Abstract
Background
Fibrates are lipid-lowering agents that act as peroxisome proliferator-activated receptor (PPAR)-α agonists. They have been associated with cancers in experimental models, but data in humans are rare, and among published studies none has investigated cancers in tissues with high PPAR-α concentrations.
Methods
A nested case–control study was performed in a French population-based healthcare database. Adults aged ≥45 years, and free of cancer for 3 years, were followed for 5 years for incident cases of melanoma, non-melanoma skin cancers, thyroid, pancreas, bladder, and kidney cancers. Cases were matched with up to ten controls for age, sex, and diseases that could increase the risk of cancers. Conditional logistic models, adjusted for drug-use as potential confounders, were used to estimate the risk (odds ratio [OR]) of cancers of interest (and individual cancers) associated with cumulative exposure to fibrates (defined daily doses [DDD]).
Results
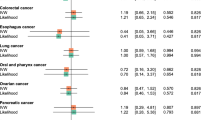
Among the 147,338 eligible subjects, 3,331 (2.3 %) cases of studied cancers were identified. Only use of fibrates >550 DDDs was associated with an increased risk (OR 1.26; 95 % CI 1.12–1.42), and similar results were found for statins (≥1,460 DDDs; OR 1.15; 95 % CI 1.03–1.28). When considering cancers individually, the association was significant for non-melanoma skin-cancer (OR 1.35; 95 % CI 1.14–1.60), and close to significance for bladder cancer (OR 1.26; 95 % CI 0.96–1.64); similar associations with the use of statins were found.
Conclusions
The associations found between fibrate exposure and cancers of tissues with high PPAR-α concentrations were most likely related to residual confounding as similar associations were found for statins.

Similar content being viewed by others
References
Mamtani R, Haynes K, Bilker WB, Vaughn DJ, Strom BL, Glanz K, et al. Association between longer therapy with thiazolidinediones and risk of bladder cancer: a cohort study. J Natl Cancer Inst. 2012;104(18):1411–21. doi:10.1093/jnci/djs328.
Azoulay L, Yin H, Filion KB, Assayag J, Majdan A, Pollak MN, et al. The use of pioglitazone and the risk of bladder cancer in people with type 2 diabetes: nested case–control study. BMJ. 2012;344:e3645. doi:10.1136/bmj.e3645.
Lewis JD, Ferrara A, Peng T, Hedderson M, Bilker WB, Quesenberry CP Jr, et al. Risk of bladder cancer among diabetic patients treated with pioglitazone: interim report of a longitudinal cohort study. Diabetes Care. 2011;34(4):916–22. doi:10.2337/dc10-1068.
Piccinni C, Motola D, Marchesini G, Poluzzi E. Assessing the association of pioglitazone use and bladder cancer through drug adverse event reporting. Diabetes Care. 2011;34(6):1369–71. doi:10.2337/dc10-2412.
Bosetti C, Rosato V, Buniato D, Zambon A, La Vecchia C, Corrao G. Cancer risk for patients using thiazolidinediones for type 2 diabetes: a meta-analysis. Oncologist. 2013;18(2):148–56. doi:10.1634/theoncologist.2012-0302.
Tseng CH. Rosiglitazone is not associated with an increased risk of bladder cancer. Cancer Epidemiol. 2013;37(4):385–9. doi:10.1016/j.canep.2013.03.013.
Hillaire-Buys D, Faillie JL. Pioglitazone and the risk of bladder cancer. BMJ. 2012;344:e3500. doi:10.1136/bmj.e3500.
Peters JM, Cheung C, Gonzalez FJ. Peroxisome proliferator-activated receptor-alpha and liver cancer: where do we stand? J Mol Med (Berl). 2005;83(10):774–85. doi:10.1007/s00109-005-0678-9.
Gelman L, Fruchart JC, Auwerx J. An update on the mechanisms of action of the peroxisome proliferator-activated receptors (PPARs) and their roles in inflammation and cancer. Cell Mol Life Sci. 1999;55(6–7):932–43.
Atarod EB, Kehrer JP. Dissociation of oxidant production by peroxisome proliferator-activated receptor ligands from cell death in human cell lines. Free Radic Biol Med. 2004;37(1):36–47. doi:10.1016/j.freeradbiomed.2004.04.015.
Tenenbaum A, Fisman EZ. Fibrates are an essential part of modern anti-dyslipidemic arsenal: spotlight on atherogenic dyslipidemia and residual risk reduction. Cardiovasc Diabetol. 2012;11:125. doi:10.1186/1475-2840-11-125.
Nesfield SR, Clarke CJ, Hoivik DJ, Miller RT, Allen JS, Selinger K, et al. Evaluation of the carcinogenic potential of clofibrate in the rasH2 mouse. Int J Toxicol. 2005;24(5):301–11. doi:10.1080/10915810500210278.
Nishimura J, Dewa Y, Okamura T, Muguruma M, Jin M, Saegusa Y, et al. Possible involvement of oxidative stress in fenofibrate-induced hepatocarcinogenesis in rats. Arch Toxicol. 2008;82(9):641–54. doi:10.1007/s00204-007-0278-2.
Cattley RC. Carcinogenicity of lipid-lowering drugs. JAMA. 1996;275(19):1479 author reply 81-82.
Newman TB, Hulley SB. Carcinogenicity of lipid-lowering drugs. JAMA. 1996;275(1):55–60.
Bonovas S, Nikolopoulos GK, Bagos PG. Use of fibrates and cancer risk: a systematic review and meta-analysis of 17 long-term randomized placebo-controlled trials. PLoS One. 2012;7(9):e45259. doi:10.1371/journal.pone.0045259.
Gardette V, Bongard V, Dallongeville J, Arveiler D, Bingham A, Ruidavets JB, et al. Ten-year all-cause mortality in presumably healthy subjects on lipid-lowering drugs (from the Prospective Epidemiological Study of Myocardial Infarction [PRIME] prospective cohort). Am J Cardiol. 2009;103(3):381–6. doi:10.1016/j.amjcard.2008.09.092.
Berard E, Bongard V, Dallongeville J, Arveiler D, Ruidavets JB, Ferrieres J. Cancer mortality according to lipid-lowering drugs and lipoproteins in a general population. Curr Med Res Opin. 2011;27(10):1963–71. doi:10.1185/03007995.2011.616191.
Oleksiewicz MB, Southgate J, Iversen L, Egerod FL. Rat urinary bladder carcinogenesis by dual-acting PPARalpha + gamma agonists. PPAR Res. 2008;2008:103167. doi:10.1155/2008/103167.
Egerod FL, Svendsen JE, Hinley J, Southgate J, Bartels A, Brunner N, et al. PPAR alpha and PPAR gamma coactivation rapidly induces Egr-1 in the nuclei of the dorsal and ventral urinary bladder and kidney pelvis urothelium of rats. Toxicol Pathol. 2009;37(7):947–58. doi:10.1177/0192623309351723.
Michalik L, Desvergne B, Wahli W. Peroxisome-proliferator-activated receptors and cancers: complex stories. Nat Rev Cancer. 2004;4(1):61–70. doi:10.1038/nrc1254.
Tachibana K, Yamasaki D, Ishimoto K, Doi T. The role of PPARs in cancer. PPAR Res. 2008;2008:102737. doi:10.1155/2008/102737.
WHO. WHO Collaborating Centre For Drug Statistics Methodology. Norwegian Institute of Public Health, Oslo. 2014. http://www.whocc.no/. Accessed 10 Jan 2014.
Kimura O, Kondo Y, Shimosegawa T. PPAR could contribute to the pathogenesis of hepatocellular carcinoma. PPAR Res. 2012;2012:574180. doi:10.1155/2012/574180.
Torra IP, Chinetti G, Duval C, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors: from transcriptional control to clinical practice. Curr Opin Lipidol. 2001;12(3):245–54.
Freeman SR, Drake AL, Heilig LF, Graber M, McNealy K, Schilling LM, et al. Statins, fibrates, and melanoma risk: a systematic review and meta-analysis. J Natl Cancer Inst. 2006;98(21):1538–46. doi:10.1093/jnci/djj412.
Neumann A, Weill A, Ricordeau P, Fagot JP, Alla F, Allemand H. Pioglitazone and risk of bladder cancer among diabetic patients in France: a population-based cohort study. Diabetologia. 2012;55(7):1953–62. doi:10.1007/s00125-012-2538-9.
Jacobs EJ, Newton CC, Gapstur SM, Thun MJ. Daily aspirin use and cancer mortality in a large US cohort. J Natl Cancer Inst. 2012;104(16):1208–17. doi:10.1093/jnci/djs318.
Rothwell PM, Price JF, Fowkes FG, Zanchetti A, Roncaglioni MC, Tognoni G, et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379(9826):1602–12. doi:10.1016/S0140-6736(11)61720-0.
Taylor ML, Wells BJ, Smolak MJ. Statins and cancer: a meta-analysis of case–control studies. Eur J Cancer Prev. 2008;17(3):259–68. doi:10.1097/CEJ.0b013e3282b721fe.
Bonovas S, Filioussi K, Tsavaris N, Sitaras NM. Statins and cancer risk: a literature-based meta-analysis and meta-regression analysis of 35 randomized controlled trials. J Clin Oncol. 2006;24(30):4808–17. doi:10.1200/jco.2006.06.3560.
Bonovas S, Nikolopoulos G, Filioussi K, Peponi E, Bagos P, Sitaras NM. Can statin therapy reduce the risk of melanoma? A meta-analysis of randomized controlled trials. Eur J Epidemiol. 2010;25(1):29–35. doi:10.1007/s10654-009-9396-x.
Browning DR, Martin RM. Statins and risk of cancer: a systematic review and metaanalysis. Int J Cancer. 2007;120(4):833–43. doi:10.1002/ijc.22366.
Cui X, Xie Y, Chen M, Li J, Liao X, Shen J, et al. Statin use and risk of pancreatic cancer: a meta-analysis. Cancer Causes Control. 2012;23(7):1099–111. doi:10.1007/s10552-012-9979-9.
Dale KM, Coleman CI, Henyan NN, Kluger J, White CM. Statins and cancer risk: a meta-analysis. JAMA. 2006;295(1):74–80. doi:10.1001/jama.295.1.74.
Farwell WR, D’Avolio LW, Scranton RE, Lawler EV, Gaziano JM. Statins and prostate cancer diagnosis and grade in a veterans population. J Natl Cancer Inst. 2011;103(11):885–92. doi:10.1093/jnci/djr108.
Farwell WR, Scranton RE, Lawler EV, Lew RA, Brophy MT, Fiore LD, et al. The association between statins and cancer incidence in a veterans population. J Natl Cancer Inst. 2008;100(2):134–9. doi:10.1093/jnci/djm286.
Kuoppala J, Lamminpaa A, Pukkala E. Statins and cancer: a systematic review and meta-analysis. Eur J Cancer. 2008;44(15):2122–32. doi:10.1016/j.ejca.2008.06.025.
Zhang XL, Geng J, Zhang XP, Peng B, Che JP, Yan Y, et al. Statin use and risk of bladder cancer: a meta-analysis. Cancer Causes Control. 2013;24(4):769–76. doi:10.1007/s10552-013-0159-3.
Assurance Maladie. MEDIC’AM 2008–2012. Assurance Maladie, Paris. 2013. http://www.ameli.fr/fileadmin/user_upload/documents/MEDICAM_2008-2012-AMELI_tous_prescripteurs.zip. Accessed 1 Jan 2013.
Acknowledgments
Francesco Salvo and Antoine Pariente had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. They agree to allow the journal to review their data if requested.
This study was supported by the French IReSP (Institut de la Recherche en Santé Publique) through a grant obtained after the 2011 Call for Research, which was part of the Plan Cancer 2009–2013. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Conflicts of interest declaration
Fabienne Bazin, Aude Kostrzewa, Christian Bandre, and Philip Robinson, have had no relationships with companies that might have an interest in the submitted work in the previous 3 years. Francesco Salvo is a consultant of YOLARX Consultants Inc. (Montreal, Canada), and has worked on studies concerning one antifungal, one anticancer drug, and paracetamol. Bernard Bégaud has received investigator-initiated research funding from the French Health Ministry (2011); he is chair of the scientific committee for two pharmacoepidemiological studies conducted by the contract research organisation LA-SER (London, UK)—one on medicines used in osteoarthritis, the other on the use of homeopathic remedies by French practitioners. Nicholas Moore, in the previous 3 years, has had specified relationships on other matters with the following drug companies that might have an interest in the submitted work: Pfizer, Servier, Pierre Fabre, Roche, Merck Serono, Novartis, AstraZeneca, Abbott, Axcan, Bristol-Myers Squibb, Celgene, Cephalon, Vivatec, Lundbeck, GlaxoSmithKline, Leo Pharma, Helsinn Healthcare, Orion, Genevrier, Takeda, Sanofi, and Johnson & Johnson; he has also had, in the previous 3 years, specified relationships on other matters with public regulatory agencies and with healthcare insurance systems that might have an interest in the submitted work. Antoine Pariente, in the previous 3 years, has had specified relationships on other matters with Novartis that might have an interest in the submitted work. The authors’ spouses, partners, or children have no financial relationships that may be relevant to the submitted work. All authors have no non-financial interests that may be relevant to the submitted work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Salvo, F., Bazin, F., Kostrzewa, A. et al. Fibrates and Risk of Cancer in Tissues with High PPAR-α Concentration: A Nested Case–Control Study. Drug Saf 37, 361–368 (2014). https://doi.org/10.1007/s40264-014-0157-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40264-014-0157-8