Abstract
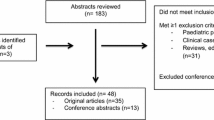
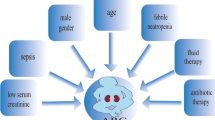
Augmented renal clearance (ARC) is a phenomenon of enhanced renal function seen in critically ill patients. ARC alters the disposition of renally eliminated medications currently used in the intensive care unit, resulting in underdosing and potential therapy failure. Our review addresses the rising concern of inadequate dosing in patients with ARC by summarizing the currently available evidence. To our knowledge, this guide is the first to provide clinicians with dose recommendation insights for renally eliminated agents in adult critically ill patients with ARC. A comprehensive literature search using MEDLINE, Embase, Cochrane Library, CINAHL, Scopus, and ProQuest Dissertations and Theses Global was conducted until 3 November 2021. Screening and data extraction were conducted in two steps: title and abstract screening followed by full-text review. Full text review resulted in a total of 51 studies included in this review. The results demonstrated the need for higher-than-standard doses for meropenem, imipenem, and vancomycin and reduced dosing intervals for ceftriaxone in patients with ARC. The potential need for increased dosing frequency in patients with ARC was also found for both enoxaparin and levetiracetam. In conclusion, ARC has been shown to influence the probability of target attainment in several medications requiring dosing changes to mitigate the risk of therapeutic failure.
Similar content being viewed by others
References
Mahmoud SH, Shen C. Augmented Renal Clearance in Critical Illness: An Important Consideration in Drug Dosing. Pharmaceutics. 2017;9(3). doi:https://doi.org/10.3390/pharmaceutics9030036.
Molina KC, Hall ST, Barletta JF, Mangram AJ, Dzandu JK, Huang V. Utilization of augmented renal clearance in trauma intensive care scoring system to improve vancomycin dosing in trauma patients at risk for augmented renal clearance. Surg Infect. 2020;21(1):43–7. https://doi.org/10.1089/sur.2019.026.
Cojutti PG, Lazzarotto D, Candoni A, Dubbini MV, Zannier ME, Fanin R, et al. Real-time TDM-based optimization of continuous-infusion meropenem for improving treatment outcome of febrile neutropenia in oncohaematological patients: results from a prospective, monocentric, interventional study. J Antimicrob Chemother. 2020;75(10):3029–37. https://doi.org/10.1093/jac/dkaa267.
Hefny F, Stuart A, Kung JY, Mahmoud SH. Prevalence and risk factors of augmented renal clearance: a systematic review and meta-analysis. Pharmaceutics. 2022;14(2). https://doi.org/10.3390/pharmaceutics14020445
Lexicomp. UpToDate, Inc. , Waltham, MA. https://online.lexi.com/lco/action/login.
Al-Shaer MH, Alghamdi WA, Graham E, Peloquin CA. Meropenem, cefepime, and piperacillin protein binding in patient samples. Ther Drug Monit. 2020;42(1):129–32. https://doi.org/10.1097/FTD.0000000000000675.
Roberts JA, Paul SK, Akova M, Bassetti M, De Waele JJ, Dimopoulos G, et al. DALI: defining antibiotic levels in intensive care unit patients: are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis. 2014;58(8):1072–83. https://doi.org/10.1093/cid/ciu027.
Guilhaumou R, Benaboud S, Bennis Y, Dahyot-Fizelier C, Dailly E, Gandia P, et al. Optimization of the treatment with beta-lactam antibiotics in critically ill patients-guidelines from the French Society of Pharmacology and Therapeutics (Societe Francaise de Pharmacologie et Therapeutique-SFPT) and the French Society of Anaesthesia and Intensive Care Medicine (Societe Francaise d’Anesthesie et Reanimation-SFAR). Crit Care. 2019;23(1):104. https://doi.org/10.1186/s13054-019-2378-9.
Kitzes-Cohen R, Farin D, Piva G, De Myttenaere-Bursztein SA. Pharmacokinetics and pharmacodynamics of meropenem in critically ill patients. Int J Antimicrob Agents. 2002;19(2):105–10.
Ehmann L, Zoller M, Minichmayr IK, Scharf C, Maier B, Schmitt MV, et al. Role of renal function in risk assessment of target non-attainment after standard dosing of meropenem in critically ill patients: a prospective observational study. Crit Care. 2017;21:1–14. https://doi.org/10.1186/s13054-017-1829-4.
Tamatsukuri T, Ohbayashi M, Kohyama N, Kobayashi Y, Yamamoto T, Fukuda K, et al. The exploration of population pharmacokinetic model for meropenem in augmented renal clearance and investigation of optimum setting of dose. J Infect Chemotherap. 2018;24(10):834–40. https://doi.org/10.1016/j.jiac.2018.07.007.
Bricheux A, Lenggenhager L, Hughes S, Karmime A, Lescuyer P, Huttner A. Therapeutic drug monitoring of imipenem and the incidence of toxicity and failure in hospitalized patients: a retrospective cohort study. Clin Microbiol Infect 2019;25(3):383.e1-e4. doi:https://doi.org/10.1016/j.cmi.2018.11.020.
Huttner A, Von Dach E, Renzoni A, Huttner BD, Affaticati M, Pagani L, et al. Augmented renal clearance, low beta-lactam concentrations and clinical outcomes in the critically ill: an observational prospective cohort study. Int J Antimicrob Agents. 2015;45(4):385–92. https://doi.org/10.1016/j.ijantimicag.2014.12.017.
Patel M, Bellanti F, Daryani NM, Noormohamed N, Hilbert DW, Young K, et al. Population pharmacokinetic/pharmacodynamic assessment of imipenem/cilastatin/relebactam in patients with hospital-acquired/ventilator-associated bacterial pneumonia. Clin Transl Sci. 2021. https://doi.org/10.1111/cts.13158.
Jamal JA, Mat-Nor MB, Mohamad-Nor FS, Udy AA, Wallis SC, Lipman J, et al. Pharmacokinetics of meropenem in critically ill patients receiving continuous venovenous haemofiltration: a randomised controlled trial of continuous infusion versus intermittent bolus administration. Int J Antimicrob Agents. 2015;45(1):41–5. https://doi.org/10.1016/j.ijantimicag.2014.09.009.
Ollivier J, Carrie C, d'Houdain N, Djabarouti S, Petit L, Xuereb F et al. Are standard dosing regimens of ceftriaxone adapted for critically ill patients with augmented creatinine clearance? Antimicrob Agents Chemotherap. 2019;63(3). doi: https://doi.org/10.1128/AAC.02134-18
Joynt GM, Lipman J, Gomersall CD, Young RJ, Wong EL, Gin T. The pharmacokinetics of once-daily dosing of ceftriaxone in critically ill patients. J Antimicrob Chemother. 2001;47(4):421–9.
Wong G, Briscoe S, McWhinney B, Ally M, Ungerer J, Lipman J, et al. Therapeutic drug monitoring of beta-lactam antibiotics in the critically ill: direct measurement of unbound drug concentrations to achieve appropriate drug exposures. J Antimicrob Chemother. 2018;73(11):3087–94. https://doi.org/10.1093/jac/dky314.
Carrie C, Chadefaux G, Sauvage N, de Courson H, Petit L, Nouette-Gaulain K, et al. Increased beta-lactams dosing regimens improve clinical outcome in critically ill patients with augmented renal clearance treated for a first episode of hospital or ventilator-acquired pneumonia: a before and after study. Crit Care (London, England). 2019;23(1):379. https://doi.org/10.1186/s13054-019-2621-4.
Carrie C, Delzor F, Roure S, Dubuisson V, Petit L, Molimard M et al. Population pharmacokinetic study of the suitability of standard dosing regimens of amikacin in critically ill patients with open-abdomen and negative-pressure wound therapy. Antimicrob Agents Chemother. 2020;64(4). doi: https://doi.org/10.1128/AAC.02098-19
Arechiga-Alvarado NA, Medellin-Garibay SE, Milan-Segovia RDC, Ortiz-Alvarez A, Magana-Aquino M, Romano-Moreno S. Population Pharmacokinetics of amikacin administered once daily in patients with different renal functions. Antimicrob Agents Chemother. 2020;64(5). https://doi.org/10.1128/AAC.02178-19
Bugs & Drugs. ©1998-2020 Alberta Health Service. https://www.bugsanddrugs.org/.
Chu Y, Luo Y, Ji S, Jiang M, Zhou B. Population pharmacokinetics of vancomycin in Chinese patients with augmented renal clearance. J Infect Public Health. 2020;13(1):68–74. https://doi.org/10.1016/j.jiph.2019.06.016.
Chen Y, Liu L, Zhu M. Effect of augmented renal clearance on the therapeutic drug monitoring of vancomycin in patients after neurosurgery. J Int Med Res. 2020;48(10):300060520949076. https://doi.org/10.1177/0300060520949076.
He J, Yang ZT, Qian X, Zhao B, Mao EQ, Chen EZ, et al. A higher dose of vancomycin is needed in critically ill patients with augmented renal clearance. Transl Androl Urol. 2020;9(5):2166–71. https://doi.org/10.21037/tau-20-1048.
Hirai K, Ishii H, Shimoshikiryo T, Shimomura T, Tsuji D, Inoue K, et al. Augmented renal clearance in patients with febrile neutropenia is associated with increased risk for subtherapeutic concentrations of vancomycin. Ther Drug Monit. 2016;38(6):706–10. https://doi.org/10.1097/FTD.0000000000000346.
Chu Y, Luo Y, Qu L, Zhao C, Jiang M. Application of vancomycin in patients with varying renal function, especially those with augmented renal clearance. Pharm Biol. 2016;54(12):2802–6. https://doi.org/10.1080/13880209.2016.1183684.
Minkute R, Briedis V, Steponaviciute R, Vitkauskiene A, Maciulaitis R. Augmented renal clearance—an evolving risk factor to consider during the treatment with vancomycin. J Clin Pharm Ther. 2013;38(6):462–7. https://doi.org/10.1111/jcpt.12088.
Zhao S, He N, Zhang Y, Wang C, Zhai S, Zhang C. Population pharmacokinetic modeling and dose optimization of vancomycin in Chinese patients with augmented renal clearance. Antibiotics (Basel). 2021;10(10). doi:https://doi.org/10.3390/antibiotics10101238.
Baptista JP, Sousa E, Martins PJ, Pimentel JM. Augmented renal clearance in septic patients and implications for vancomycin optimisation. Int J Antimicrob Agents. 2012;39(5):420–3. https://doi.org/10.1016/j.ijantimicag.2011.12.011.
Campassi ML, Gonzalez MC, Masevicius FD, Vazquez AR, Moseinco M, Navarro NC, et al. Augmented renal clearance in critically ill patients: incidence, associated factors and effects on vancomycin treatment. Incremento da depuracao renal em pacientes gravemente enfermos: incidencia, fatores associados e efeitos no tratamento com vancomicina. 2014;26(1):13–20.
Helset E, Nordøy I, Sporsem H, Bakke VD, Bugge JF, Gammelsrud KW, et al. Factors increasing the risk of inappropriate vancomycin therapy in ICU patients: a prospective observational study. Acta Anaesthesiol Scand. 2020;64(9):1295–304. https://doi.org/10.1111/aas.13658.
Baptista JP, Roberts JA, Sousa E, Freitas R, Deveza N, Pimentel J. Decreasing the time to achieve therapeutic vancomycin concentrations in critically ill patients: developing and testing of a dosing nomogram. Crit Care (London, England). 2014;18(6):654. https://doi.org/10.1186/s13054-014-0654-2.
Ishii H, Hirai K, Sugiyama K, Nakatani E, Kimura M, Itoh K. Validation of a nomogram for achieving target trough concentration of vancomycin: accuracy in patients with augmented renal function. Ther Drug Monit. 2018;40(6):693–8. https://doi.org/10.1097/FTD.0000000000000562.
Barrasa H, Soraluce A, Uson E, Sainz J, Martin A, Sanchez-Izquierdo JA, et al. Impact of augmented renal clearance on the pharmacokinetics of linezolid: advantages of continuous infusion from a pharmacokinetic/pharmacodynamic perspective. Int J Infect Dis. 2020;93:329–38. https://doi.org/10.1016/j.ijid.2020.02.044.
Millard J, Pertinez H, Bonnett L, Hodel EM, Dartois V, Johnson JL, et al. Linezolid pharmacokinetics in MDR-TB: a systematic review, meta-analysis and Monte Carlo simulation. J Antimicrob Chemother. 2018;73(7):1755–62. https://doi.org/10.1093/jac/dky096.
Srivastava S, Magombedze G, Koeuth T, Sherman C, Pasipanodya JG, Raj P et al. Linezolid dose that maximizes sterilizing effect while minimizing toxicity and resistance emergence for tuberculosis. Antimicrob Agents Chemother. 2017;61(8). doi:https://doi.org/10.1128/AAC.00751-17.
Alsultan A, Peloquin CA. Therapeutic drug monitoring in the treatment of tuberculosis: an update. Drugs. 2014;74(8):839–54. https://doi.org/10.1007/s40265-014-0222-8.
Wu CC, Tai CH, Liao WY, Wang CC, Kuo CH, Lin SW, et al. Augmented renal clearance is associated with inadequate antibiotic pharmacokinetic/pharmacodynamic target in Asian ICU population: a prospective observational study. Infect Drug Resist. 2019;12:2531–41. https://doi.org/10.2147/IDR.S213183.
Carrie C, Petit L, d’Houdain N, Sauvage N, Cottenceau V, Lafitte M, et al. Association between augmented renal clearance, antibiotic exposure and clinical outcome in critically ill septic patients receiving high doses of beta-lactams administered by continuous infusion: a prospective observational study. Int J Antimicrob Agents. 2018;51(3):443–9. https://doi.org/10.1016/j.ijantimicag.2017.11.013.
Andersen MG, Thorsted A, Storgaard M, Kristoffersson AN, Friberg LE, Obrink-Hansen K. Population Pharmacokinetics of piperacillin in sepsis patients: should alternative dosing strategies be considered? Antimicrob Agents Chemother. 2018;62(5). doi:https:https://doi.org/10.1128/AAC.02306-17.
Weber N, Jackson K, McWhinney B, Ungerer J, Kennedy G, Lipman J, et al. Evaluation of pharmacokinetic/pharmacodynamic and clinical outcomes with 6-hourly empiric piperacillin-tazobactam dosing in hematological malignancy patients with febrile neutropenia. J Infect Chemother. 2019;25(7):503–8. https://doi.org/10.1016/j.jiac.2019.02.014.
Akers KS, Niece KL, Chung KK, Cannon JW, Cota JM, Murray CK. Modified augmented renal clearance score predicts rapid piperacillin and tazobactam clearance in critically ill surgery and trauma patients. J Trauma Acute Care Surg. 2014;77(3 Suppl 2):S163–70. https://doi.org/10.1097/TA.0000000000000191.
Carlier M, Carrette S, Roberts JA, Stove V, Verstraete A, Hoste E, et al. Meropenem and piperacillin/tazobactam prescribing in critically ill patients: does augmented renal clearance affect pharmacokinetic/pharmacodynamic target attainment when extended infusions are used? Crit Care (London, England). 2013;17(3):R84. https://doi.org/10.1186/cc12705.
Carrie C, Legeron R, Petit L, Ollivier J, Cottenceau V, d’Houdain N, et al. Higher than standard dosing regimen are needed to achieve optimal antibiotic exposure in critically ill patients with augmented renal clearance receiving piperacillin-tazobactam administered by continuous infusion. J Crit Care. 2018;48:66–71. https://doi.org/10.1016/j.jcrc.2018.08.026.
Dhaese SAM, Roberts JA, Carlier M, Verstraete AG, Stove V, De Waele JJ. Population pharmacokinetics of continuous infusion of piperacillin in critically ill patients. Int J Antimicrob Agents. 2018;51(4):594–600. https://doi.org/10.1016/j.ijantimicag.2017.12.015.
Udy AA, Dulhunty JM, Roberts JA, Davis JS, Webb SAR, Bellomo R, et al. Association between augmented renal clearance and clinical outcomes in patients receiving beta-lactam antibiotic therapy by continuous or intermittent infusion: a nested cohort study of the BLING-II randomised, placebo-controlled, clinical trial. Int J Antimicrob Agents. 2017;49(5):624–30. https://doi.org/10.1016/j.ijantimicag.2016.12.022.
Nicolau DP, De Waele J, Kuti JL, Caro L, Larson KB, Yu B, et al. Pharmacokinetics and pharmacodynamics of ceftolozane/tazobactam in critically ill patients with augmented renal clearance. Int J Antimicrob Agents. 2021;57(4): 106299. https://doi.org/10.1016/j.ijantimicag.2021.106299.
Shorr AF, Bruno CJ, Zhang Z, Jensen E, Gao W, Feng HP, et al. Ceftolozane/tazobactam probability of target attainment and outcomes in participants with augmented renal clearance from the randomized phase 3 ASPECT-NP trial. Crit Care. 2021;25(1):354. https://doi.org/10.1186/s13054-021-03773-5.
Ley EJ, Brown CVR, Moore EE, Sava JA, Peck K, Ciesla DJ, et al. Updated guidelines to reduce venous thromboembolism in trauma patients: a Western Trauma Association critical decisions algorithm. J Trauma Acute Care Surg. 2020;89(5):971–81. https://doi.org/10.1097/TA.0000000000002830.
Abdel El Naeem HEM, Abdelhamid MHE, Atteya DAM. Impact of augmented renal clearance on enoxaparin therapy in critically ill patients. Egypt J Anaesth. 2017;33(1):113-7. doi:https://doi.org/10.1016/j.egja.2016.11.001.
Hernandez-Mitre MP, Medellin-Garibay SE, Rodriguez-Leyva I, Rodriguez-Pinal CJ, Zarazua S, Jung-Cook HH, et al. Population pharmacokinetics and dosing recommendations of levetiracetam in adult and elderly patients with epilepsy. J Pharm Sci. 2020;109(6):2070–8. https://doi.org/10.1016/j.xphs.2020.02.018.
Jarvie D, Mahmoud SH. Therapeutic drug monitoring of levetiracetam in select populations. J Pharm Pharm Sci. 2018;21(1s):149s-s176. https://doi.org/10.18433/jpps30081.
Ong CLJ, Goh PSJ, Teo MM, Lim TP, Goh KKK, Ang XY, et al. Pharmacokinetics of levetiracetam in neurosurgical ICU patients. J Crit Care. 2021;64:255–61. https://doi.org/10.1016/j.jcrc.2021.04.013.
La MK, Morbitzer KA, Cook A, Hatton-Kolpek J, Jordan JD, Nelson NR, et al. Levetiracetam pharmacokinetics and dose optimization for seizure prophylaxis in TBI. Neurocrit Care. 2018;29(1 Supplement):S106. https://doi.org/10.1007/s12028-018-0606-9.
May C, Arora S, Parli S, Bastin MT, Cook A. Levetiracetam pharmacokinetics in subarachnoid hemorrhage patients with augmented renal clearance: a Monte Carlo simulation. Pharmacotherapy. 2014;34(10):e261–2. https://doi.org/10.1002/phar.1497.
Spencer DD, Jacobi J, Juenke JM, Fleck JD, Kays MB. Steady-state pharmacokinetics of intravenous levetiracetam in neurocritical care patients. Pharmacotherapy. 2011;31(10):934–41. https://doi.org/10.1592/phco.31.10.934.
Bilbao-Meseguer I, Barrasa H, Asin-Prieto E, Alarcia-Lacalle A, Rodriguez-Gascon A, Maynar J et al. Population pharmacokinetics of levetiracetam and dosing evaluation in critically ill patients with normal or augmented renal function. Pharmaceutics. 2021;13(10). doi:https://doi.org/10.3390/pharmaceutics13101690.
Sime FB, Roberts JA, Jeffree RL, Pandey S, Adiraju S, Livermore A, et al. Population pharmacokinetics of levetiracetam in patients with traumatic brain injury and subarachnoid hemorrhage exhibiting augmented renal clearance. Clin Pharmacokinet. 2021;60(5):655–64. https://doi.org/10.1007/s40262-020-00979-8.
Forsberg J, Bedard E, Mahmoud SH. Bioavailability of orally administered drugs in critically ill patients. J Pharm Pract. 2022:8971900221100205. doi:https://doi.org/10.1177/08971900221100205.
Al-Hwiesh A, Alhwiesh A, Abdul-Rahman IS, Al-Harbi A, Mousa D, Skiker S, et al. Meropenem at recommended dose is a potential risk for seizure in hemodialysis patient. Saudi J Kidney Dis Transpl. 2020;31(6):1427–31. https://doi.org/10.4103/1319-2442.308364.
Izumisawa T, Kaneko T, Soma M, Imai M, Wakui N, Hasegawa H, et al. Augmented renal clearance of vancomycin in hematologic malignancy patients. Biol Pharm Bull. 2019;42(12):2089–94. https://doi.org/10.1248/bpb.b19-00652.
Villanueva RD, Talledo O, Neely S, White B, Celii A, Cross A, et al. Vancomycin dosing in critically ill trauma patients: the VANCTIC study. J Trauma Acute Care Surg. 2019;87(5):1164–71. https://doi.org/10.1097/TA.0000000000002492.
Vermis K, Steel E, Vandenbroucke J. Prevalence of augmented renal clearance in haematological patients and the impact on vancomycin dosing. J Oncol Pharm Pract. 2014;20(3 SUPPL. 1):7. https://doi.org/10.1177/1078155214523700.
Chu Y, Luo Y, Jiang M, Zhou B. Application of vancomycin in patients with augmented renal clearance. Eur J Hosp Pharm. 2020;27(5):276–9. https://doi.org/10.1136/ejhpharm-2018-001781.
Mikami R, Imai S, Hayakawa M, Sugawara M, Takekuma Y. Clinical applicability of urinary creatinine clearance for determining the initial dose of vancomycin in critically ill patients. J Infect Chemother. 2022;28(2):199–205. https://doi.org/10.1016/j.jiac.2021.10.008.
Weigel J, Egal M, Lima A, Koch B, Hunfeld NG, Van Gelder T, et al. Vancomycin is underdosed in patients with high estimated glomerular filtration rate. Intensive Care Med. 2014;40(1 SUPPL. 1):S252. https://doi.org/10.1007/s00134-013-3451-5.
Sridharan K, Pasha SAA, Qader AM, Hasan HM, ElSeirafi MM. Drug utilization in critically ill adults with augmented renal clearance compared to normal renal clearance: implications for use of antimicrobials with predominant renal excretion. Curr Clin Pharmacol. 2020. https://doi.org/10.2174/1574884715666200810095225.
Ramos A, Dogliotti A, Pires N, Lovesio C, Latasa D, Perezlindo M et al. Enoxaparin pharmacokinetics in patients with augmented renal clearance, preliminary results of a single center study. Crit Care. 2018;22(Supplement 1). https://doi.org/10.1186/s13054-018-1973-5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
There is no funding associated with this work.
Conflict of interest
All authors declare that they have no conflict of interest.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Data availability
All data generated during this review are included in this published article (and its supplementary information files).
Code availability
Not applicable.
Author contributions
Conceptualization and design, S.H.M.; database search, J.Y.K.; study screening and selection, S.H.M., A.S., S.S.; data extraction and summarization, F.H., C.M., S.S., A.S.; resolution of conflict in study selection and interpretation, S.H.M.; drafting the first version of the manuscript; C.M., S.S.; revision and approval of the final manuscript, all authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hefny, F., Sambhi, S., Morris, C. et al. Drug Dosing in Critically Ill Adult Patients with Augmented Renal Clearance. Eur J Drug Metab Pharmacokinet 47, 607–620 (2022). https://doi.org/10.1007/s13318-022-00779-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-022-00779-4