Abstract
Objectives
The objective of the study was to determine the relative bioavailability of an extended-release multilayer bead formulation of methylphenidate hydrochloride (MPH-MLR) 80 mg vs. methylphenidate immediate-release (IR; Ritalin®) tablets as single and multiple doses in the fed state.
Methods
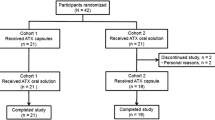
A single-center, multiple-dose, randomized, open-label, two-period crossover study conducted in 26 healthy adults assigned to 4 days of once-daily MPH-MLR 80 mg or IR methylphenidate 25 mg three times daily.
Results
MPH-MLR 80 mg produced reproducible biphasic profiles of plasma methylphenidate concentrations characterized by a rapid initial peak, followed by a moderate decline reaching a plateau ~5 h post dose, then a gradual increase culminating in an attenuated second peak ~7 h post dose. Maximum concentration was lower for MPH-MLR 80 mg than IR methylphenidate 25 mg three times daily on day 1 (23.70 vs. 31.47 ng/mL); exposure was similar. The geometric mean ratios (MPH-MLR/IR methylphenidate [90 % CI]) of log-transformed area under the plasma drug concentration-time curve to the last measurable observation (day 1: 0.88 [84.75–91.80]; day 4: 0.84 [81.16–86.94]), and area under the plasma drug concentration extrapolated to infinity (day 1: 0.93 [88.57–97.28]; day 4: 0.88 [84.48–91.17]) were within the 80–125 % bioequivalence range. The mean ± SD MPH-MLR 80-mg capsule day 4 area under the plasma drug concentration vs. time curve from 0 to 4 h (74.5 ± 15.2 ng·h/mL) was greater than IR methylphenidate 25 mg three times daily (66.0 ± 17.4 ng·h/mL), confirming steady-state levels during the study period. All treatment regimens were safe and well tolerated.
Conclusion
MPH-MLR 80-mg capsule once daily or IR methylphenidate 25 mg three times daily provides comparable maximum methylphenidate concentrations and systemic exposure in the fed state.



Similar content being viewed by others
Notes
Rhodes Pharmaceuticals L.P. has received conditional acceptance from the US Food and Drug Administration to use the name Aptensio XR™ for this extended-release methylphenidate product.
References
Lecendreux M, Konofal E, Faraone SV. Prevalence of attention deficit hyperactivity disorder and associated features among children in France. J Atten Disord. 2011;15:516–24.
Centers for Disease Control and Prevention. Increasing prevalence of parent-reported attention-deficit/hyperactivity disorder among children–United States, 2003 and 2007. MMWR Morb Mortal Wkly Rep. 2010;59:1439–43.
Döpfner M, Breuer D, Wille N, Erhart M, Ravens-Sieberer U. How often do children meet ICD-10/DSM-IV criteria of attention deficit-/hyperactivity disorder and hyperkinetic disorder? Parent-based prevalence rates in a national sample: results of the BELLA study. Eur Child Adolesc Psychiatry. 2008;17(suppl 1):59–70.
Froehlich TE, Lanphear BP, Epstein JN, Barbaresi WJ, Katusic SK, Kahn RS. Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Arch Pediatr Adolesc Med. 2007;161:857–64.
Fayyad J, De Graaf R, Kessler R, et al. Cross-national prevalence and correlates of adult attention-deficit hyperactivity disorder. Br J Psychiatry. 2007;190:402–9.
Kessler RC, Adler L, Barkley R, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163:716–23.
Simon V, Czobor P, Balint S, Mészáros A, Bitter I. Prevalence and correlates of adult attention-deficit hyperactivity disorder: meta-analysis. Br J Psychiatry. 2009;194:204–11.
Greenhill LL, Pliszka S, Dulcan MK, et al. Summary of the practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry. 2001;40:1352–5.
Wolraich M, Brown L, Brown RT, et al. ADHD: clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics. 2011;128:1007–22.
National Institute for Health and Care Excellence. Attention deficit hyperactivity disorder: diagnosis and management of ADHD in children, young people and adults. 2008. http://publications.nice.org.uk/attention-deficit-hyperactivity-disorder-cg72. Accessed 12 Feb 2014.
Rothenberger A, Döpfner M. Editorial: Observational studies in ADHD: the effects of switching to modified-release methylphenidate preparations on clinical outcomes and adherence. Eur Child Adolesc Psychiatry. 2011;20(suppl 2):S235–42.
Markowitz JS, Straughn AB, Patrick KS. Advances in the pharmacotherapy of attention-deficit-hyperactivity disorder: focus on methylphenidate formulations. Pharmacotherapy. 2003;23:1281–99.
Maldonado R. Comparison of the pharmacokinetics and clinical efficacy of new extended-release formulations of methylphenidate. Expert Opin Drug Metab Toxicol. 2013;9:1001–14.
Quillivant XR (methylphenidate hydrochloride) [prescribing information]. New York: NextWave Pharmaceuticals; 2013.
Brams M, Turnbow J, Pestreich L, et al. A randomized, double-blind study of 30 versus 20 mg dexmethylphenidate extended-release in children with attention-deficit/hyperactivity disorder: late-day symptom control. J Clin Psychopharmacol. 2012;32:637–44.
Fischer R, Schutz H, Grossmann M, Leis HJ, Ammer R. Bioequivalence of a methylphenidate hydrochloride extended-release preparation: comparison of an intact capsule and an opened capsule sprinkled on applesauce. Int J Clin Pharmacol Ther. 2006;44:135–41.
González MA, Pentikis HS, Anderl N, et al. Methylphenidate bioavailability from two extended-release formulations. Int J Clin Pharmacol Ther. 2002;40:175–84.
Schutz H, Fischer R, Grossmann M, Mazur D, Leis HJ, Ammer R. Lack of bioequivalence between two methylphenidate extended modified release formulations in healthy volunteers. Int J Clin Pharmacol Ther. 2009;47:761–9.
Swanson J, Gupta S, Guinta D, et al. Acute tolerance to methylphenidate in the treatment of attention deficit hyperactivity disorder in children. Clin Pharmacol Ther. 1999;66:295–305.
Swanson JM, Volkow ND. Pharmacokinetic and pharmacodynamic properties of stimulants: implications for the design of new treatments for ADHD. Behav Brain Res. 2002;130:73–8.
Polli JE, Cook JA, Davit BM, et al. Summary workshop report: facilitating oral product development and reducing regulatory burden through novel approaches to assess bioavailability/bioequivalence. AAPS J. 2012;14:627–38.
Swanson JM, Wigal SB, Wigal T, et al. COMACS Study Group: a comparison of once-daily extended-release methylphenidate formulations in children with attention-deficit/hyperactivity disorder in the laboratory school (the Comacs Study). Pediatrics. 2004;113:e206–16.
Quinn D, Bode T, Reiz JL, Donnelly GA, Darke AC. Single-dose pharmacokinetics of multilayer-release methylphenidate and immediate-release methylphenidate in children with attention-deficit/hyperactivity disorder. J Clin Pharmacol. 2007;47:760–6.
Reiz JL, Donnelly GA, Michalko K. Comparative bioavailability of single-dose methylphenidate from a multilayer-release bead formulation and an osmotic system: a two-way crossover study in healthy young adults. Clin Ther. 2008;30:59–69.
Weiss M, Hechtman L, Turgay A, et al. Once-daily multilayer-release methylphenidate in a double-blind, crossover comparison to immediate-release methylphenidate in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2007;17:675–88.
Schachar R, Ickowicz A, Crosbie J, et al. Cognitive and behavioral effects of multilayer-release methylphenidate in the treatment of children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2008;18:11–24.
Jain U, Hechtman L, Weiss M, et al. Efficacy of a novel biphasic controlled-release methylphenidate formula in adults with attention-deficit/hyperactivity disorder: results of a double-blind, placebo-controlled crossover study. J Clin Psychiatry. 2007;68:268–77.
Metropolitan Life Insurance Company. Metropolitan height and weight tables. Stat Bull Metrop Life Insur Co. 1999.
Adjei A, Teuscher NS, Kupper RJ, et al. Single-dose pharmacokinetics of methylphenidate extended-release administered as intact capsule or sprikles versus methylphenidate immediate-release tablets (Ritalin®) in healthy adult volunteers. J Child Adolesc Psychopharmacol. 2014 (in press).
Greenhill LL. Pharmacologic treatment of attention deficit hyperactivity disorder. Psychiatr Clin North Am. 1992;15:1–27.
Patrick KS, Markowitz JS. Pharmacology of methylphenidate, amphetamine enantiomers and pemoline in attention-deficit hyperactivity disorder. Hum Psychopharmacol. 1997;12:527–46.
Stein MA, Blondis TA, Schnitzler ER, et al. Methylphenidate dosing: twice daily versus three times daily. Pediatrics. 1996;98:748–56.
Greenhill LL, Halperin JM, Abikoff H. Stimulant medications. J Am Acad Child Adolesc Psychiatry. 1999;38:503–12.
Acknowledgments
Dr. Adjei is Executive Director of Product Development at Rhodes Pharmaceuticals L.P. and was Study Director for this study. This study was conducted at Frontage Laboratories, Exton, PA, USA. The authors acknowledge the contribution of Lisa Diamond, PhD, for her contribution to the conduct of this study. Medical writing support was provided by Linda Wagner and Malcolm Darkes, medical writers at Excel Scientific Solutions, and funded by Rhodes Pharmaceuticals L.P.
Conflicts of interest
Dr. Adjei is the Executive Director of Product Development at Rhodes Pharmaceuticals L.P. and was study director for this study. Dr. Kupper is an employee of Rhodes Pharmaceuticals L.P. Dr. Teuscher is a consultant for Rhodes Pharmaceuticals L.P. Dr. Wigal is an advisor board member/consultant/speakers bureau member for Eli Lilly, Ironshore, Neos, NextWave, Noven, NuTec, Pfizer, Purdue, Rhodes Pharmaceuticals L.P., Shionogi, Shire, and Tris and has received grant and research support from Eli Lilly, Forest Laboratories, the National Institutes of Health, NextWave, Noven, NuTec, Rhodes Pharmaceuticals L.P., Shire, and Sunovion. Dr. Sallee is advisory board member/consultant/speakers bureau member for Ironshore, Neos, NextWave, Impax Labs, Otsuka Pharmaceutical Development and Commercialization, Purdue, Rhodes Pharmaceuticals L.P., Shionogi, and Shire, and has received grant and research support from the National Institutes of Health, Rhodes Pharmaceuticals L.P., and Shire. Dr. Sallee has an equity interest in and is member board of directors for P2D Bioscience Inc. Dr. Childress is an advisory board member/consultant/speakers bureau member for Bristol-Myers Squibb, Ironshore, NextWave, Novartis, Pfizer, Shionogi, and Shire, and has received research support from Arbor, Bristol-Myers Squibb, Forest Research Institute, Johnson & Johnson Pharmaceutical Research & Development, Lilly USA, Neos, Neurovance, NextWave, Novartis, Noven, Otsuka, Pfizer, Rhodes Pharmaceuticals L.P., Sepracor, Shionogi, Shire, Sunovion, Theravance, and Tris. Dr. Kollins has received research support and/or consulting fees from the following sources: Akili Interactive, Alcobra, Arbor, Atentiv, the Environmental Protection Agency, the National Institutes of Health (National Institute on Drug Abuse, National Institute of Environmental Health Sciences), Neos, Otsuka, Pfizer, Purdue, Rhodes Pharmaceuticals L.P., Shire, and Tris. Dr. Greenhill has received research support from the National Institutes of Health (National Institute on Drug Abuse) and Shire and is on the advisory board for BioBehavioral Diagnostics.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adjei, A., Kupper, R.J., Teuscher, N.S. et al. Steady-State Bioavailability of Extended-Release Methylphenidate (MPH-MLR) Capsule vs. Immediate-Release Methylphenidate Tablets in Healthy Adult Volunteers. Clin Drug Investig 34, 795–805 (2014). https://doi.org/10.1007/s40261-014-0234-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-014-0234-x