Abstract
Background
Traditionally, renal function in critically ill patients has been assessed to identify renal dysfunction, and dose adjustment is generally accepted in such a context. Nevertheless, augmented renal clearance (ARC) is a less well-studied phenomenon that could lead to faster elimination of drugs, resulting in subtherapeutic concentrations and poorer clinical outcomes when standard dosage guidelines are followed.
Objective
The aim of this systematic review was to gather and summarise all the available evidence on ARC in critically ill patients, including its definition, underlying mechanisms, epidemiology, diagnosis and impact on both drug pharmacokinetics and clinical outcomes.
Method
A systematic review was conducted to include all the original studies that provided information on ARC in critically ill patients, and is reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Results
Augmented renal clearance, defined as a creatinine clearance (CrCl) > 130 mL/min/1.73 m2, preferably measured in urine, is present in 20–65% of critically ill patients. Younger age, polytrauma and lower severity illness have been identified as risk factors. An influence of ARC on antimicrobial pharmacokinetics has been observed, with ARC consistently being associated with subtherapeutic antibiotic plasma concentrations.
Conclusion
ARC is a prevalent condition in critically ill patients, especially in young people, with urinary CrCl being the best diagnostic method because mathematical estimates tend to underestimate CrCl. ARC increases renal drug elimination and has a clear influence on certain antimicrobial plasma levels, but is yet to define its impact on clinical outcomes and on pharmacokinetics of other types of drugs. Research on the need to stage ARC and establish specific dosing guidelines is warranted.
Similar content being viewed by others
Augmented renal clearance (ARC), defined as a creatinine clearance (CrCl) > 130 mL/min/1.73 m2, is present in 20–65% of critically ill patients. The best diagnostic method for the identification of critically ill patients with ARC is measured urinary CrCl. |
Younger age, polytrauma and lower severity illness have been identified as risk factors for ARC. |
ARC has been consistently associated with subtherapeutic antimicrobial plasma concentrations. |
1 Introduction
Antimicrobial treatment in critically ill patients remains challenging. During critical illness, physiological changes and therapeutic interventions can alter drug pharmacokinetics, making the standard dosage guidelines unsuitable. Drugs in critically ill patients usually have a greater volume of distribution (Vd) due to capillary leak, inflammatory response and aggressive fluid loading. Increased Vd has been demonstrated for hydrophilic antimicrobials such as aminoglycosides, β-lactams, daptomycin, linezolid and glycopeptides [1, 2]. Hypoalbuminaemia, also frequently found in this population, might change the unbound drug fraction in blood, which in turn would be likely to influence the pharmacokinetics of antimicrobials that are highly protein bound (> 90%) and have high extraction rates. For a drug that is highly protein bound, hypoalbuminaemia is likely to lead to a high free fraction of antimicrobial in the early stage of the dosing interval, which might result in advantageously high unbound concentrations. On the other hand, changes in Vd and protein binding can lead to low unbound concentrations later in the dosing interval, which could reduce the effectiveness of time-dependent antimicrobials [1,2,3]. These alterations, together with some intensive care procedures such as continuous renal replacement therapies, could lead to lower plasma levels of antimicrobials [1,2,3]. In contrast, kidney or liver impairment can result in an accumulation of the drugs in plasma and therefore higher plasma concentrations [1,2,3].
Traditionally, renal function in critically ill patients has been routinely assessed with the objective of detecting renal impairment and adjusting drug doses. Nevertheless, augmented renal clearance (ARC) has also been identified in intensive care unit (ICU) patients. As a result, renal drug clearance can be increased in these patients compared with noncritically ill patients. This may be particularly important for antibacterial agents that are eliminated by the kidney and whose activity is time-dependent, such as β-lactams. Patients with ARC could be at risk of suboptimal antimicrobial exposure when conventional dosage regimens are used.
Changes in antimicrobial pharmacokinetics that take place in the critically ill can lead to clinical failure or an increased risk of adverse effects. In this context, individualised antimicrobial dosing and the application of pharmacokinetic/pharmacodynamic (PK/PD) principles are recommended [1,2,3]. The use of PK/PD analysis increases the probability of treatment success, minimises the emergence of resistance and reduces adverse effects [3]. The combination of the PK/PD analysis with Monte Carlo simulation can guide antimicrobial prescribing, considering the individual characteristics of patients and adjusting the antimicrobial therapy to their clinical status, which is especially relevant in certain subpopulations such as critically ill patients with ARC. Monte Carlo simulation is a statistical modelling tool that allows expanding the sample size, considering the variability of the PK and PD parameters in the estimation of the PK/PD indices [3]. It allows individualisation of antimicrobial therapy and simulation of different scenarios (higher doses, extended or continuous infusions, etc.) to support decision making and thereby improve clinical outcome. One of the principal requirements to perform Monte Carlo simulations is a validated population PK model including PK parameters, their variability and a covariate model [3]. For these reasons, it is important to investigate the pharmacokinetic alterations that take place in the intensive care setting and their influence on antimicrobial treatment.
In line with the fact that ARC is a relatively new concept, and the difficulty of conducting research in the intensive care setting, the evidence available to date regarding ARC is scarce and diverse. The aim of this review was to gather and summarise all the evidence on ARC in critically ill patients, including its definition, underlying mechanisms, epidemiology, diagnosis, and impact on drug pharmacokinetics and clinical outcomes.
2 Methods
2.1 Adherence to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Guidelines
This systematic review is reported following the applicable criteria of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement guidelines [4].
2.2 Search Strategy
The MEDLINE, EMBASE and International Pharmaceutical Abstracts (IPA) databases were systematically searched, from inception until May 2017, for all studies that reported information on ARC in critically ill patients. The following terms were used: (augmented renal clearance OR hyperfiltration) AND (critic* OR intensive). The search was additionally limited to English-language articles. Secondary literature was identified using the references included from the first search.
2.3 Eligibility Criteria
All references that reported information on underlying mechanisms, epidemiology, diagnosis, or impact of ARC in critically ill patients were included. Articles were excluded if they assessed paediatric patients or were clinical cases, reviews, letters or editorials.
2.4 Study Selection
Records obtained from the MEDLINE, EMBASE and IPA databases were compared and duplicates were eliminated. Abstracts of all records were screened to identify relevant publications according to the selection criteria. If there was insufficient information in the abstract, the full text was retrieved and assessed.
2.5 Data Collection Process and Analysis
For each record, the following data regarding ARC, when reported, were extracted: definition of ARC, proposed mechanism(s), frequency, course, related factors, method of diagnosis, and impact on both drug pharmacokinetics and clinical outcome. Given the nature of the topic studied, that ARC is a fairly new concept and that randomised trials were not expected, we conducted a descriptive critical analysis of the records included.
3 Results
3.1 Study Selection
As described in Fig. 1, we reviewed the abstracts of the 183 records obtained. Of these, 131 were not included as they did not meet the selection criteria. Additionally, seven conference abstracts were excluded because they were based on the same study and gave the same results as an original article published subsequently and included in this review. Of the 45 records included, 32 were original articles [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36] and 13 were conference abstracts [37,38,39,40,41,42,43,44,45,46,47,48,49]. An additional three original articles were identified from the reference lists of selected papers [50,51,52].
3.2 Definition of Augmented Renal Clearance (ARC)
ARC refers to enhanced elimination of solutes compared with an expected baseline, a process that involves changes in glomerular filtration and renal tubular function. Glomerular filtration rate (GFR) is generally accepted as the best overall index of kidney function, and ARC has been associated with elevated urinary creatinine clearance (CrCl); hence, this parameter is used to define ARC [21, 53].
The normal GFR in young adults is approximately 125 mL/min/1.73 m2 [53]. ARC is a fairly new concept and does not have a standard definition. Nevertheless, there is currently a broad consensus in considering 130 mL/min/1.73 m2 as the lower limit of CrCl for the diagnosis of ARC, since there are studies linking CrCl > 130 mL/min/1.73 m2 with subtherapeutic antimicrobial concentration [15, 18, 24, 26, 31, 32, 48].
Assessing the presence of ARC in critically ill patients is still challenging. GFR measured as the clearance of an exogenous filtration marker is the best overall index of kidney function. The ‘gold standard’ method is the urinary clearance of inulin during a continuous intravenous infusion. However, this is an invasive and expensive method, and, to simplify the procedure, alternative endogenous filtration markers are used in clinical practice, mainly creatinine and cystatin C. In the general population, GFR estimating equations to derive GFR from serum creatinine are preferred over relying on serum creatinine concentration alone. These equations have been developed from large epidemiological studies with the aim of diagnosing and monitoring patients with chronic kidney disease and stable renal function. As they all assume that endogenous serum markers are in steady state and this cannot be assumed in critically ill patients, the use of measured CrCl in urine is generally preferred in this setting. A good correlation has been observed between measured GFR using inulin or radioactive iothalamate and urine CrCl in critically ill patients [16, 51]. In summary, ARC is defined as a CrCl > 130 mL/min/1.73 m2, preferably calculated by measuring CrCl in urine (urinary CrCl).
3.3 Mechanism of ARC in Critically Ill Patients
No articles were found whose main objective was to establish the mechanism(s) underlying ARC. The physiological mechanism responsible for ARC in critically ill patients is not well-defined and the propositions put forward to date need to be studied further. It has been postulated that systemic inflammatory response syndrome (SIRS), a clinical syndrome resulting from the general and nonspecific activation of the immune system, could be associated with ARC [25]. SIRS may occur in several conditions that may or may not be related to infection, including sepsis, severe trauma, major surgery and burns. The release of cytokines and pro-inflammatory mediators leads to decreased vascular resistance and increased cardiac output, which, together with intensive fluid therapy and inotropic drugs commonly used in critically ill patients, may increase renal blood flow and GFR [31, 32, 34].
Nevertheless, trials have been unable to establish a statistically and clinically significant relationship between cardiac index, fluid balance or use of vasopressors and ARC. Although a weak correlation has been noted between cardiac index and CrCl, it has been shown to be of little use in identifying patients at risk of ARC [28].
Other theories suggest that renal functional reserve may play a role in ARC. The concept of renal functional reserve refers to the capacity of the kidney to increase GFR in response to certain physiological or pathological stimuli [54]. In clinical conditions in which ARC is present (pregnant women, kidney donors or critically ill patients), renal functional reserve may be used to achieve normal or supranormal renal function. Renal functional reserve can be assessed after a protein load and seems to be significantly lower in the elderly than in young healthy individuals. This would explain some of the demographic characteristics that have most consistently been linked to the presence of ARC in critically ill patients, such as young age and diagnosis of polytrauma [28].
The combination of systemic inflammation coupled with a greater physiological reserve, rather than any single mechanism, has been accepted by several authors as a possible mechanism for ARC [19, 23]. ARC has even been considered a marker of a good prognosis as it may predict a host’s increased ability to adapt to and withstand severe infection [5, 15].
In critically ill patients with severe traumatic brain injury, Dias et al. [10] documented a relationship between brain autoregulation impairment and estimated kidney GFR. Autoregulation of blood flow is the inherent capacity of the vascular bed to maintain constant perfusion despite variations in arterial blood pressure (ABP) and intracranial pressure (ICP), and is an important mechanism for maintaining cerebral and kidney blood flow constant. In the aforementioned study, CrCl was found to be negatively correlated with the cerebrovascular pressure reactivity index (PRx), which expresses the correlation between ABP and ICP. For each 10 mL/min increase in estimated CrCl, a mean decrease in PRx of 0.01 was expected, i.e. the higher the CrCl, the better the cerebrovascular reactivity. Furthermore, the mean PRx value for a fatal outcome was significantly greater than the mean PRx for a nonfatal outcome. Udy et al. [36] have also recently explored the potential mechanisms of ARC in patients with traumatic brain injury and found significantly elevated atrial natriuretic peptide (ANP) levels compared with those reported in healthy volunteers. ARC is a common finding in neurocritic patients and some theories to explain this relationship have also been postulated. The usual management of these patients with vasopressors and hypertonic solutions or the presence of neuroendocrine factors, such as ANP, is suggested to explain the high incidence of ARC in this population. These studies open a new line of research on the mechanism of ARC in patients with traumatic brain injury, and further studies are needed to understand the pathophysiological mechanism between brain and kidney autoregulation and the practical implications of this relationship.
3.4 Epidemiology of ARC in Critically Ill Patients
3.4.1 Frequency and Course
Observational studies show that ARC is present in 20–65% of critically ill patients [5,6,7,8,9, 11, 12, 15, 17,18,19, 22,23,24,25,26,27,28, 30, 32,33,34, 37, 44, 45, 49, 52], and that it seems to be more common in certain conditions, such as traumatic brain injury (85%) [10, 36, 50], subarachnoid haemorrhage (100%) [35] and burns (65%) [51].
Most studies define patients with ARC as those in which a single measurement of urinary CrCl is greater than a given limit (120–130 mL/min/1.73 m2). In some studies, patients have been considered to have ARC if more than 50% of the CrCl measurements during admission had been higher than 130 mL/min/1.73 m2. These studies have shown that between 55.4 and 74% [22, 23] of patients who have CrCl higher than 130 mL/min/1.73 m2 in one measurement are found to have values higher than this level in more than 50% of measurements. De Waele et al. [12] found that 59% of patients found to have CrCl higher than 130 mL/min/1.73 m2 once, had ARC throughout their ICU stay. Another study showed that ARC was permanently present in 23% of patients and was transient (lasting 1 day) in 35% of patients with one CrCl value higher than 130 mL/min/1.73 m2 [27], while Grootaert et al. [44] found that 40% of patients who had one CrCl value higher than 120 mL/min/1.73 m2 had episodes of CrCl higher than this level for at least 5 days, and that 5 days was also the relative duration of ARC per patient. In addition, we have identified two studies that describe ARC prevalence over time in patients admitted to the ICU. In both studies, the highest prevalence of ARC is observed on day 5 after admission [23, 34].
3.4.2 Related Factors
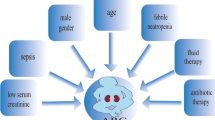
ARC has been associated with a wide range of factors (Fig. 2). One that has most consistently been linked to a high risk of ARC, in both univariate and multivariate analysis, is younger age [5, 7,8,9, 11, 12, 15, 19, 22, 23, 26,27,28, 32, 34, 38, 44, 51, 52]. Most studies show a difference of 10–20 years between patients with and without ARC. The mean or median age of patients with ARC is between 34 and 50 years in most studies, while in the case of patients without ARC, it is always over 50 years, and, in most studies, over 60 years. Just two studies have not found significant differences in age, probably because the majority of participants were young (mean age < 40 years) [6, 17].
Trauma has also been described as a risk factor for developing ARC in several studies [8, 11, 15, 19, 23, 28, 32, 52]. Publications that provide information on demographic characteristics by reason for admission [23, 28, 52] indicate that patients admitted for trauma are significantly younger. On the other hand, trauma admission has been identified as a significant risk factor in multivariate analysis, when also considering age [11, 28, 52], and hence its biological influence remains uncertain.
Research has also focused on the relationship of ARC with illness severity, assessed by the Acute Physiology And Chronic Health Evaluation II (APACHE II) score, Simplified Acute Physiology Score (SAPS II) and/or Sequential Organ Failure Assessment (SOFA) score. Some studies have found a significant relationship between lower severity and ARC [5, 15, 28, 32, 34, 52]. This relationship has not been observed in other studies [22, 23, 27] or has only been observed using the SAPS II and APACHE II score, but not the SOFA score [8, 11, 19]. It should be considered that the SAPS II and APACHE II scores are influenced by age.
Other factors for which associations with ARC have been found in univariate analysis, but not subsequently confirmed, include male sex [5, 7, 22, 23, 28, 52], mechanical ventilation [23, 26], high diastolic blood pressure [34], elevated cardiac index [28], high [26, 50] or low [12] vasopressor use, low use of furosemide [19, 23], high diuretic volumes [19, 34, 52] and a less-positive fluid balance [19, 34].
3.5 Identification of ARC in Critically Ill Patients
3.5.1 Estimated Versus Measured Creatinine Clearance
Over recent years, several observational studies have been conducted to establish the usefulness of GFR estimating equations in the diagnosis of critically ill patients with ARC. A detailed overview of the studies identified is provided in Table 1. The conclusions should be interpreted with caution because the comparator used is CrCl measured in urine, which, despite being a pragmatic alternative, is not the ‘gold standard’. All the equations mentioned are given in Table 2.
Baptista et al. [33] were the first to characterise the accuracy of four commonly used estimating equations—Cockcroft–Gault (CG), Modified CG, 4-variable Modification of Diet in Renal Disease (MDRD-4) and 6-variable Modification of Diet in Renal Disease (MDRD-6). In 86 critically ill patients with ARC (CrCl > 130 mL/min/1.73 m2), all the equations, except MDRD-6, yielded values that were statistically significantly but weakly correlated with measured urinary CrCl (r2 < 0.3, p < 0.05). They all significantly underestimated the measured value of CrCl, with a bias of between 39 mL/min/1.73 m2 (for CG) and 84 mL/min/1.73 m2 (for modified CG), and a precision of ± 70–75 mL/min/1.73 m2, which is clinically unacceptable. Grootaert et al. [30] conducted a similar study, retrospectively comparing the validity of two estimating equations—the CG and the updated MDRD-4 (MDRD-4-IDMS)—in 1679 samples from 390 critically ill adults with a measured CrCl of 120 mL/min/1.73 m2 or more. Estimates showed poor agreement with measured CrCl values, with a bias between 11.2 mL/min (for CG) and 19.9 mL/min/1.73 m2 (for MDRD-4-IDMS), and a precision of ± 61 mL/min and ± 77 mL/min/1.73 m2, respectively. In contrast to Baptista et al., estimates predicted higher CrCl than the measured values, which was attributed to differences in the population (older, with lower body weight, and more severely ill), which could lead to falsely high renal function when estimated.
Udy et al. [25] assessed the performance of the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI), CG and MDRD-4-IDMS equations in a prospective, observational study in which they included 110 critically ill patients with plasma creatinine concentration within the normal range. In the subgroup analysis, the Udy et al. observed that for CrCl < 120 mL/min/1.73 m2, the equations tend to overestimate the CrCl, while the opposite occurred for CrCl ≥ 120 mL/min/1.73 m2. Although a moderate correlation was found for CKD-EPI (r2 = 0.46, p = 0.005), CG (r2 = 0.399, p = 0.009) and MDRD-4-IDMS (r2 = 0.427, p = 0.009) in patients with measured CrCl ≥ 150 mL/min/1.73 m2, there was no significant correlation in patients with measured CrCl between 120 and 149 mL/min/1.73 m2. All of the equations underestimated the measured value of CrCl with significant bias and imprecision (29.2 ± 10.8 mL/min/1.73 m2 for CKD-EPI, 6.62 ± 23.9 mL/min/1.73 m2 for CG and 22.7 ± 26.1 mL/min/1.73 m2 for MDRD-4-IDMS) in patients with measured CrCl between 120 and 149 mL/min/1.73 m2. Bias and imprecision were even higher for patients with measured CrCl ≥ 150 mL/min/1.73 m2.
Similar results have been obtained in other studies, namely weak correlations and significant bias and imprecision, in critically ill patients with serum creatinine concentration within the normal range for CG [9, 11, 17, 22, 35, 43], MDRD-4-IDMS [9, 11, 22] and CKD-EPI [9, 11, 22]. In all cases, equations tended to underestimate CrCl, compared with measured urinary CrCl, when there was ARC.
Steinke et al. [14] compared the agreement of the estimated CrCl using equations based on plasma creatinine (CG and CKD-EPI) or cystatin C (Hoek) with measured urinary CrCl. This retrospective analysis included 100 critically ill patients from two pharmacokinetic studies, 16 of whom had ARC (urinary CrCl > 130 mL/min/1.73 m2). Both the Hoek and CKD-EPI equations significantly underestimated CrCl in patients with ARC. The specificity to detect patients with ARC was 0.81 (95% confidence interval [CI] 0.71–0.89), 0.96 (95% CI 0.90–0.99) and 0.96 (95% CI 0.90–0.99) for the CG, CKD-EPI and Hoek equations, respectively, but sensitivity was only 0.69 (95% CI 0.41–0.89), 0.25 (95% CI 0.07–0.52) and 0.38 (95% CI 0.15–0.65), respectively. Similar results were obtained by Baptista et al. [46] regarding the inaccuracy of the Hoek and Larson cystatin C-derived equations when applied to ICU patients with ARC.
Only two studies have been identified in which an exogenous marker is used to assess GFR in patients at risk of ARC. The first, conducted by Loirat et al. [51], found a close correlation between 125I-iothalamate clearance and CrCl (r2 = 0.93, p < 0.001) and between inulin clearance and CrCl (r2 = 0.74, p < 0.001) in 20 burn patients, 13 of whom had ARC. More recently, Udy et al. [21] used sinistrin clearance as a marker of GFR and compared it with measured urinary CrCl and the CKD-EPI equation. They found that sinistrin clearance was highly correlated with measured CrCl (r2 = 0.7, p < 0.01). Both measured CrCl and the CKD-EPI-estimated value tended to underestimate sinistrin clearance, although the bias was smaller in the measured value.
Given the current evidence, measuring urinary CrCl should be considered the method of choice for identifying critically ill patients with ARC. Nevertheless, in most ICUs, renal function is still determined based on estimating equations or serum creatinine values. In England, for instance, nearly 60% of ICUs use serum creatinine [40].
3.5.2 ARC Diagnostic Scores
The limited usefulness of CrCl estimating equations has motivated the creation of scales with greater sensitivity and specificity for identifying patients at risk of ARC. As reported in an abstract at the 2014 Congress of the European Society of Intensive Care Medicine, Baptista et al. [41] presented a retrospective analysis of urine samples of patients admitted to the ICU of a tertiary university hospital in 2012. They excluded urine samples with contemporaneous serum creatinine ≥ 1.2 mg/dL and grouped patients according to their measured urinary CrCl (< 60 mL/min/1.73 m2, 60–130 mL/min/1.73 m2 and > 130 mL/min/1.73 m2). Overall, they analysed 4271 urine samples from 477 patients, 33% of whom had ARC and 20% had renal dysfunction. The best diagnostic value for ARC was obtained using the combination of urinary creatinine > 45 mg/mL and age < 65 years, with a specificity of 0.88 but low sensitivity (0.60).
Udy et al. [28] conducted a study that included 71 critically ill patients with trauma (n = 28) or sepsis (n = 43), enrolled in a wider pharmacokinetic study on antimicrobials, who had serum creatinine within the normal range (< 1.3 mg/dL). ARC (urinary CrCl > 130 mL/min/1.73 m2) was present in 58% of the patients. Based on the results of the multivariate analysis, they created a scoring system to identify ARC patients, in which modified SOFA score ≤ 4 was given 1 point, admission post-trauma was given 3 points and age ≤ 50 years was given 6 points. Scores were then summed and patients grouped into categories of low (0–3), medium (4–6) or high (7–10) risk of ARC. Higher scores were strongly associated with a greater prevalence of ARC, with an area under the receiver operating characteristic curve (AUCROC) of 0.89 (p < 0.001).
Recently, Barletta et al. [7] developed the Augmented Renal Clearance in Trauma Intensive Care (ARCTIC) scoring system to predict ARC in trauma patients. They included 133 trauma patients with serum creatinine within the normal range (< 1.3 mg/dL) and performed a multivariate analysis to identify independent predictors of ARC. The risk factors included in the final ARCTIC score were age below 56 years (4 points), age between 56 and 75 years (3 points), serum creatinine < 0.7 mg/dL (3 points) and male sex (2 points). The score had an AUCROC of 0.813 (p < 0.001) and an ARCTIC score of 6 or higher had a sensitivity of 0.84 and a specificity of 0.68.
We must bear in mind that all these studies select patients with serum creatinine within the normal range. Therefore, the application of ARC scores makes little sense in patients with serum creatinine higher than 1.3 mg/dL, despite creatinine levels not being included in the scores. Scores to detect patients at risk of ARC are useful and easy to apply in ICUs. They can help identify patients at the highest risk of ARC, and, based on the level of risk, indicate the need to measure urinary CrCl to obtain a definitive diagnosis.
3.6 Impact of ARC on Antimicrobial Treatment
The presence of ARC in critically ill patients may have a negative impact on the attainment of therapeutic levels of many drugs. For example, the activity of enoxaparin has been shown to be shorter in patients with ARC [6]; however, almost all of the scarce references published about this subject are focused on antimicrobial therapy, where ARC is very important because it could condition not only the drug efficacy but also the emergence of resistance.
ARC can influence the pharmacokinetic profile of antimicrobial drugs that are renally cleared and known to have a direct correlation between their renal clearance and CrCl, such as β-lactams, vancomycin or aminoglycosides. According to their activity pattern, antimicrobial drugs can be classified into three groups: concentration-dependent killing along with prolonged effects (aminoglycosides, fluoroquinolones, polymyxins, daptomycin or metronidazole), time-dependent activity with no or very short persistent effects (β-lactams) and concentration-independent killing with prolonged persistent effects (tetracyclines, tigecycline, macrolides, azithromycin, clindamycin, linezolid, chloramphenicol, trimethoprim, sulphonamides and vancomycin). For the first and the third groups, the PK/PD indexes that best correlated with efficacy are the maximum serum concentration (Cmax)/minimum inhibitory concentration (MIC) ratio or the area under the concentration-time curve (AUC)/MIC ratio, because the prolonged persistent effects protect against regrowth when the active drug concentration falls below the MIC. For the second group, time-dependent activity, the PK/PD index that best correlated with efficacy is the duration of time that free antimicrobial concentrations exceeded the MIC.
Enhanced drug clearance will lead to a shorter half-life, lower Cmax and smaller AUC of renally cleared drugs compromising their effectiveness [2, 3]. Some research has been conducted attempting to assess the influence of ARC on antimicrobial pharmacokinetics and clinical outcomes in critically ill patients, and the main findings are outlined below.
3.6.1 Impact of ARC on Vancomycin Pharmacokinetics
Vancomycin is a glycopeptide that is primarily eliminated by the kidneys (90%) and whose clearance is directly related to CrCl. It is bactericidal and exhibits concentration-independent bacterial killing. Clinically, an AUC/MIC ratio > 400 has been linked to efficacy of this drug [3]. Several studies have been conducted to determine the influence of ARC on the plasma concentration of vancomycin [13, 18, 19, 26, 32, 48]. Baptista et al. [32] evaluated the effect of ARC (urinary CrCl > 130 mL/min/1.73 m2) in 93 critically ill septic patients who started empirical or directed treatment that included vancomycin by continuous infusion. Patients with ARC (40% of the study population, n = 37) reached between 25 and 30% lower vancomycin levels (p < 0.05), and ARC was strongly associated with subtherapeutic serum concentrations of vancomycin on the first 3 days of treatment. In a subsequent study [18], these same authors developed a nomogram for dosing vancomycin administered by continuous infusion during the first 24 h of treatment. First, they retrospectively analysed 79 patients, of whom 36% (n = 29) had ARC, treated with the standard hospital protocol; only 28% (n = 8) of the patients with ARC reached the target level of 20–30 mg/L, compared with 64% (n = 32) of those who did not have ARC (p = 0.092). Then, using these data, they developed a predictive equation for vancomycin clearance and a dosing nomogram based on 8-h urine collections to measure urinary CrCl, and tested it in 25 patients. Applying the nomogram, 84% of patients, including all those with ARC, reached the target level.
Campassi et al. [19] conducted a prospective study to determine the effect of ARC on vancomycin concentrations. Of the 44 patients treated with vancomycin, 12 had ARC (urinary CrCl > 120 mL/min/1.73 m2). None of the patients with ARC reached the target level by 24 h after starting treatment, and they had lower vancomycin plasma concentrations during the first 48 h after the start of the treatment (p < 0.05). Furthermore, they needed higher doses of the drug to finally reach the target level than non-ARC patients (p < 0.05). Another study, conducted by Spadaro et al. [13], aimed to estimate the efficacy of a vancomycin dosing protocol in critically ill patients with and without kidney dysfunction. It was found that 50, 66 and 80% of patients with subtherapeutic levels of vancomycin had ARC (urinary CrCl > 130 mL/min/1.73 m2) at the first (day 2), second (day 4) and third (day 6) monitoring tests, respectively. Similar findings were obtained by Minkute et al. [26], who concluded that the risk of subtherapeutic vancomycin levels is doubled in patients with ARC (estimated CrCl > 130 mL/min, p = 0.011).
3.6.2 Impact of ARC on β-Lactam Pharmacokinetics
β-lactam antibacterials are primarily eliminated by the kidneys and have time-dependent antibacterial activity. Their efficacy is best predicted by the duration of time for which the free drug plasma concentration remains above the MIC (fT > MIC). Traditionally, an fT > MIC of between 40 and 70% (depending on the agent) of the dosing interval has been accepted as a PK/PD target, although it has also been suggested that greater drug exposure, up to four times the MIC for the entire dosing interval, could improve clinical outcomes in critically ill patients [3, 55].
Udy et al. [31] retrospectively analysed 52 trough concentrations of β-lactam obtained in 48 critically ill patients. Only 58 and 31% of patients had trough concentrations above the MIC and four times above the MIC, respectively. Patients having ARC (urinary CrCl > 130 mL/min/1.73 m2) was associated with trough concentrations lower than the MIC or lower than four times the MIC in 82 and 72% of cases, respectively (p < 0.01). The multivariate analysis confirmed that CrCl contributed significantly to the likelihood of obtaining subtherapeutic levels of β-lactams, and a 25 mL/min/1.73 m2 increase in the measured CrCl was associated with a mean 60% reduction in the probability of achieving a trough concentration greater than or equal to four times the MIC.
Carlier et al. [24] assessed the influence of ARC (urinary CrCl > 130 mL/min/1.73 m2) on PK/PD target attainment in critically ill patients receiving meropenem or piperacillin/tazobactam administered as an extended infusion. Overall, only 33 of 60 patients reached the PK/PD target of 100% fT > MIC. ARC patients less often reached the PK/PD targets of 100% fT > MIC (24 vs. 84%, p < 0.001) and 50% fT > MIC (63 vs. 94%, p < 0.01). Furthermore, the mean percentage of fT > MIC in ARC patients was lower (61 vs. 94%, p < 0.001). Multivariate analysis demonstrated that CrCl was an independent predictor of not achieving the PK/PD target.
Akers et al. [20] studied ARC as a predictor of subtherapeutic levels of piperacillin and tazobactam. They included 13 critically ill patients treated with piperacillin/tazobactam and with an estimated CrCl of > 90 mL/min/1.73 m2 according to the MDRD-4-IDMS equation. Patients were classified as low risk (0–6 points) or high risk (> 6 points) based on the ARC score proposed by Udy et al. [28]. The score had a sensitivity of 1 (95% CI 0.52–1) and a specificity of 0.71 (95% CI 0.30–0.95) for detecting increased clearance, increased Vd and decreased AUC. The ARC score also had a sensitivity of 1 (95% CI 0.52–1) for predicting subtherapeutic levels of piperacillin/tazobactam (considering as PK/PD target, free piperacillin concentrations greater than the MIC for at least 50% of the dose interval) at an MIC of 16 μg/mL.
ARC patients often need higher doses of β-lactams and there is a strong relationship between ARC and subtherapeutic levels of these antimicrobials, as has been observed in several studies [15, 39, 42, 47]. In this context, the individualisation of dosage regimens, for example, by the administration of antimicrobials in extended infusion can be useful, as demonstrated by Roberts and Lipman [29]. They describe the population pharmacokinetics of doripenem in critically ill patients with nosocomial pneumonia and found that doripenem clearance was correlated with CrCl and peripheral Vd was correlated with patient body weight. Then they performed Monte Carlo dosing simulations to optimise dosing schedules. Extended infusions were found to maximise the likelihood of achieving target blood concentrations, especially in patients with ARC or obesity and with infections caused by organisms with borderline susceptibility.
3.6.3 Impact of ARC on Clinical Outcomes in Patients Treated with Antimicrobials
Studies investigating the relationship between ARC and clinical outcome in patients treated with antimicrobial drugs are scarce. Claus et al. [27] conducted an observational prospective study in which they investigated the impact of ARC on clinical outcome in critically ill patients treated with antimicrobial agents. Of the 128 patients included, 51.6% (n = 66) had ARC, with this being permanently present, throughout the antimicrobial treatment, in 23% (n = 15) of patients, and transient, lasting just one day, in 35% (n = 23) of patients. The rate of treatment failure was higher in patients who had ARC than those who did not have ARC (27.3 vs. 12.9%, p = 0.04), and also tends to be higher in those with permanent rather than transient ARC (33.3 vs. 17.4%, p = 0.436), although the difference was not significant, probably due to the small number of patients in this subgroup.
In another observational prospective study, Huttner et al. [15] investigated the relationship between ARC, plasma concentrations of β-lactam antibacterials and clinical outcome in critically ill patients. They recruited 100 critically ill patients with suspected or documented severe bacterial infection for which treatment with intravenous imipenem/cilastatin, meropenem, piperacillin/tazobactam or cefepime was initiated. Overall, 64% (n = 64) of the patients had ARC. Despite ARC strongly predicting undetectable trough concentrations (odds ratio [OR] 3.3, 95% CI 1.11–9.94], no link was observed between ARC and clinical failure.
Recently, Udy et al. [5] performed a substudy of the BLING-II trial seeking to explore the relationship between ARC and clinical outcomes in 254 critically ill patients with severe sepsis, among whom 45 (17.7%) had ARC (urinary CrCl > 130 mL/min/1.73 m2). They found no differences in ICU-free days at day 28 or in 90-day mortality. On the contrary, they found that the clinical cure rate at 14 days after ceasing antimicrobial administration was significantly higher in patients with ARC (73.3 vs. 55%, p = 0.024). Nevertheless, this association was lost in the multivariate analysis adjusted for age, modified SOFA and dosing strategy. They also found no difference between ARC status and clinical outcomes according to the dosing strategy employed (continuous infusion vs. intermittent infusion).
4 Discussion
Critically ill patients undergo physiological changes that can alter drug pharmacokinetics. Traditionally, the main focus of assessing kidney function has been to adjust antimicrobial dosing in renal impairment. However, ARC has recently begun to be recognised as an alteration that can lead to accelerated drug elimination and suboptimal drug levels. Although there is no standardised definition of ARC, there is a broad consensus among authors to consider it as a CrCl higher than 130 mL/min/1.73 m2. Even if changes in renal tubular function are also expected [21], this definition seems reasonable considering that GFR is recognised as the best overall index of renal function, that the normal GFR values in young adult patients are approximately 125 mL/min/1.73 m2 [53], and the emerging evidence linking CrCl higher than 130 mL/min/1.73 m2 with subtherapeutic antimicrobial concentrations [15, 18, 24, 26, 31, 32, 48]. Current evidence indicates that, in critically ill patients, renal function should be evaluated by measuring urinary CrCl. Several diagnostic scores [7, 28, 41] have been published that may help to identify critically ill patients at increased risk of developing ARC, but they are unable to establish a definitive diagnosis.
The phenomenon of ARC is not negligible in the intensive care setting, being present in 20–65% of patients [5,6,7,8,9, 11, 12, 15, 17,18,19, 22,23,24,25,26,27,28, 30, 32,33,34, 37, 44, 45, 49, 52], and significantly more common in young patients [5, 7,8,9, 11, 12, 15, 19, 22, 23, 26,27,28, 32, 34, 38, 44, 51, 52]. ARC has been significantly and consistently related to subtherapeutic β-lactam [15, 20, 24, 29, 31, 39, 42, 47] and vancomycin [13, 18, 19, 26, 32, 48] levels, which could potentially lead to the appearance of resistances and therapeutic failure [56, 57]. Despite the fact that the evidence is scarce, it is expected that the influence of this phenomenon is not restricted to β-lactams and vancomycin, but will also affect other antimicrobials such as aminoglycosides, fluoroquinolones or daptomycin [38, 51, 58,59,60], and other types of drugs, such as anticoagulants [6] or antiepileptics.
We found only three studies evaluating the effect of ARC on clinical outcomes, and the results are discordant. Claus et al. [27] found a higher rate of treatment failure in patients with ARC (23.7 vs. 8%, p = 0.04), whereas Huttner et al. [15] and Udy et al. [5] found no relationship between ARC and clinical outcomes. Huttner et al. are the only authors who performed plasma monitoring of antimicrobials. On the other hand, they did not provide information on the MIC of isolated microorganisms and they use EUCAST’s nonspecies-related thresholds to establish subtherapeutic concentrations. Furthermore, they found no relationship between undetectable trough levels and clinical outcomes. As stated by the authors, this apparent lack of relationship might reflect their low-resistance setting, where some pathogens may have such low MICs that they lie beneath the limit of plasma antimicrobial detection, and thus even patients with seemingly undetectable plasma concentrations may be attaining the PK/PD target of 100% fT > MIC.
It is difficult to establish a relationship between ARC and clinical outcomes in critically ill patients due to the complexity and variability of this population. The physiological mechanism responsible for ARC in critically ill patients is still not well-defined, but a possible mechanism, accepted by several authors, is the combination of systemic inflammation together with a greater physiological renal reserve. In this sense, it should be noted that although ARC can increase antimicrobial elimination, increasing the risk of therapeutic failure, it has also been considered a marker of a good prognosis as it may predict a host’s increased ability to adapt to and withstand severe infection [5, 15].
Overall, when ARC is present in critically ill patients, two scenarios should be considered for future research. On the one hand is the possibility that critically ill patients with ARC could be less likely to develop certain organ dysfunction such as acute kidney injury (AKI). Patients with both sepsis and AKI are widely recognised as having an unacceptably high mortality rate [61, 62] and the same occurs with trauma patients [63, 64]. The development of AKI is a marker of bad prognosis [65,66,67,68], while the development of ARC could reflect the opposite situation. On the other hand, although the ARC itself may not be a factor of poor prognosis in the critical patient, its influence on drug pharmacokinetics is clear. The success of antimicrobial treatment in ICU depends on early initiation, correct drug selection and the use of a suitable dosage regimen to attain the PK/PD target [69]. Currently, there is great evidence on the importance of therapeutic drug monitoring and the application of PK/PD criteria in the antimicrobial treatment of ICU patients [55, 70,71,72]. An increase in antimicrobial clearance can have negative consequences but could be overcome with alternative dosing strategies that optimise drug exposure, such as higher daily doses, continuous/extended infusions or loading doses [73,74,75,76,77,78]. Recently, several guidelines and consensus documents, such as the Surviving Sepsis Campaign [69], the AGORA project for intra-abdominal infections [79], or Infectious Diseases Society of America (IDSA) guidelines for the management of adults with hospital-acquired and ventilator-associated pneumonia [80], have made specific mention to ARC and include recommendations on the use of dosing strategies based on the PK/PD principles.
Renal impairment is successfully staged in chronic kidney disease according to GFR, defining a normal GFR as ≥ 90 mL/min/1.73 m2 [53]. The use of reduced doses in patients with impaired renal function is widely accepted, however the appearance of the phenomenon of ARC in critically ill patients could raise the need to establish dose recommendations based on increasing GFR. In 2012, the European Medicines Agency published a press release recommending to double the dose of Doribax® (doripenem) for the treatment of nosocomial pneumonia in patients with ARC and/or with infections caused by nonfermenting gram-negative pathogens [81]. The reason was the preliminary results from a clinical trial in which patients treated with Doribax® were less likely to recover than patients in the control group. The Agency’s Committee for Medicinal Products for Human Use considered that factors such as ARC and infections involving specific types of bacteria might influence the effectiveness of treatment with Doribax®. However, the influence of ARC is not limited to antimicrobials, and, similarly, recently marketed drugs such as edoxaban [82, 83] already include in their summary of product characteristics (SmPC) specific recommendations or warnings about reduced efficacy in nonvalvular atrial fibrillation patients with increased CrCl.
Given the high frequency of ARC in the intensive care setting, further studies in this subgroup of critically ill patients are warranted in order to explore the need to stage the ARC and make dosage recommendations. Similar to AKI, ARC could be a dynamic and temporary situation in critically ill patients, therefore a continuous evaluation of the renal function would be necessary.
4.1 Limitations
All the included studies are observational, with relatively few patients and mostly from single centres. They also present a great deal of variability in terms of patient type, selection criteria and definition of the study variables. In addition, not all the studies define ARC in the same way or detect it with the same diagnostic techniques. For these reasons, only a descriptive analysis has been performed and a synthesis of the results has not been considered appropriate. Nevertheless, we consider that this descriptive study has allowed us to focus on the main features of ARC and that this global vision of the problem will be very useful for designing future clinical studies. Finally, another limitation in our search strategy was the English-language restriction, and hence information may have been overlooked if it was published in other languages.
5 Conclusions
ARC is a prevalent condition in critically ill patients, especially in young people. The use of GFR estimating equations leads to the underdiagnosis of ARC in the intensive care setting, therefore urinary CrCl measurement is recommended. The presence of ARC has a clear influence on antimicrobial plasma levels but further research is needed to define its impact on clinical outcomes in patients treated with antimicrobials or other types of drugs.
As happens with acute renal failure, ARC is a dynamic condition and modulation of dosing according to the daily variations in renal clearance would be necessary. More trials with greater statistical power need to be undertaken to develop a validated pharmacokinetic population model and drug dosing guidelines for critically ill patients with ARC. PK/PD analysis and Monte Carlo simulation can be applied in this setting to simulate different antimicrobial dosage regimens (e.g. higher doses and extended or continuous infusions) and establish the optimal approach to enhance clinical outcomes.
The concept of ARC is becoming increasingly relevant and is even included in the SmPC of some new drugs. In the near future, patients with ARC could be considered as a special subpopulation with specific dosage adjustments in the SmPC.
References
Roberts JA, Abdul-Aziz MH, Lipman J, Mouton JW, Vinks AA, Felton TW, et al. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis. 2014;14(6):498–509.
Udy AA, Roberts JA, Lipman J. Clinical implications of antibiotic pharmacokinetic principles in the critically ill. Intensive Care Med. 2013;39(12):2070–82.
Asín-Prieto E, Rodríguez-Gascón A, Isla A. Applications of the pharmacokinetic/pharmacodynamic (PK/PD) analysis of antimicrobial agents. J Infect Chemother. 2015;21(5):319–29.
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
Udy AA, Dulhunty JM, Roberts JA, Davis JS, Webb SAR, Bellomo R, et al. Association between augmented renal clearance and clinical outcomes in patients receiving beta-lactam antibiotic therapy by continuous or intermittent infusion: a nested cohort study of the BLING-II randomised, placebo-controlled, clinical trial. Int J Antimicrob Agents. 2017;49:624–30.
Abdel El Naeem HEM, Abdelhamid MHE, Atteya DAM. Impact of augmented renal clearance on enoxaparin therapy in critically ill patients. Egypt J Anaesth. 2017;33:113–7.
Barletta JF, Mangram AJ, Byrne M, Sucher JF, Hollingworth AK, Ali-Osman FR, et al. Identifying augmented renal clearance in trauma patients: validation of the augmented renal clearance in trauma intensive care scoring system. J Trauma Acute Care Surg. 2017;82(4):665–71.
Kawano Y, Morimoto S, Izutani Y, Muranishi K, Kaneyama H, Hoshino K, et al. Augmented renal clearance in Japanese intensive care unit patients: a prospective study. J Intensive Care. 2016;4:62.
Barletta JF, Mangram AJ, Byrne M, Hollingworth AK, Sucher JF, Ali-Osman FR, et al. The importance of empiric antibiotic dosing in critically ill trauma patients: are we under-dosing based on augmented renal clearance and inaccurate renal clearance estimates? J Trauma Acute Care Surg. 2016;81(6):1115–21.
Dias C, Gaio AR, Monteiro E, Barbosa S, Cerejo A, Donnelly J, et al. Kidney-brain link in traumatic brain injury patients? A preliminary report. Neurocrit Care. 2015;22(2):192–201.
Ruiz S, Minville V, Asehnoune K, Virtos M, Georges B, Fourcade O, et al. Screening of patients with augmented renal clearance in ICU: taking into account the CKD-EPI equation, the age, and the cause of admission. Ann Intensive Care. 2015;5(1):49.
De Waele JJ, Dumoulin A, Janssen A, Hoste EAJ. Epidemiology of augmented renal clearance in mixed ICU patients. Minerva Anestesiol. 2015;81(10):1079–85.
Spadaro S, Berselli A, Fogagnolo A, Capuzzo M, Ragazi R, Marangoni E, et al. Evaluation of a protocol for vancomycin administration in critically patients with and without kidney dysfunction. BMC Anesthesiol. 2015;15:95.
Steinke T, Moritz S, Beck S, Gnewuch C, Kees MG. Estimation of creatinine clearance using plasma creatinine or cystatin C: a secondary analysis of two pharmacokinetic studies in surgical ICU patients. BMC Anesthesiol. 2015;15:62.
Huttner A, Von Dach E, Renzoni A, Huttner BD, Affaticati M, Pagani L, et al. Augmented renal clearance, low beta-lactam concentrations and clinical outcomes in the critically ill: an observational prospective cohort study. Int J Antimicrob Agents. 2015;45(4):385–92.
Carlier M, Dumoulin A, Janssen A, Picavet S, Vanthuyne S, Van Eynde R, et al. Comparison of different equations to assess glomerular filtration in critically ill patients. Intensive Care Med. 2015;41(3):427–35.
Adnan S, Ratnam S, Kumar S, Paterson D, Lipman J, Roberts J, et al. Select critically ill patients at risk of augmented renal clearance: experience in a Malaysian intensive care unit. Anaesth Intensive Care. 2014;42(6):715–22.
Baptista JP, Roberts JA, Sousa E, Freitas R, Deveza N, Pimentel J. Decreasing the time to achieve therapeutic vancomycin concentrations in critically ill patients: developing and testing of a dosing nomogram. Crit Care. 2014;18:654.
Campassi ML, Gonzalez MC, Masevicius FD, Vazquez AR, Moseinco M, Navarro NC, et al. Augmented renal clearance in critically ill patients: incidence, associated factors and effects on vancomycin treatment. Rev Bras Ter Intensiva. 2014;26(1):13–20.
Akers KS, Niece KL, Chung KK, Cannon JW, Cota JM, Murray CK. Modified Augmented Renal Clearance score predicts rapid piperacillin and tazobactam clearance in critically ill surgery and trauma patients. J Trauma Acute Care Surg. 2014;77(3 Suppl 2):S163–70.
Udy AA, Jarrett P, Stuart J, Lassig-Smith M, Starr T, Dunlop R, et al. Determining the mechanisms underlying augmented renal drug clearance in the critically ill: use of exogenous marker compounds. Crit Care. 2014;18:657.
Baptista JP, Neves M, Rodrigues L, Teixeira L, Pinho J, Pimentel J. Accuracy of the estimation of glomerular filtration rate within a population of critically ill patients. J Nephrol. 2014;27(4):403–10.
Udy AA, Baptista JP, Lim NL, Joynt GM, Jarret P, Wockner L, et al. Augmented renal clearance in the ICU: results of a multicenter observational study of renal function in critically ill patients with normal plasma creatinine concentrations. Crit Care Med. 2014;42(3):520–7.
Carlier M, Carrette S, Roberts JA, Stove V, Verstraete A, Hoste E, et al. Meropenem and piperacillin/tazobactam prescribing in critically ill patients: does augmented renal clearance affect pharmacokinetic/pharmacodynamic target attainment when extended infusions are used? Crit Care. 2013;17(3):R84.
Udy AA, Morton FJA, Nguyen-Pham S, Jarret P, Lassig-Smith M, Stuart J, et al. A comparison of CKD-EPI estimated glomerular filtration rate and measured creatinine clearance in recently admitted critically ill patients with normal plasma creatinine concentrations. BMC Nephrol. 2013;14:250.
Minkute R, Briedis V, Steponaviciute R, Vitkauskiene A, Maciulaitis R. Augmented renal clearance: an evolving risk factor to consider during the treatment with vancomycin. J Clin Pharm Ther. 2013;38(6):462–7.
Claus BOM, Hoste EA, Colpaert K, Robays H, Decruyenaere J, De Waele JJ. Augmented renal clearance is a common finding with worse clinical outcome in critically ill patients receiving antimicrobial therapy. J Crit Care. 2013;28(5):695–700.
Udy AA, Roberts JA, Shorr AF, Boots RJ, Lipman J. Augmented renal clearance in septic and traumatized patients with normal plasma creatinine concentrations: identifying at-risk patients. Crit Care. 2013;17(1):R35.
Roberts JA, Lipman J. Optimal doripenem dosing simulations in critically ill nosocomial pneumonia patients with obesity, augmented renal clearance, and decreased bacterial susceptibility. Crit Care Med. 2013;41(2):489–95.
Grootaert V, Willems L, Debaveye Y, Meyfroidt G, Spriet I. Augmented renal clearance in the critically ill: how to assess kidney function. Ann Pharmacother. 2012;46:952–9.
Udy AA, Varghese JM, Altukroni M, Briscoe S, McWhinney BC, Ungerer JP, et al. Subtherapeutic initial beta-lactam concentrations in select critically Ill patients: association between augmented renal clearance and low trough drug concentrations. Chest. 2012;142(1):30–9.
Baptista JP, Sousa E, Martins PJ, Pimentel JM. Augmented renal clearance in septic patients and implications for vancomycin optimisation. Int J Antimicrob Agents. 2012;39(5):420–3.
Baptista JP, Udy AA, Sousa E, Pimentel J, Wang L, Roberts JA, et al. A comparison of estimates of glomerular filtration in critically ill patients with augmented renal clearance. Crit Care. 2011;15(3):R139.
Fuster-Lluch O, Geronimo-Pardo M, Peyro-Garcia R, Lizan-Garcia M. Glomerular hyperfiltration and albuminuria in critically ill patients. Anaesth Intensive Care. 2008;36:674–80.
May CC, Arora S, Parli SE, Fraser JF, Thompson Bastin M, Cook AM. Augmented renal clearance in patients with subarachnoid hemorrhage. Neurocrit Care. 2015;23:274–9.
Udy AA, Jarrett P, Lassig-Smith M, Stuart J, Starr T, Dunlop R, et al. Augmented renal clearance in traumatic brain injury: a single-center observational study of atrial natriuretic peptide, cardiac output, and creatinine clearance. J Neurotrauma. 2017;34(1):137–44.
Sporsem H, Lao Y, Von Der Lippe E, Bakke V, Helset E. Vancomycin trough serum concentrations are frequently subtherapeutic in a population of critically ill patients: a prospective observational study. Int J Clin Pharm. 2017;39:217.
Goboova M, Kuzelova M, Fazekas T, Kissova V, Kakosova V, Salkovska L. The impact of therapeutic drug monitoring (TDM) in optimizing dosage regimens of gentamicin in patients with augmented renal clearance. Int J Clin Pharm. 2016;38:596.
Caro L, Larson K, Nicolau D, DeWaele J, Kuti J, Gadzicki E, et al. PK/PD and safety of 3 G ceftolozane/tazobactam in critically ill augmented renal clearance patients. Crit Care Med. 2016;44(12 Suppl 1):241.
Dunning J, Roberts J. Assessment of renal function in dosing antibiotics in septic patients: a survey of current practice within critical care units in England. Anaesthesia. 2015;70:11–91.
Baptista JP, Silva N, Costa E, Fontes F, Marques M, Ribeiro G, et al. Identification of the critically ill patient with augmented renal clearance: make do with what you have! Intensive Care Med. 2014;40(Suppl 1):S110.
Antonucci E, Knoop C, Rondelet B, Beumier M, Wolff F, Vincent JL, et al. Beta-lactams concentrations after lung transplantation. Crit Care Med 2013;41(12):A244–A245.
Neves M, Baptista JP, Rodrigues L, Pinho J, Teixeira L, Pimentel J. Correlation between estimated glomerular filtration rate and measured renal creatinine clearance in critically ill patients with normal serum creatinine. Nephrol Dial Transplant. 2013;28(S1):i345.
Grootaert V, Spriet I, Decoutere L, Debaveye Y, Meyfroidt G, Willems L. Augmented renal clearance in the critically ill: fiction or fact? In J Clin Pharm. 2012;34:143.
Bhattacharyya M, Kumar R, Todi S. Assessment of glomerular filtration rate in trauma patients in early resuscitation phase. Crit Care. 2012;16(S1):S128.
Baptista JP, Teixeira SC, Pimentel J. Are serum cystatin-C-based estimates better than those derived from serum creatinine in critically ill patients? Crit Care. 2012;16(S1):S128.
Drust A, Troger U, Martens-Lobenhoffer J, Tanev I, Braun-Dullaeus C, Bode-Boger SM. Therapeutic drug monitoring of meropenem is mandatory for critically ill patients with glomerular hyperfiltration. Br J Clin Pharmacol. 2011;72(S1):18.
Weigel J, Egal M, Lima A, Koch B, Hunfeld NG, Van Gelder T, et al. Vancomycin is underdosed in patients with high estimated glomerular filtration rate. Intensive Care Med. 2014;40(Suppl 1):S252.
Pham N, Lautrette A, Tixier V, Heng AE, Deteix P, Souweine B. Does glomerular hyperfiltration exist in ICU? Nephron Physiol. 2011;188(S1):11.
Udy AA, Boots R, Sethuran S, et al. Augmented creatinine clearance in traumatic brain injury. Anesth Analg. 2010;111:1505–10.
Loirat P, Rohan J, Baillet A, et al. Increased glomerular filtration rate in patients with major burns and its effect on the pharmacokinetics of tobramycin. N Engl J Med. 1978;299(17):915–9.
Minville V, Asehnoune K, Ruiz S, et al. Increased creatinine clearance in polytrauma patients with normal serum creatinine: a retrospective observational study. Crit Care. 2011;15:R49.
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150.
Sharma A, Mucino MJ, Ronco C. Renal functional reserve and renal recovery after acute kidney injury. Nephron Clin Pract. 2014;127:94–100.
Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, et al. DALI: defining antibiotic levels in intensive care unit patients: are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis. 2014;58:1072–83.
Bergen PJ, Bulitta JB, Kirkpatrick CMJ, Rogers KE, McGregor MJ, Wallis SC, et al. Substantial impact of altered pharmacokinetics in critically ill patients on the antibacterial effects of meropenem evaluated via the dynamic hollow-fiber infection model. Antimicrob Agents Chemother. 2017;61(5):e02642-16.
Bergen PJ, Bulitta JB, Kirkpatrick CM, Rogers KE, McGregor MJ, et al. Effect of different renal function on antibacterial effects of piperacillin against Pseudomonas aeruginosa evaluated via the hollow-fibre infection model and mechanism-based modelling. J Antimicrob Chemother. 2016;71(9):2509–20.
Conil JM, Georges B, Breden A, Segonds C, Lavit M, Seguin T, et al. Increased amikacin dosage requirements in burn patients receiving a once-daily regimen. Int J Antimicrob Agents. 2006;28(3):226–30.
Pai MP, Cojutti P, Pea F. Levofloxacin dosing regimen in severely morbidly obese patients (BMI ≥ 40 kg/m(2)) should be guided by creatinine clearance estimates based on ideal body weight and optimized by therapeutic drug monitoring. Clin Pharmacokinet. 2014;53(8):753–62.
Falcone M, Russo A, Venditti M, Novelli A, Pai MP. Considerations for higher doses of daptomycin in critically ill patients with methicillin-resistant Staphylococcus aureus bacteremia. Clin Infect Dis. 2013;57(11):1568–76.
Bagshaw SM, George C, Bellomo R. Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care. 2008;12(2):R47.
Doi K. Role of kidney injury in sepsis. J Intensive Care. 2016;4:17.
Podoll AS, Kozar R, Holcomb JB, Finkel KW. Incidence and outcome of early acute kidney injury in critically-ill trauma patients. PLoS One. 2013;8(10):e77376.
Brandt MM, Falvo AJ, Rubinfeld IS, Blyden D, Durrani NK, Horst HM. Renal dysfunction in trauma: even a little costs a lot. J Trauma. 2007;62(6):1362–4.
Metnitz PG, Krenn CG, Steltzer H, Lang T, Ploder J, Lenz K, et al. Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med. 2002;30(9):2051–8.
Mandelbaum T, Scott DJ, Lee J, Mark RG, Malhotra A, Waikar SS, et al. Outcome of critically ill patients with acute kidney injury using the acute kidney injury network criteria. Crit Care Med. 2011;39(12):2659–64.
Clermont G, Acker CG, Angus DC, Sirio CA, Pinsky MR, Johnson JP. Renal failure in the ICU: comparison of the impact of acute renal failure and end-stage renal disease on ICU outcomes. Kidney Int. 2002;62(3):986–96.
Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813–8.
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45(3):486–552.
Zelenitsky S, Rubinstein E, Ariano R, Iacovides H, Dodek P, Mirzanejad Y, et al. Vancomycin pharmacodynamics and survival in patients with methicillin-resistant Staphylococcus aureus-associated septic shock. Int J Antimicrob Agents. 2013;41(3):255–60.
Drusano GL, Preston SL, Fowler C, Corrado M, Weisinger B, Kahn J. Relationship between fluoroquinolone area under the curve: minimum inhibitory concentration ratio and the probability of eradication of the infecting pathogen, in patients with nosocomial pneumonia. J Infect Dis. 2004;189(9):1590–7.
Barza M, Ioannidis JP, Cappelleri JC, Lau J. Single or multiple daily doses of aminoglycosides: a meta-analysis. BMJ. 1996;312(7027):338–45.
Lomaestro BM, Drusano GL. Pharmacodynamic evaluation of extending the administration time of meropenem using a Monte Carlo simulation. Antimicrob Agents Chemother. 2005;49(1):461–3.
Zelenitsky SA, Ariano RE, Zhanel GG. Pharmacodynamics of empirical antibiotic monotherapies for an intensive care unit (ICU) population based on Canadian surveillance data. J Antimicrob Chemother. 2011;66(2):343–9.
Martin JH, Norris R, Barras M, Roberts J, Morris R, Doogue M, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society Of Infectious Diseases Pharmacists. Clin Biochem Rev. 2010;31(1):21–4.
Vardakas KZ, Voulgaris GL, Maliaros A, Samonis G, Falagas ME. Prolonged versus short-term intravenous infusion of antipseudomonal β-lactams for patients with sepsis: a systematic review and meta-analysis of randomised trials. Lancet Infect Dis. 2018;18(1):108–20.
Falagas ME, Tansarli GS, Ikawa K, Vardakas KZ. Clinical outcomes with extended or continuous versus short-term intravenous infusion of carbapenems and piperacillin/tazobactam: a systematic review and meta-analysis. Clin Infect Dis. 2013;56(2):272–82.
Kasiakou SK, Sermaides GJ, Michalopoulos A, Soteriades ES, Falagas ME. Continuous versus intermittent intravenous administration of antibiotics: a meta-analysis of randomised controlled trials. Lancet Infect Dis. 2005;5(9):581–9.
Sartelli M, Weber DG, Ruppé E, Bassetti M, Wright BJ, Ansaloni L, et al. Antimicrobials: a global alliance for optimizing their rational use in intra-abdominal infections (AGORA). World J Emerg Surg. 2016;11:33.
Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases society of america and the american thoracic society. Clin Infect Dis. 2016;63(5):e61–111.
EMA/CHMP/413801/2012. European Medicines Agency advises doctors treating patients with nosocomial pneumonia with Doribax. http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2012/06/WC500129087.pdf. Accessed Dec 2017.
Lixiana® summary of product characteristics approved by European Medicines Agency. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002629/WC500189045.pdf. Accessed Dec 2017.
Savaysa® summary of product characteristics approved by Food & Drug Administration. https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206316lbl.pdf. Accessed Dec 2017.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
Idoia Bilbao-Meseguer, Alicia Rodríguez-Gascón, Helena Barrasa, Arantxazu Isla and María Ángeles Solinís have no conflicts of interest that are relevant to the content of this review.
Funding
This study was financially supported by the University of the Basque Country (UPV/EHU) (PPG17/65).
Rights and permissions
About this article
Cite this article
Bilbao-Meseguer, I., Rodríguez-Gascón, A., Barrasa, H. et al. Augmented Renal Clearance in Critically Ill Patients: A Systematic Review. Clin Pharmacokinet 57, 1107–1121 (2018). https://doi.org/10.1007/s40262-018-0636-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40262-018-0636-7