most people with CUD use other drugs. However, it was ensured that crack was the “primary drug”, both from the participant’s own point of view and from the assessment of the study team in the screening stage.
Informed consent was obtained from all participants and indicated through signing the informed consent forms.
Outcomes and Procedures
The primary outcomes were safety and tolerability, frequency of crack use, adverse events, physical health symptoms, and craving (urge and recall-induced craving).
To perform all the study procedures, the participants needed to attend the Reference Center for Drugs and Vulnerabilities (Centro de Referência sobre Drogas e Vulnerabilidades Associadas), a research center composed of a multidisciplinary team at the University of Brasília (UnB; Federal District, Brazil), once per week for 10 weeks to receive the medication kit, answer the questionnaires, receive a brief intervention, meet the physician, and provide urine for the toxicological tests. All study procedures were performed in private offices to ensure the confidentiality and comfort of participants. At each meeting, participants received ~US$ 5.00 to cover transportation expenses.
The purpose of performing an out-of-hospital double-blind RCT without rigorous control of abstinence from crack and other drugs employed in the study was to reproduce treatment dynamics according to the model adopted in the Brazilian public healthcare system, primarily performed at community-based drug treatment services (CAPS-AD; Gallassi et al., 2016). This method also reproduces the real-life situation of some individuals with CUD, characterized mainly by precarious living and work conditions, and polydrug use.
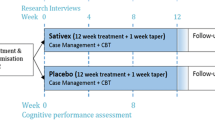
The study was composed of four questionnaires for data collection, consisting of (i) the initial questionnaire addressing the participant’s sociodemographic profile, substance use history, routine impacted by the substance use, and expectations about the treatment; (ii) the weekly questionnaire addressing crack use, the intensity of crack craving and recall-induced craving, routine impacted by crack use, adverse events of medications, and adherence to medication use (correct medications intake, possible forgetfulness, possible use of medications other than the study medications) in the previous week; (iii) the monthly questionnaire contained the same questions of the weekly questionnaire for crack use, adding information about the frequency and quantity of the use of other drugs, and physical and psychiatric symptoms in the last month; and (iv) the final questionnaire included the questions of the weekly and monthly questionnaires, and the participant’s satisfaction and perceived progress during the treatment period (Fig. 1I).
Crack Use
Crack use was assessed once per week during the 10-week study through the weekly questionnaire. The questions were developed based on the Composite International Diagnostic Interview (CIDI; Kessler & Üstün, 2004), and on the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST; Henrique et al., 2004), both validated instruments for the Brazilian population. Participants were asked about the frequency of crack use in the last 7 days (no-use/1 or 2 times/3 to 4 times/daily), the highest number of consecutive days without crack use, the highest number of consecutive days with crack use, the average number of “stones”/grams used on days of use, and the highest number of “stones”/grams used in a single day.
Adverse Events
Adverse events of the medications used in the study were assessed once per week during the weeks of pharmacological treatment—week 1 (T0) to week 9 (T2)—using the weekly questionnaire. The list of adverse events was built according to the scientific literature (Chan et al., 2019; Fischer, Kuganesan, et al., 2015; Lazarini-Lopes et al., 2020; Morgan et al., 2013; Rodrigues et al., 2020; Ronsley et al., 2020) and the medications’ leaflets, such as headache, diarrhea, nausea, sleepiness, dizziness, and anxiety. Participants were asked if they had experienced any adverse events related to taking the medication in the last 7 days. If they answered yes, they were asked to describe the events. Then, these events were marked on the appropriate items of the questionnaire, and if there was no appropriate option, they were described and registered as “other”.
Physical Health Symptoms
The questions of this parameter were assessed once per month using the monthly questionnaire. It was built based on the CIDI (Kessler & Üstün, 2004) to verify the general health status of the participants regarding clinical and/or psychiatric complaints as well as the decrease in food intake and its relationship with crack use, considering that this is one of the main “physical symptoms” reported by people with CUD. Participants were asked how they rated their health in the last 30 days (bad/regular/good/very good/excellent) and whether they had any health complaints (clinical complaints, such as generalized pain, fatigue; psychiatric/emotional complaints, such as sadness, anxiety, depression; or both clinical and psychiatric complaints). They also answered questions about their food intake (no decrease/slight decrease/moderate decrease/severe decrease) and whether the decrease in food intake was due to crack use (never/sometimes/many times).
Craving and Recall-Induced Craving
Craving and recall-induced cravings for crack were assessed once per week during the 10-week study by the weekly questionnaire. The two questions were based on the Craving Cocaine Questionnaire (Weiss et al., 1997) to address the intensity (weak/intermediate/ strong) of the crack craving and the recall-induced cravings in the previous week. Participants were asked to rate the intensity of their craving for crack in the last 7 days, and if they were in a typical crack use situation/environment with the presence of triggers (e.g., pipe, lighter, beer, people using/selling), what would be the intensity of the craving resulting from this imagined environment?
Toxicological Test
The participants’ urine samples were collected once per week during the 10-week study. The samples were stored in 10-mL polypropylene tubes at –80°C before laboratory analysis at the Criminalistic Institute of the Federal District Civil Police. The detection method was liquid chromatography coupled with tandem mass spectrometry. The limit of detection of the method was 1 ng/mL. The monitoring of crack use was performed by the determination of benzoylecgonine (BZE). The cutoff level for BZE considered positive for crack use was ≥ 100 ng/mL (Nickley et al., 2017; West et al., 2011).
Intervention
Participants in both groups, CBD and control, were treated for 10 weeks after the screening (T-1). At the screening stage, it was assessed whether the participants met the inclusion/exclusion criteria and whether they were taking any medications to treat CUD for washout procedures (1-week washout period). The study time points are as follows:
-
Week 1 (T0) to week 9 (T2): pharmacological treatment;
-
Week 5: half of the study (T1);
-
Week 9 (T2) to 10 (T3): follow-up with the suspension of the medications;
-
Week 10 (T3): final study procedures (de-blinding and referral for continuity of treatment in CAPS-AD; Fig. 1I).
The intervention group received CBD oil, Isodiolex® 600 mg (50 mg/ml CBD), which consisted of pharmaceutical-grade coconut oil infused in THC-free hemp concentrate refined for > 99% purity of natural CBD with a strawberry aroma. A dose of 300 mg was used in the first week (week 1—T0) and in the last week of the pharmacological treatment (week 8 to week 9—T2); a dose of 600 mg was used from week 2 until week 7 to 8 (Fig. 1)II.
The control group received the pharmacological treatment as usual, which was based on the (i) symptoms commonly experienced by people with CUD (Fischer, Blanken, et al., 2015; Rodrigues et al., 2020)—insomnia, paranoia, depression, inappetence, and anxiety—(ii) on the clinical practice performed in the CAPS-AD in Brazil, which takes into consideration the availability of the medications provided by Brazil’s universal health system (SUS, Sistema Único de Saúde; Carvalho et al., 2021), and (iii) by an interview conducted with CAPS-AD physicians to verify the medication management commonly applied in cases of CUD. The medications used were as follows:
-
Fluoxetine: 20 mg from week 1 (T0) until the last week of the pharmacological treatment (week 8 to 9—T2), one capsule/day;
-
Valproic acid: 250 mg in the first week (week 1—T0) and in the last week of the pharmacological treatment (week 8 to 9—T2) and 500 mg from week 2 until week 7 to 8, in both doses two capsules/day;
-
Clonazepam: 2 mg from week 1 until 6, 1 mg in week 7, and 0.5 mg in week 8, one capsule/day (Fig. 1II).
In addition to receiving the medication, urine collection, and answering the questionnaires at each weekly meeting, both groups received a brief intervention (~20 min) delivered by the study’s multidisciplinary team—composed of a psychologist, occupational therapist, and pharmacist—to inquire how the participant felt in the last week, the difficulties and benefits observed with the possible reduction/interruption or even increasing of crack use, and to motivate them to follow the treatment. After the brief intervention, the participants received a medical appointment where they were asked about the use of the medications, possible adverse events, and any questions/complaints they raised related to the study medications.
Adherence to medication use was also measured by the weekly questionnaire between weeks 1 and 9 (pharmacological treatment), which asked about the correct administration of each of the study’s medications (Fig. 1II; A, B, C, and D) in the previous week as described in the guidance leaflet, whether and why they had forgotten to take some of the pills/oil, and possible use of medications other than those provided through the study. At each meeting, participants also brought the medication kit received in the previous week for checking/counting the pills/amount of oil not taken. In order to improve adherence to the medication and to the study, weekly text messages were sent to the participants reminding them about the next appointment and how to correctly take the medications.
Randomization and Blinding
Randomization for parallel allocation (1:1) was performed in blocks of two or four individuals using a free-access website (https://www.random.org). The information on the allocation of the participants to the treatment groups was stored in an envelope with two copies—one for the researcher in charge of the randomization and one for the project coordinator—along with a digital version with restricted access.
As the treatments had distinct presentations (oil and capsules), “double-dummy” blinding was employed. The intervention group received the CBD and placebo capsules, whereas the control group received active capsules and a placebo oil. The medications for the control group and the placebo capsules were acquired from a commercial compounding pharmacy authorized by the local governmental health agency. For valproic acid, a commercially available brand was used due to the specificities of the substance (gelatin capsule). The placebo oil was prepared at the Pharmacy School of the UnB-hospital. It was composed of a blend of coconut and corn oil (1:1) plus a strawberry aroma similar to CBD oil. All medications were filled/refilled in opaque bottles to ensure blinding.
Participants and Public Involvement
The study design and goal were built based on previous studies performed with clients who attend CAPS-AD (Carvalho et al., 2021; Fonseca & Gallassi, 2021; Gallassi et al., 2016) and by conducting a systematic review (Rodrigues et al., 2020) to incorporate input from participants at an early stage. Also, before the participants were part of this study, they received all relevant information about CBD and the other study’s medications at the T-1 stage (screening). In the end, all participants were contacted by phone call/text message (even those who interrupted the study) to schedule an in-person meeting with the research team to receive a debrief about their participation and to be referred to CAPS-AD to continue the treatment.
Statistical Analyses
The per-protocol analysis of participants who did not deviate from the study protocol was applied comparing the control and CBD treatment groups. Missing values of those participants who, at a certain time, had not yet dropped out of the study (missed 1 week, but returned the following week) were imputed according to the Last Observation Carry Forward (LOCF) technique. However, the LOCF was not applied after the participant definitively left the study (skipped and never returned), which suggests that the participant has lost the intention to treat/decrease/discontinue crack use, which means a protocol deviation.
Quantitative variables were expressed as mean and standard deviation (SD), and categorical variables were expressed as frequency and percentages. Differences between the control and CBD groups (analysis inter-group) were performed using the Mann–Whitney test comparing the periods in the study (T0 (week 1) vs. T1 (week 5); T0 vs. T2 (week 9); and T2 vs. T3 (week 10)). Differences intra-group (control group and CBD compared to themselves) were conducted using the Wilcoxon test. Pearson’s Chi-square test (by Monte Carlo simulation for small expected frequencies) or Fisher’s exact test was performed to compare the frequency of demographic data between control and CBD groups and to compare the data from participants who completed at least half of the study (week 5—T1) with those who dropped out before it. The software IBM SPSS© (Statistical Product and Service Solutions), version 22, was used to perform the analyses, and the significance level was set at 5% (p < 0.05).
The results of the urine analyses were presented descriptively as “detected” or “not detected”. In order to verify whether the answers given by the participants about crack use were equivalent to the findings in the toxicological test, a “veracity index” (VI) was created to confirm the questionnaire answers through the toxicological test result (counterevidence). For the VI calculation, the proportion of weeks in which a participant had a consistent report is calculated. Consistent reporting is when a participant with benzoylecgonine detected (≥ 100 ng/mL) reported crack use in the last week or when a participant with BZE non-detected (< 100 ng/mL) reported no crack use in the last week. Finally, the VI is obtained by averaging the consistent report proportion of the group of participants. The closer the score of the VI to 1, the more reliable the answer about crack use/non-use.
Results
Of the 90 who met the eligibility criteria, 17 were excluded for not returning, and 73 were randomly allocated to one of the two groups (n = 37 in the control group and 36 in the CBD group; Fig. 2). Thirty-four reached at least the half-way point of the study (week 5—T1; 14 in the control group and 20 in the CBD group), and 25 participants completed the study protocol through to week 10—T3 (10 in the control group and 15 in the CBD group). Recruitment and follow-up occurred over two moments, (i) from August 2019 to mid-March 2020, being interrupted until July 2021 due to the COVID-19 pandemic and the suspension of all face-to-face activities at the university, and (ii) from October 2021 to May 2022.
Safety/Tolerability, and Adherence
The medications used in both the control and CBD groups presented safety and tolerability results that were considered adequate. This was verified by the frequency of adverse events reported (“Adverse events” section) and the medication adherence rate.
Regarding medication adherence outcomes, 10%, 17.8%, 18.8%, and 19.8% reported not taking medications A, B, C, and D, respectively, at least once among those who completed at least half of the study (week 5—T1). The most cited reasons, in order of frequency, were simply that they forget, “the oil had a bad taste”, being away from home, being busy, or experiencing a change in daily routine.
Sociodemographic Profile
Among the analyzed sample—who completed at least half of the study (week 5—T1; 34 participants)—82.4% were men, the majority was Black/multiracial (pardo) (67.6%), between 30 and 49 years of age (52.9%), unemployed (41.2%), with income between 1 and 2 minimum wages (1 MW ~US$ 240.00/month; 38.2%), and had a complete high school education (55.9%; Table 1). The characteristics of participants who dropped out of the study did not differ significantly (p > 0.05) from those who remained for at least half of the study (Table S1, Supplementary Material).
Crack Use
Regarding the decrease in crack use, a comparison was performed between T0 (week 1) and T1 (week 5; half of the study), T0 and T2 (week 9), and T2 and T3 (follow-up; week 10) inter-groups (control vs. CBD), and intra-group (control group and CBD compared to themselves). In the intra-group analyses, a significant reduction in crack use was found in the CBD group between T0 and T1 (p = 0.016) and T0 and T2 (p = 0.028). In the control group, a difference was detected between T0 and T2 (p = 0.039). In the inter-group analyses, no significant differences in the reduction of crack use between the control and CBD groups were observed (Table 2).
Adverse Events
The mean of the weekly frequency of adverse events reported by participants in the weekly questionnaire was used to compare differences inter-group. There were significant differences between the CBD group in comparison to the control group, with fewer episodes in the CBD group regarding diarrhea (p = 0.019), constipation (p = 0.018), nausea (p = 0.025), dizziness (p = 0.001), memory impairment (p = 0.043), low concentration (p = 0.047), tremor (p = 0.030), ataxia (p = 0.001), and nasal congestion (p = 0.007) (Table 3).
Physical Health Symptoms
The participants were asked about aspects related to their physical health. A comparison was performed between T0 (week 1) and T1 (half of the study; week 5), T0 and T2 (week 9), and T2 and T3 (follow-up; week 10) inter-group and intra-group. In the inter-group analyses, the reduction of clinical and psychiatric complaints between T0 and T1 was significantly more pronounced in the control group than in the CBD group (p = 0.008). In the intra-group comparison, the control group presented a significant improvement between T0 and T1, with fewer clinical and psychiatric complaints (p = 0.022), and no decrease in food intake (p = 0.007). Between T0 and T2, the control group presented no food intake decrease due to crack use (p = 0.038). The CBD group demonstrated significant improvement between T0 and T1 with fewer clinical and psychiatric complaints (p = 0.023), no decrease in food intake (p = 0.023), and no food intake decrease due to crack use (p = 0.009). Between T0 and T2, the CBD group showed significant improvement in overall health ratings (p = 0.030), no decrease in food intake (p = 0.033), and no food intake decrease due to crack use (p = 0.006; Table 4).
Craving and Recall-Induced Craving
The participants were also asked about the intensity of the crack craving and the recall-induced craving in the last week with three ordinal answer possibilities (weak, intermediate, or strong). A comparison was performed between T0 (week 1) and T1 (week 5; half of the study), T0 and T2 (week 9), and T2 and T3 (follow-up; week 10) inter-group and intra-group. In the intra-group comparison, a significant improvement (increased frequency of the “weak” response) was found in the control group (p = 0.014/T0–T1; p = 0.025/T0–T2) and in the CBD group (p = 0.004/T0–T1; p = 0.010/T0–T2), as well in recall-induced craving in the control group (p = 0.021/T0–T1; p = 0.033/T0–T2) and in the CBD group (p = 0.047/T0–T1; p = 0.002/T0–T2). In the inter-group analyses, no difference was detected in the comparison between the two groups (Table 5).
Toxicological Analysis
The collection of urine and the questionnaire about crack use were administered weekly. From the VI of the control group (0.758), the VI of the CBD group (0.816), and the total VI (0.787), it is possible to affirm that the answers given by the participants of the study are reliable regarding the use/non-use of crack (Table S2, Supplementary Material).
Discussion
The CBD is a safe/tolerable product that presented significantly fewer adverse events compared to the control group, which in turn had fewer clinical and psychiatric complaints than the CBD group (from T0 to T1). The CBD group performed better on additional measures, including reduction of crack use and not reducing food intake due to crack use between (from T0 to T1), as well as for self-rated health and not reducing food intake (from T0 to T2).
Although the two significant results in the inter-group analyses (the primary outcome in RCT) were favorable for both groups, one for each, the CBD group had significant results in more parameters than the control group in the intra-group analyses (a secondary outcome), one of them being regarding the decrease in crack use. Therefore, the results of this study are encouraging from the perspective of assessing the feasibility, safety/tolerability, and preliminary efficacy of a novel pharmacological treatment for CUD. In addition to all parameters analyzed, the study also provided a relevant contribution to the existing literature regarding the reliability of the answers reported by the participants about the use or non-use of crack with the construction of the VI using the toxicology test results.
The safety and tolerability of CBD have been studied in RCTs for several medical conditions and in healthy individuals, and the results show that CBD was generally well-tolerated (Hosseini et al., 2021; Perkins et al., 2020; Taylor et al., 2018), contributing to improved adherence to treatment. Adherence to medications is particularly challenging in patients with SUD. In a systematic review of community-based interventions to improve oral chronic disease medication regimen adherence among individuals with SUD, the results demonstrated that interventions that were effective in this population were varied and included video information sessions, motivational interviewing, cognitive behavioral therapy (CBT), assertive community treatment, and bidirectional texting (Clements et al., 2018), some of which were used to guide the approach in the present RCT (e.g., motivational interviewing, CBT, and assertive community treatment).
To investigate the therapeutic effects of CBD among people with SUD, several studies have been conducted in animal and human models. Socías et al. (2017) found that the intentional use of cannabis in nature by people experiencing homelessness was associated with a reduction in crack use. For other substances than crack/cocaine, CBD was more effective than a placebo for a reduction in drug use and increasing days of abstinence among individuals with cannabis use disorder (Freeman et al., 2020), and low doses of inhaled CBD were found to reduce the number of cigarettes smoked among individuals with nicotine use disorder (Morgan et al., 2013). Luján et al. (2020) demonstrated that repeated treatment with CBD reduced the self-administration of cocaine in mice.
Commonly reported adverse events in studies conducted with CBD are considered mild, and include diarrhea, headache (Capano et al., 2020), tiredness/fatigue (Levin et al., 2011), sleepiness, nausea, dry mouth, an increase in nighttime anxiety, and disturbed sleep (Capano et al., 2020; Hurd et al., 2015). On the other hand, events such as sleepiness and dizziness are common in treatments involving valproic acid (Product information, 2014) and clonazepam (Product Information: Klonopin(r), 2010), while ataxia is commonly found in people taking fluoxetine (Product Information: Prozac(r), 2013) and valproic acid (Martin et al., 2009). Additionally, depression and feelings of anguish are commonly found in clients treated with valproic acid (Martin et al., 2009) and clonazepam (Harkins et al., 1991), and nasal congestion, cold symptoms, and recurrent airway infections are often associated with the use of clonazepam (Harkins et al., 1991). Fluoxetine has mild cardiotoxicity, which can lead to arrhythmias/palpitations (Allhoff et al., 2001).
CBD has been found to reduce cue-induced craving and anxiety in individuals in withdrawal from heroin use disorder (Hurd et al., 2018), as well as cravings among people who use nicotine and want to quit smoking (Morgan et al., 2013). Similarly, dronabinol (synthetic THC) improved withdrawal symptoms in people with cannabis use disorder (Levin et al., 2011). In contrast, in rats with the chronic use of cocaine and morphine, a reduction in the “reward-facilitating effect” was only found for morphine (Katsidoni et al., 2013). Other studies found no effect of CBD regarding the reduction of craving among individuals with cocaine/CUD compared to placebo (Meneses-Gaya et al., 2020; Mongeau-Pérusse et al., 2021).
Several studies have shown that cannabis compounds play an important role in appetite-related functions and general well-being. In a study of children with autism spectrum disorder, the CBD group showed a significant improvement in psychomotor agitation, accepted more meals per day, improved social interaction, and was less anxious when compared to children in the placebo group (da Silva Junior et al., 2022). Patients with lung cancer who received nabilone (THC) increased their caloric intake and had a significantly higher intake of carbohydrates compared to placebo. Additionally, quality of life (emotional functioning, social functioning, pain, and insomnia) significantly improved in patients taking nabilone while no changes were found in the control group (Turcott et al., 2018). Finally, in a study of children and adolescents with autism that compared a whole-plant cannabinoids group to a placebo group, the whole-plant cannabinoids group demonstrated better sleep outcomes, which subsequently resulted in improvements in quality of life and well-being (Schnapp et al., 2022).
In addition to the clinical efficacy of novel medications tested, the costs involved with RCT have been considered by researchers and funders during trial planning and funding approval processes. For example, the UK Medical Research Council and the US National Institute of Health (NIH) routinely request the inclusion of economic assessments before funding large-scale multicentric trials (Glick et al., 2014). Although this study is a feasibility RCT, in which the costs are lower than a multicenter phase III study, the stage for collecting and analyzing biological material is the most expensive due to the number and frequency of samples collected. Therefore, this study made an important contribution by finding that self-reporting on the use/non-use of crack can be reliable and might replace the need for “counterevidence” offered through toxicological tests, thereby reducing the costs involved and maintaining the same standard of reliability of the results.
Study Limitations
This study has some limitations that should be mentioned. Although it was initially designed to stratify the sample by sex, there was considerable difficulty in finding female participants, making the stratification not viable. Additionally, part of the study took place when the COVID-19 pandemic was not yet under control, the number of cases was still high, and social distancing measures were being adopted. The pandemic likely contributed to the participant dropout rate. Another factor that affected adherence was the social vulnerability of the participants. Even though the study provided money for transportation to come to the treatment, this was often not sufficient to remain participants in the study, as some lived in distant suburbs, others in shelters, and still others were experiencing homelessness. Although the team emphasized that they would be excluded from the study if absent, many did not have enough motivation to go every week probably due to these unfavorable social contexts. Social vulnerability may have had the greatest impact on participants’ adherence. Finally, as this was a real-life study, we did not have full control over how these participants took their medication, so they could take, for example, all the capsules in one day if they wanted to, although none of them pointed out the possibility of this happening.
Future Directions
Given our experiences in this feasibility, safety/tolerability, and preliminary efficacy double-blind RCT, we will implement the following adjustments in the next stage (with the enlargement of the sample size): (1) participants will be screened and monitored more rigorously to avoid early dropouts; (2) to improve the demand for volunteers and recruitment, the dissemination of the study information will be widespread; (3) employ targeted recruitment strategies to address the difficulty in finding female participants; and (4) provide a better contribution/reward for participation in the study (e.g., food, clothing, hygiene kit), respecting the ethical assumptions established by Brazilian law, to try to improve the adherence of those who are in extremely vulnerable conditions.
Conclusion
CBD has a broad spectrum of pharmacological properties that affect multiple targets, making it difficult to outline its mechanism of action for each health condition. In contrast, the multiple-target action seems important for performing a wide range of therapeutic properties. CBD decreases endocannabinoid receptor signaling and inhibits fatty acid amide hydrolase, which may reduce craving and decrease relapse rates in people with CUD.
CBD is a safe/tolerable product that presented significantly fewer adverse events compared to the control group, which performed better in reducing clinical and psychiatric complaints. In the intra-group analyses, the CBD group performed better in more parameters than the control group, reducing crack use, not reducing food intake due to crack use, and greater improvements in self-rated health. Also, the study demonstrated how self-reports for drug use can be reliable and implemented in real situations to collect valid evidence outside classic laboratory and hospital settings.
In this sense, the main implications of this study point to CBD as a powerful and promising therapeutic tool for people with CUD. CBD seems to mitigate the primary symptoms reported by the participants, such as lack of appetite, difficulty in reducing crack use, and the feeling of poor health. In addition, CBD stands out primarily for presenting mild adverse events, the main complaints associated with the use of usual psychotropic drugs. Clinical practice shows that the adverse events of traditional psychotropic drugs may be even more pronounced in people with CUD due to the excessive dosage and combination of these medications. This is because they are seen as clients having problems with a drug that is “too heavy,” and, therefore, they need to be “overmedicated.” In other words, adverse events end up contributing to low adherence to treatment of people with CUD in health services, which would favor broad access to CBD, including as adjuvant therapy.
Future studies should focus on increasing the sample size and retention strategies (e.g., more rigorous screening and monitoring of the participants, dissemination of study information, specific strategies to recruit female participants, and better contribution/reward for participation in the study) to determine whether the indications of benefits are maintained or even improved. In addition, by reducing stress and environmental factors while improving emotional regulation, implementing strategies to support these individuals, and using other cannabinoid compositions (e.g., full-spectrum CBD and/or THC), these effects may be enhanced. If so, this would be an important advance in the pharmacological treatment of stimulant use disorders.
Data Availability
The dataset is available at http://alcooledrogas.unb.br/nossas-publicacoes (Planilha Estudo Crack).
References
Allhoff, T., Bender, S., Banger, M., Sack, S., Erbel, R., Rehlinghaus, U., et al. (2001). Atrial arrhythmia in a woman treated with fluoxetine: Is there a causal relationship (letter). Annals of Emergency Medicine, 37(1), 116–117. https://doi.org/10.1067/mem.2001.111869
Amundsen, E. J., & Reid, M. J. (2014). Self-reports of consumption of amphetamines, cocaine and heroin in a survey among marginalized drug users. Science of the Total Environment, 487, 740–745. https://doi.org/10.1016/j.scitotenv.2013.12.098
An, D., Peigneur, S., Hendrickx, L. A., & Tytgat, J. (2020). Targeting cannabinoid receptors: Current status and prospects of natural products. International Journal of Molecular Sciences, 21(14), 5064. https://doi.org/10.3390/ijms21145064
Bastos, F. I. P. M., & Bertoni, N. (2014). Pesquisa Nacional sobre o uso de crack: Quem são os usuários de crack e/ou similares no Brasil? Quantos são nas capitais brasileiras?, ed. ICICT/FIOCRUZ Available online https://www.arca.fiocruz.br/handle/icict/10019
Capano, A., Weaver, R., & Burkman, E. (2020). Evaluation of the effects of CBD hemp extract on opioid use and quality of life indicators in chronic pain patients: A prospective cohort study. Postgraduate Medicine, 132(1), 56–61. https://doi.org/10.1080/00325481.2019.1685298
Carvalho, L. F., Pandossio, J. E., Rodrigues, L. A., & Gallassi, A. D. (2021). Análise crítica sobre medicamentos prescritos para o uso problemático de crack. Psicologia: Teoria e pesquisa, 37, e3725115. https://doi.org/10.1590/0102.3772e372515
Carvalho, S. R., Miranda, F. A. N., Belmiro, S. S. D. R., Moura, I. B. L., & Santos, R. C. A. (2016). Tratamento medicamentoso do craving em usuários de cocaína/crack: Revisão integrativa. Rev Enferm UFPE, 10(1), 163–171. https://doi.org/10.5205/reuol.8557-74661-1-SM1002201621
Chan, B., Kondo, K., Freeman, M., Ayers, C., Montgomery, J., & Kansagara, D. (2019). Pharmacotherapy for cocaine use disorder-a systematic review and meta-analysis. Journal of General Internal Medicine, 34(12), 2858–2873. https://doi.org/10.1007/s11606-019-05074-8
Clements, K. M., Hydery, T., Tesell, M. A., Greenwood, B. C., & Angelini, M. C. (2018). A systematic review of community-based interventions to improve oral chronic disease medication regimen adherence among individuals with substance use disorder. Drug and Alcohol Dependence, 188, 141–152.
Cristino, L., Bisogno, T., & Di Marzo, V. (2020). Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nature Reviews Neurology, 16(1), 9–29. https://doi.org/10.1038/s41582-019-0284-z
da Silva Junior, E. A., Medeiros, W. M. B., Dos Santos, J. P. M., de Sousa, J. M. M., da Costa, F. B., Pontes, K. M., et al. (2022). Evaluation of the efficacy and safety of cannabidiol-rich cannabis extract in children with autism spectrum disorder: Randomized, double-blind and controlled placebo clinical trial. Trends in Psychiatry and Psychotherapy. https://doi.org/10.47626/2237-6089-2021-0396
Englund, A., Morrison, P. D., Nottage, J., Hague, D., Kane, F., Bonaccorso, S., et al. (2013). Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. Journal of Psychopharmacology, 27(1), 19–27. https://doi.org/10.1177/0269881112460109
Fischer, B., Blanken, P., Da Silveira, D., Gallassi, A., Goldner, E. M., Rehm, J., et al. (2015). Effectiveness of secondary prevention and treatment interventions for crack-cocaine abuse: A comprehensive narrative overview of English-language studies. International Journal of Drug Policy, 26(4), 352–363. https://doi.org/10.1016/j.drugpo.2015.01.002
Fischer, B., Kuganesan, S., Gallassi, A., Malcher-Lopes, R., van den Brink, W., & Wood, E. (2015). Addressing the stimulant treatment gap: A call to investigate the therapeutic benefits potential of cannabinoids for crack-cocaine use. The International Journal on Drug Policy, 26(12), 1177–1182. https://doi.org/10.1016/j.drugpo.2015.09.005
Fonseca, R. M. A. M., & Gallassi, A. D. (2021). Práticas de cuidado extramuros nos Centros de Atenção Psicossocial Álcool e outras Drogas: A ocupação cidadã. Interface-Comunicação, Saúde, Educação, 25, e200369. https://doi.org/10.1590/interface.200369
Freeman, T. P., Hindocha, C., Baio, G., Shaban, N. D. C., Thomas, E. M., Astbury, D., et al. (2020). Cannabidiol for the treatment of cannabis use disorder: A phase 2a, double-blind, placebo-controlled, randomised, adaptive Bayesian trial. The Lancet Psychiatry, 7(10), 865–874. https://doi.org/10.1016/S2215-0366(20)30290-X
Galaj, E., Bi, G. H., Yang, H. J., & Xi, Z. X. (2020). Cannabidiol attenuates the rewarding effects of cocaine in rats by CB2, 5-HT1A and TRPV1 receptor mechanisms. Neuropharmacology, 167, 107740. https://doi.org/10.1016/j.neuropharm.2019.107740
Gallassi, A. D., Nakano, E. Y., Wagner, G. A., & Fischer, B. (2016). Characteristics of clients using a community-based drug treatment service (‘CAPS-AD’) in Brazil: An exploratory study. International Journal of Drug Policy, 31, 99–103. https://doi.org/10.1016/j.drugpo.2016.01.020
Glick, H. A., Doshi, J. A., Sonnad, S. S., & Polsky, D. (2014). Economic evaluation in clinical trials. OUP Oxford.
Gonçalves, J. R., & Nappo, S. A. (2015). Factors that lead to the use of crack cocaine in combination with marijuana in Brazil: A qualitative study. BMC Public Health, 15(1), 1–8. https://doi.org/10.1186/s12889-015-2063-0
Harkins, S., Linford, J., Cohen, J., Kramer, T., & Cueva, L. (1991). Administration of clonazepam in the treatment of TMD and associated myofascial pain: A double-blind pilot study. Journal of Craniomandibular Disorders, 5(3), 179–186 https://pubmed.ncbi.nlm.nih.gov/1812146/
Henrique, I. F. S., De Micheli, D., Lacerda, R. B. D., Lacerda, L. A. D., & Formigoni, M. L. O. D. S. (2004). Validação da versão brasileira do teste de triagem do envolvimento com álcool, cigarro e outras substâncias (ASSIST). Revista da Associacao Medica Brasileira, 50(2), 199–206. https://doi.org/10.1590/S0104-42302004000200039
Hosseini, A., McLachlan, A. J., & Lickliter, J. D. (2021). A phase I trial of the safety, tolerability and pharmacokinetics of cannabidiol administered as single-dose oil solution and single and multiple doses of a sublingual wafer in healthy volunteers. British Journal of Clinical Pharmacology, 87(4), 2070–2077. https://doi.org/10.1111/bcp.14617
Hurd, Y. L., Spriggs, S., Alishayev, J., Winkel, G., Gurgov, K., Kudrich, C., et al. (2018). Cannabidiol for the reduction of cue-induced craving and anxiety in drug-abstinent individuals with heroin use disorder: A double-blind randomized placebo-controlled trial. The American Journal of Psychiatry, 176(11), 911–922. https://doi.org/10.1176/appi.ajp.2019.18101191
Hurd, Y. L., Yoon, M., Manini, A. F., Hernandez, S., Olmedo, R., Ostman, M., et al. (2015). Early phase in the development of cannabidiol as a treatment for addiction: Opioid relapse takes initial center stage. Neurotherapeutics, 12(4), 807–815. https://doi.org/10.1007/s13311-015-0373-7
Katsidoni, V., Anagnostou, I., & Panagis, G. (2013). Cannabidiol inhibits the reward-facilitating effect of morphine: Involvement of 5-HT1A receptors in the dorsal raphe nucleus. Addiction Biology, 18(2), 286–296. https://doi.org/10.1111/j.1369-1600.2012.00483.x
Kessler, R. C., & Üstün, T. B. (2004). The World Mental Health (WMH) survey initiative version of the World Health Organization (WHO) composite international diagnostic interview (CIDI). International Journal of Methods in Psychiatric Research, 13(2), 93–121. https://doi.org/10.1002/mpr.168
Lazarini-Lopes, W., Do Val-da Silva, R. A., da Silva-Júnior, R. M., Leite, J. P., & Garcia-Cairasco, N. (2020). The anticonvulsant effects of cannabidiol in experimental models of epileptic seizures: From behavior and mechanisms to clinical insights. Neuroscience & Biobehavioral Reviews, 111, 166–182. https://doi.org/10.1016/j.neubiorev.2020.01.014
Levin, F. R., Mariani, J. J., Brooks, D. J., Pavlicova, M., Cheng, W., & Nunes, E. V. (2011). Dronabinol for the treatment of cannabis dependence: A randomized, double-blind, placebo-controlled trial. Drug and Alcohol Dependence, 116(1-3), 142–150. https://doi.org/10.1016/j.drugalcdep.2010.12.010
Luján, M. Á., Cantacorps, L., & Valverde, O. (2020). The pharmacological reduction of hippocampal neurogenesis attenuates the protective effects of cannabidiol on cocaine voluntary intake. Addiction Biology, 25(4), e12778. https://doi.org/10.1111/adb.12778
Martin, C. K., Han, H., Anton, S. D., Greenway, F. L., & Smith, S. R. (2009). Effect of valproic acid on body weight, food intake, physical activity and hormones: Results of a randomized controlled trial. Journal of Psychopharmacology, 23(7), 814–825. https://doi.org/10.1177/0269881108091595
Mechoulam, R., & Parker, L. A. (2013). The endocannabinoid system and the brain. Annual Review of Psychology, 64, 21–47. https://doi.org/10.1146/annurev-psych-113011-143739
Meneses-Gaya, C., Crippa, J. A., Hallak, J. E., Miguel, A. Q., Laranjeira, R., Bressan, R. A., et al. (2020). Cannabidiol for the treatment of crack-cocaine craving: An exploratory double-blind study. Brazilian Journal of Psychiatry, 30, S1516–S44462020005037203. https://doi.org/10.1590/1516-4446-2020-1416
Mongeau-Pérusse, V., Brissette, S., Bruneau, J., Conrod, P., Dubreucq, S., Gazil, G., et al. (2021). Cannabidiol as a treatment for craving and relapse in individuals with cocaine use disorder: A randomized placebo-controlled trial. Addiction, 116(9), 2431–2442. https://doi.org/10.1111/add.15417
Morgan, C. J. A., Das, R. K., Joye, A., Curran, H. V., & Kamboj, S. K. (2013). Cannabidiol reduces cigarette consumption in tobacco smokers: Preliminary findings. Addictive Behaviors, 38(9), 2433–2436. https://doi.org/10.1016/j.addbeh.2013.03.011
Nickley, J., Pesce, A. J., & Krock, K. (2017). A sensitive assay for urinary cocaine metabolite benzoylecgonine shows more positive results and longer half-lives than those using traditional cut-offs. Drug Testing and Analysis, 9(8), 1214–1216. https://doi.org/10.1002/dta.2153
Pereira, A. S., & Wurfel, R. F. (2011). O uso de maconha como estratégia de redução de danos em dependentes de crack. Aletheia, 34, 163–174 http://pepsic.bvsalud.org/scielo.php?script=sci_arttext&pid=S1413-03942011000100013&lng=pt&nrm=iso
Perkins, D., Butler, J., Ong, K., Nguyen, T. H., Cox, S., Francis, B., et al. (2020). A phase 1, randomised, placebo-controlled, dose escalation study to investigate the safety, tolerability and pharmacokinetics of cannabidiol in fed healthy volunteers. European Journal of Drug Metabolism and Pharmacokinetics, 45, 575–586. https://doi.org/10.1007/s13318-020-00624-6
Pertwee, R. G. (2008). The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. British Journal of Pharmacology, 153(2), 199–215. https://doi.org/10.1038/sj.bjp.0707442
Product Information. (2010). Klonopin(r) tablets, Klonopin(r) wafers oral tablets, orally disintegrating tablets, clonazepam oral tablets, orally disintegrating tablets. CA, Genentech USA, Inc..
Product information: Prozac(r) oral pulvules, oral delayed-release capsules, fluoxetine HCl oral pulvules, oral delayed-release capsules. Indianapolis, IN, Lilly USA, LLC (per FDA), 2013.
Product information. (2014). Depakene oral capsules, oral solution, valproic acid oral capsules, oral solution. AbbVie Inc..
Renard, J., Loureiro, M., Rosen, L. G., Zunder, J., De Oliveira, C., Schmid, S., et al. (2016). Cannabidiol counteracts amphetamine-induced neuronal and behavioral sensitization of the mesolimbic dopamine pathway through a novel mTOR/p70S6 kinase signaling pathway. Journal of Neuroscience, 36(18), 5160–5169. https://doi.org/10.1523/JNEUROSCI.3387-15.2016
Roberts, C. A., Jager, G., Christiansen, P., & Kirkham, T. C. (2019). Exploring the munchies: An online survey of users’ experiences of cannabis effects on appetite and the development of a cannabinoid eating experience questionnaire. Journal of Psychopharmacology, 33(9), 1149–1159. https://doi.org/10.1177/0269881119862526
Rodrigues, L. A., Caroba, M. E. S., Taba, F. K., Filev, R., & Gallassi, A. D. (2020). Evaluation of the potential use of cannabidiol in the treatment of cocaine use disorder: A systematic review. Pharmacology, Biochemistry, and Behavior, 196, 172982. https://doi.org/10.1016/j.pbb.2020.172982
Ronsley, C., Nolan, S., Knight, R., Hayashi, K., Klimas, J., Walley, A., et al. (2020). Treatment of stimulant use disorder: A systematic review of reviews. PLoS One, 15(6), e0234809. https://doi.org/10.1371/journal.pone.0234809
Schnapp, A., Harel, M., Cayam-Rand, D., Cassuto, H., Polyansky, L., & Aran, A. (2022). A placebo-controlled trial of cannabinoid treatment for disruptive behavior in children and adolescents with autism spectrum disorder: Effects on sleep parameters as measured by the CSHQ. Biomedicines, 10(7), 1685. https://doi.org/10.3390/biomedicines10071685
Serafini, G., Pompili, M., Innamorati, M., Dwivedi, Y., Brahmachari, G., & Girardi, P. (2013). Pharmacological properties of glutamatergic drugs targeting NMDA receptors and their application in major depression. Current Pharmaceutical Design, 19(10), 1898–1922. https://doi.org/10.2174/13816128113199990293
Shannon, S., Lewis, N., Lee, H., & Hughes, S. (2019). Cannabidiol in anxiety and sleep: A large case series. The Permanente Journal, 23. https://doi.org/10.7812/TPP/18-041
Skelley, J. W., Deas, C. M., Curren, Z., & Ennis, J. (2020). Use of cannabidiol in anxiety and anxiety-related disorders. Journal of the American Pharmacists Association, 60(1), 253–261. https://doi.org/10.1016/j.japh.2019.11.008
Socías, M. E., Kerr, T., Wood, E., Dong, H., Lake, S., Hayashi, K., et al. (2017). Intentional cannabis use to reduce crack cocaine use in a Canadian setting: A longitudinal analysis. Addictive Behaviors, 72, 138–143. https://doi.org/10.1016/j.addbeh.2017.04.006
Spanagel, R. (2020). Cannabinoids and the endocannabinoid system in reward processing and addiction: From mechanisms to interventions. Dialogues in Clinical Neuroscience, 22(3), 241–250. https://doi.org/10.31887/DCNS.2020.22.3/rspanagel
Taylor, L., Gidal, B., Blakey, G., Tayo, B., & Morrison, G. (2018). A phase I, randomized, double-blind, placebo-controlled, single ascending dose, multiple dose, and food effect trial of the safety, tolerability and pharmacokinetics of highly purified cannabidiol in healthy subjects. CNS Drugs, 32, 1053–1067. https://doi.org/10.1007/s40263-018-0578-5
Turcott, J. G., del Rocío Guillen Núñez, M., Flores-Estrada, D., Oñate-Ocaña, L. F., Zatarain-Barrón, Z. L., Barrón, F., & Arrieta, O. (2018). The effect of nabilone on appetite, nutritional status, and quality of life in lung cancer patients: A randomized, double-blind clinical trial. Supportive Care in Cancer, 26, 3029–3038. https://doi.org/10.1007/s00520-018-4154-9
United Nations Office on Drugs and Crime (UNDOC) (2020). World drug report 2020 (United Nations publication, Sales no. E.20.XI.6). Available online https://wdr.unodc.org/uploads/wdr2020/documents/WDR20_Booklet_2.pdf.
Walsh, Z., Mollaahmetoglu, O. M., Rootman, J., Golsof, S., Keeler, J., Marsh, B., Nutt, D. J., & Morgan, C. J. A. (2021). Ketamine for the treatment of mental health and substance use disorders: Comprehensive systematic review. BJPsych Open, 8(1), e19. https://doi.org/10.1192/bjo.2021.1061
Weiss, R. D., Griffin, M. L., Hufford, C., Muenz, L. R., Najavits, L. M., Jansson, S. B., et al. (1997). Early prediction of initiation of abstinence from cocaine: Use of a craving questionnaire. The American Journal on Addictions, 6(3), 224–231 https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1521-0391.1997.tb00401.x?sid=nlm%3Apubmed
West, R., Pesce, A. J., Crews, B., Mikel, C., Rosenthal, M., Almazan, P., et al. (2011). Determination of illicit drug cutoff values in a pain patient population. Clinica Chimica Acta, 412(17-18), 1589–1593. https://doi.org/10.1016/j.cca.2011.05.004
Zanos, P., & Gould, T. (2018). Mechanisms of ketamine action as an antidepressant. Molecular Psychiatry, 23, 801–811. https://doi.org/10.1038/mp.2017.255
Acknowledgements
Special thanks to the participants and their families/advisors, the team of the CAPS-AD Ceilândia, with great consideration to the physician Dr. Daniel Dornelas Batista do Couto, who facilitated the recruitment and medication plan for the control group. The authors also thank the director of the Faculty of Ceilândia, especially Jean Carlos Soares, for his financial, executive, and logistical coordination in this project. We would also like to thank Prof. Noemia Tavares and Lorena Malaquias from the Pharmacy School of the UnB for all their support in the organization and preparation of the medications, Prof. Josenaide Engracia dos Santos for the training of this trial’s research team in brief intervention, and Jéssica Lohana Aquino for the contributions related to the pharmacological component of the study.
Funding
This work was supported by the Federal District Research Support Foundation (Fundação de Apoio à Pesquisa do Distrito Federal – FAPDF) grant number 0193.001493/2016. Also, the Brazilian fostering agency Coordination for the Advancement of Higher Education Personnel (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – CAPES) supported with one master’s degree scholarship and the UnB with five scholarships by the Scientific Initiation fellow (PIBIC). This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Gallassi AD: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, software, validation, visualization, and writing—original draft preparation, review and editing; De Oliveira AWC and Rodrigues LA: data curation, formal analysis, investigation, methodology, visualization, and writing—original draft preparation, review, and editing; Nakano EY: formal analysis, methodology, software, validation, visualization, and writing—original draft preparation, review and editing; Ruas PAS and La Mata AI: data curation, investigation, validation, visualization, and writing—original draft preparation, review, and editing; Júnior EF and Gomes JA: formal analysis (urine sample), methodology, software, validation, visualization, and writing—original draft preparation, review, and editing; Caroba MES, Silva MGS, Vieira MGQ, Reis JGGR, Leite JLM, Lima GHA, Lima JM, Lima YPV, Ribas JAA, Chagas NAL, Magalhães MA, and Silva MF: data curation, investigation, validation, visualization, and writing—original draft preparation, review, and editing; Filev R: methodology, validation, visualization, and writing—original draft preparation, review, and editing; MalcherLopes RM: conceptualization, validation, visualization, and writing—original draft preparation, review, and editing.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Faculty of Ceilândia Research Ethics Committee of the UnB approved this study (certificate number CAAE: 82559418.5.0000.8093/2018; available to consult at https://plataformabrasil.saude.gov.br/login.jsf). The National Health Surveillance Agency authorized the importation and storage of the substances—Anvisa do Brasil (AEP/n°005/2019).
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all participants to be included in the study. The Clinical Trial Protocol was built according to the recommendations of the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT), and the preparation of the manuscript followed the guidelines of the Consolidated Standards of Reporting Trials (CONSORT), which establishes evidence-based recommendations for reporting clinical trials (CONSORT checklist available after the “References” section).
Consent for Publication
All authors have participated in the study and approved this submission.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 22.1 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gallassi, A.D., de Oliveira, A.W.C., Rodrigues, L.A. et al. Cannabidiol Compared to Pharmacological Treatment as Usual for Crack Use Disorder: A Feasibility, Preliminary Efficacy, Parallel, Double-Blind, Randomized Clinical Trial. Int J Ment Health Addiction (2024). https://doi.org/10.1007/s11469-024-01287-z
Accepted:
Published:
DOI: https://doi.org/10.1007/s11469-024-01287-z