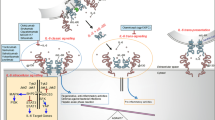
Abstract
Interleukin-6 (IL-6) signaling is a critical target in inflammatory pathways. Today, tocilizumab (TCZ) and sarilumab (SAR), two IL-6 receptor-inhibiting monoclonal antibodies, are widely used in the treatment of rheumatoid arthritis (RA), with a favorable efficacy/safety profile. Successful introduction of such agents in the treatment of RA has encouraged the development of other agents targeting different points of the pathway. Sirukumab (SRK), a human anti-IL-6 monoclonal antibody, has been evaluated in clinical trials and showed largely similar clinical efficacy compared with TCZ and other IL-6 pathway-targeting agents. Furthermore, the drug safety profile seemed to reflect the profile of adverse effects and laboratory abnormalities seen in other inhibitors of the IL-6 pathway. However, increased death rates under SRK treatment compared with placebo raised safety concerns, which led to the decision by the FDA to decline the approval of SRK in August 2017. However, during the 18-week true placebo-controlled period, mortality rates were identical in the placebo- and SRK-treated patients. Comparisons after week 18 may be confounded by some factors, and also the ‘crossover’ design resulted in various treatment groups with varying drug exposure periods. The limited placebo exposure relative to SRK exposure makes interpretation of mortality rates difficult. We do not know whether the imbalance in mortality rates seen for SRK is a true safety signal or a result of bias due to the study design. Therefore, further long-term clinical data as well as basic research is needed to allow deeper insight into IL-6 signaling.




Similar content being viewed by others
References
Schett G. Physiological effects of modulating the interleukin-6 axis. Rheumatology (Oxford). 2018;57:ii43-ii50.
Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–57.
Calabrese LH, Rose-John S. IL-6 biology: implications for clinical targeting in rheumatic disease. Nat Rev Rheumatol. 2014;10:720–7.
Yao X, Huang J, Zhong H, et al. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol Ther. 2014;141:125–39.
Hirano T. Revisiting the 1986 molecular cloning of interleukin 6. Front Immunol. 2014;5:456.
Naka T, Nishimoto N, Kishimoto T. The paradigm of IL-6: from basic science to medicine. Arthritis Res. 2002;4(Suppl 3):S233–42.
Schett G, Elewaut D, McInnes IB, Dayer JM, Neurath MF. How cytokine networks fuel inflammation: toward a cytokine-based disease taxonomy. Nat Med. 2013;19:822–4.
Bethin KE, Vogt SK, Muglia LJ. Interleukin-6 is an essential, corticotropin-releasing hormone-independent stimulator of the adrenal axis during immune system activation. Proc Natl Acad Sci USA. 2000;97:9317–22.
Kraakman MJ, Kammoun HL, Allen TL, et al. Blocking IL-6 trans-signaling prevents high-fat diet-induced adipose tissue macrophage recruitment but does not improve insulin resistance. Cell Metab. 2015;21:403–16.
Rose-John S, Winthrop K, Calabrese L. The role of IL-6 in host defence against infections: immunobiology and clinical implications. Nat Rev Rheumatol. 2017;13:399–409.
Schaper F, Rose-John S. Interleukin-6: Biology, signaling and strategies of blockade. Cytokine Growth Factor Rev. 2015;26:475–87.
Lehmann U, Schmitz J, Weissenbach M, et al. SHP2 and SOCS3 contribute to Tyr-759-dependent attenuation of interleukin-6 signaling through gp130. J Biol Chem. 2003;278:661–71.
Taga T, Hibi M, Hirata Y, et al. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell. 1989;58:573–81.
Mullberg J, Schooltink H, Stoyan T, et al. The soluble interleukin-6 receptor is generated by shedding. Eur J Immunol. 1993;23:473–80.
Heink S, Yogev N, Garbers C, et al. Trans-presentation of IL-6 by dendritic cells is required for the priming of pathogenic TH17 cells. Nat Immunol. 2017;18:74–85.
Scheller J, Rose-John S. The interleukin 6 pathway and atherosclerosis. Lancet. 2012;380:338.
Scheller J, Garbers C, Rose-John S. Interleukin-6: from basic biology to selective blockade of pro-inflammatory activities. Semin Immunol. 2014;26:2–12.
Jones SA, Scheller J, Rose-John S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J Clin Invest. 2011;121:3375–83.
Rafiq S, Frayling TM, Murray A, et al. A common variant of the interleukin 6 receptor (IL-6r) gene increases IL-6r and IL-6 levels, without other inflammatory effects. Genes Immun. 2007;8:552–9.
Ferreira RC, Freitag DF, Cutler AJ, et al. Functional IL6R 358Ala allele impairs classical IL-6 receptor signaling and influences risk of diverse inflammatory diseases. PLoS Genet. 2013;9:e1003444.
Jostock T, Mullberg J, Ozbek S, et al. Soluble gp130 is the natural inhibitor of soluble interleukin-6 receptor transsignaling responses. Eur J Biochem. 2001;268:160–7.
Atreya R, Mudter J, Finotto S, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in Crohn disease and experimental colitis in vivo. Nat Med. 2000;6:583–8.
Hurst SM, Wilkinson TS, McLoughlin RM, et al. Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity. 2001;14:705–14.
Nowell MA, Richards PJ, Horiuchi S, et al. Soluble IL-6 receptor governs IL-6 activity in experimental arthritis: blockade of arthritis severity by soluble glycoprotein 130. J Immunol. 2003;171:3202–9.
Mitsuyama K, Matsumoto S, Rose-John S, et al. STAT3 activation via interleukin 6 trans-signalling contributes to ileitis in SAMP1/Yit mice. Gut. 2006;55:1263–9.
Nowell MA, Williams AS, Carty SA, et al. Therapeutic targeting of IL-6 trans signaling counteracts STAT3 control of experimental inflammatory arthritis. J Immunol. 2009;182:613–22.
Matsumoto S, Hara T, Mitsuyama K, et al. Essential roles of IL-6 trans-signaling in colonic epithelial cells, induced by the IL-6/soluble-IL-6 receptor derived from lamina propria macrophages, on the development of colitis-associated premalignant cancer in a murine model. J Immunol. 2010;184:1543–51.
Becker C, Fantini MC, Schramm C, et al. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21:491–501.
Doganci A, Eigenbrod T, Krug N, et al. The IL-6R alpha chain controls lung CD4 + CD25 + Treg development and function during allergic airway inflammation in vivo. J Clin Invest. 2005;115:313–25.
Hoge J, Yan I, Janner N, et al. IL-6 controls the innate immune response against Listeria monocytogenes via classical IL-6 signaling. J Immunol. 2013;190:703–11.
Sodenkamp J, Waetzig GH, Scheller J, et al. Therapeutic targeting of interleukin-6 trans-signaling does not affect the outcome of experimental tuberculosis. Immunobiology. 2012;217:996–1004.
Safety and efficacy of TJ301 IV in participants with active ulcerative colitis. from https://clinicaltrials.gov/ct2/show/NCT03235752?cond=FE+999301&rank=1. Retrieved 27 May 2018.
Dasgupta B, Corkill M, Kirkham B, Gibson T, Panayi G. Serial estimation of interleukin 6 as a measure of systemic disease in rheumatoid arthritis. J Rheumatol. 1992;19:22–5.
Robak T, Gladalska A, Stepien H, Robak E. Serum levels of interleukin-6 type cytokines and soluble interleukin-6 receptor in patients with rheumatoid arthritis. Mediators Inflamm. 1998;7:347–53.
Houssiau FA, Devogelaer JP, Van Damme J, de Deuxchaisnes CN, Van Snick J. Interleukin-6 in synovial fluid and serum of patients with rheumatoid arthritis and other inflammatory arthritides. Arthritis Rheum. 1988;31:784–8.
Madhok R, Crilly A, Watson J, Capell HA. Serum interleukin 6 levels in rheumatoid arthritis: correlations with clinical and laboratory indices of disease activity. Ann Rheum Dis. 1993;52:232–4.
Straub RH, Muller-Ladner U, Lichtinger T, et al. Decrease of interleukin 6 during the first 12 months is a prognostic marker for clinical outcome during 36 months treatment with disease-modifying anti-rheumatic drugs. Br J Rheumatol. 1997;36:1298–303.
Atsumi T, Ishihara K, Kamimura D, et al. A point mutation of Tyr-759 in interleukin 6 family cytokine receptor subunit gp130 causes autoimmune arthritis. J Exp Med. 2002;196:979–90.
Sasai M, Saeki Y, Ohshima S, et al. Delayed onset and reduced severity of collagen-induced arthritis in interleukin-6-deficient mice. Arthritis Rheum. 1999;42:1635–43.
Hata H, Sakaguchi N, Yoshitomi H, et al. Distinct contribution of IL-6, TNF-alpha, IL-1, and IL-10 to T cell-mediated spontaneous autoimmune arthritis in mice. J Clin Invest. 2004;114:582–8.
Garbers C, Heink S, Korn T, Rose-John S. Interleukin-6: designing specific therapeutics for a complex cytokine. Nat Rev Drug Discov. 2018;17:395–412.
Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8.
McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–42.
Briso EM, Dienz O, Rincon M. Cutting edge: soluble IL-6R is produced by IL-6R ectodomain shedding in activated CD4 T cells. J Immunol. 2008;180:7102–6.
Dominitzki S, Fantini MC, Neufert C, et al. Cutting edge: trans-signaling via the soluble IL-6R abrogates the induction of FoxP3 in naive CD4 + CD25 T cells. J Immunol. 2007;179:2041–5.
Thiolat A, Semerano L, Pers YM, et al. Interleukin-6 receptor blockade enhances CD39 + regulatory T cell development in rheumatoid arthritis and in experimental arthritis. Arthritis Rheumatol. 2014;66:273–83.
Sirukumab Presentation to the Arthritis Advisory Committee August 2, 2017 Janssen R&D, LLC. https://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/arthritisadvisorycommittee/ucm570357.pdf. Retrieved 27 May 2018.
Smolen JS, Weinblatt ME, Sheng S, Zhuang Y, Hsu B. Sirukumab, a human anti-interleukin-6 monoclonal antibody: a randomised, 2-part (proof-of-concept and dose-finding), phase II study in patients with active rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis. 2014;73:1616–25.
Burmester GR, Rubbert-Roth A, Cantagrel A, et al. A randomised, double-blind, parallel-group study of the safety and efficacy of subcutaneous tocilizumab versus intravenous tocilizumab in combination with traditional disease-modifying antirheumatic drugs in patients with moderate to severe rheumatoid arthritis (SUMMACTA study). Ann Rheum Dis. 2014;73:69–74.
Kivitz A, Olech E, Borofsky M, et al. Subcutaneous tocilizumab versus placebo in combination with disease-modifying antirheumatic drugs in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). 2014;66:1653–61.
Abdallah H, Hsu JC, Lu P, et al. Pharmacokinetic and pharmacodynamic analysis of subcutaneous tocilizumab in patients with rheumatoid arthritis from 2 randomized, controlled trials: SUMMACTA and BREVACTA. J Clin Pharmacol. 2017;57:459–68.
Enevold C, Baslund B, Linde L, et al. Interleukin-6-receptor polymorphisms rs12083537, rs2228145, and rs4329505 as predictors of response to tocilizumab in rheumatoid arthritis. Pharmacogenet Genomics. 2014;24:401–5.
Schuster B, Kovaleva M, Sun Y, et al. Signaling of human ciliary neurotrophic factor (CNTF) revisited. The interleukin-6 receptor can serve as an alpha-receptor for CTNF. J Biol Chem. 2003;278:9528–35.
Garbers C, Spudy B, Aparicio-Siegmund S, et al. An interleukin-6 receptor-dependent molecular switch mediates signal transduction of the IL-27 cytokine subunit p28 (IL-30) via a gp130 protein receptor homodimer. J Biol Chem. 2013;288:4346–54.
Miller RG, Bryan WW, Dietz MA, et al. Toxicity and tolerability of recombinant human ciliary neurotrophic factor in patients with amyotrophic lateral sclerosis. Neurology. 1996;47:1329–31.
Petes C, Mintsopoulos V, Finnen RL, Banfield BW, Gee K: The effects of CD14 and IL-27 on induction of endotoxin tolerance in human monocytes and macrophages. J Biol Chem. 2018.
Kalliolias GD, Gordon RA, Ivashkiv LB. Suppression of TNF-alpha and IL-1 signaling identifies a mechanism of homeostatic regulation of macrophages by IL-27. J Immunol. 2010;185:7047–56.
Petes C, Mariani MK, Yang Y, Grandvaux N, Gee K. Interleukin (IL)-6 inhibits IL-27- and IL-30-mediated inflammatory responses in human monocytes. Front Immunol. 2018;9:256.
Zhang C, Xin H, Zhang W, et al. CD5 binds to interleukin-6 and induces a feed-forward loop with the transcription factor STAT3 in B cells to promote cancer. Immunity. 2016;44:913–23.
McFarland-Mancini MM, Funk HM, Paluch AM, et al. Differences in wound healing in mice with deficiency of IL-6 versus IL-6 receptor. J Immunol. 2010;184:7219–28.
Lazzerini PE, Capecchi PL, Guidelli GM, et al. Spotlight on sirukumab for the treatment of rheumatoid arthritis: the evidence to date. Drug Des Devel Ther. 2016;10:3083–98.
Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–57.
Matcham F, Rayner L, Steer S, Hotopf M. The prevalence of depression in rheumatoid arthritis: a systematic review and meta-analysis. Rheumatology (Oxford). 2013;52:2136–48.
Hsu B, Wang D, Sun Y, Chen G. Improvement in measures of depressed mood and anhedonia, and fatigue, in a randomized, placebo-controlled, phase 2 study of sirukumab, a human anti-interleukin-6 antibody, in patients with rheumatoid arthritis. Ann Rheum Dis. 2015;74(Suppl 2):720–1. https://doi.org/10.1136/annrheumdis-2015-eular.4081.
Zhou AJ, Lee Y, Salvadore G, et al. Sirukumab: a potential treatment for mood disorders? Adv Ther. 2017;34:78–90.
Choy EHS, Calabrese LH: Neuroendocrine and neurophysiological effects of interleukin 6 in rheumatoid arthritis. Rheumatology (Oxford). 2017.
An efficacy and safety study of sirukumab in participants with major depressive disorder. https://clinicaltrials.gov/ct2/results?cond=&term=NCT02473289&cntry=&state=&city=&dist=. Retrieved 27 May 2018.
Feaver R, Collado S, Hoang S, et al. The anti-IL-6 antibody sirukumab inhibits vascular inflammation in a human surrogate model of atherosclerosis. Abstract number: 439, ACR/ARHP annual meeting, 2014.
Feaver R, Collado S, Hoang S, et al. Neutralization of IL6 by sirukumab (SIR) inhibits inflammation and cellular stress in a human vascular surrogate system of atherosclerosis. Ann Rheum Dis. 2015;74:444–5. https://doi.org/10.1136/annrheumdis-2015-eular.5132.
Szepietowski JC, Nilganuwong S, Wozniacka A, et al. Phase I, randomized, double-blind, placebo-controlled, multiple intravenous, dose-ascending study of sirukumab in cutaneous or systemic lupus erythematosus. Arthritis Rheum. 2013;65:2661–71.
Rovin BH, van Vollenhoven RF, Aranow C, et al. A multicenter, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of treatment with sirukumab (CNTO 136) in patients with active lupus nephritis. Arthritis Rheumatol. 2016;68:2174–83.
Nishimoto N, Hashimoto J, Miyasaka N, et al. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an X ray reader-blinded randomised controlled trial of tocilizumab. Ann Rheum Dis. 2007;66:1162–7.
Jones G, Sebba A, Gu J, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis. 2010;69:88–96.
Burmester GR, Rigby WF, van Vollenhoven RF, et al. Tocilizumab in early progressive rheumatoid arthritis: FUNCTION, a randomised controlled trial. Ann Rheum Dis. 2016;75:1081–91.
Burmester GR, Rigby WF, van Vollenhoven RF, et al. Tocilizumab combination therapy or monotherapy or methotrexate monotherapy in methotrexate-naive patients with early rheumatoid arthritis: 2-year clinical and radiographic results from the randomised, placebo-controlled FUNCTION trial. Ann Rheum Dis. 2017;76:1279–84.
Kremer JM, Blanco R, Brzosko M, et al. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: results from the double-blind treatment phase of a randomized placebo-controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis Rheum. 2011;63:609–21.
Kremer JM, Blanco R, Halland AM, et al. Clinical efficacy and safety maintained up to 5 years in patients with rheumatoid arthritis treated with tocilizumab in a randomised trial. Clin Exp Rheumatol. 2016;34:625–33.
Gabay C, Emery P, van Vollenhoven R, et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet. 2013;381:1541–50.
Kim GW, Lee NR, Pi RH, et al. IL-6 inhibitors for treatment of rheumatoid arthritis: past, present, and future. Arch Pharm Res. 2015;38:575–84.
Rafique A, Martin J, Blome M, Huang T, Ouyang A, Papadopoulos N. Evaluation of the binding kinetics and functional bioassay activity of sarilumab and tocilizumab to the human IL-6 receptor (IL-6R) alpha. Ann Rheum Dis. 2013;72(Suppl3):797.
Huizinga TW, Fleischmann RM, Jasson M, et al. Sarilumab, a fully human monoclonal antibody against IL-6Ralpha in patients with rheumatoid arthritis and an inadequate response to methotrexate: efficacy and safety results from the randomised SARIL-RA-MOBILITY Part A trial. Ann Rheum Dis. 2014;73:1626–34.
Genovese MC, Fleischmann R, Kivitz AJ, et al. Sarilumab plus methotrexate in patients with active rheumatoid arthritis and inadequate response to methotrexate: results of a phase III study. Arthritis Rheumatol. 2015;67:1424–37.
Strand V, Kosinski M, Chen CI, et al. Sarilumab plus methotrexate improves patient-reported outcomes in patients with active rheumatoid arthritis and inadequate responses to methotrexate: results of a phase III trial. Arthritis Res Ther. 2016;18:198.
Fleischmann R, van Adelsberg J, Lin Y, et al. Sarilumab and nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis and inadequate response or intolerance to tumor necrosis factor inhibitors. Arthritis Rheumatol. 2017;69:277–90.
Burmester GR, Lin Y, Patel R, et al. Efficacy and safety of sarilumab monotherapy versus adalimumab monotherapy for the treatment of patients with active rheumatoid arthritis (MONARCH): a randomised, double-blind, parallel-group phase III trial. Ann Rheum Dis. 2017;76:840–7.
Raimondo MG, Biggioggero M, Crotti C, Becciolini A, Favalli EG. Profile of sarilumab and its potential in the treatment of rheumatoid arthritis. Drug Des Devel Ther. 2017;11:1593–603.
Hamers-Casterman C, Atarhouch T, Muyldermans S, et al. Naturally occurring antibodies devoid of light chains. Nature. 1993;363:446–8.
Rissiek B, Koch-Nolte F, Magnus T. Nanobodies as modulators of inflammation: potential applications for acute brain injury. Front Cell Neurosci. 2014;8:344.
Muyldermans S. Nanobodies: natural single-domain antibodies. Annu Rev Biochem. 2013;82:775–97.
Van Roy M, Ververken C, Beirnaert E, et al. The preclinical pharmacology of the high affinity anti-IL-6R nanobody(R) ALX-0061 supports its clinical development in rheumatoid arthritis. Arthritis Res Ther. 2015;17:135.
Holz JB, Sargentini-Maier L, De Bruyn S, Gachályi B, Udvaros I, Rojkovich B, Bruk S, Sramek P, Korkosz M, Krause K, Schoen P, D’Artois J, Verschueren K, Willems W, De Swert K, Arold G. Twenty-four weeks of treatment with a novel anti-IL-6 receptor nanobody® (aALX-0061) resulted in 84% ACR20 improvement and 58% DAS28 remission in a phase I/II study in RA. Ann Rheum Dis. 2013;72:A64.
http://www.ablynx.com/rd-portfolio/clinical-programmes/vobarilizumab/. Retrieved 07 June 2018.
Mayer CL, Xie L, Bandekar R, et al. Dose selection of siltuximab for multicentric Castleman’s disease. Cancer Chemother Pharmacol. 2015;75:1037–45.
van Rhee F, Wong RS, Munshi N, et al. Siltuximab for multicentric Castleman’s disease: a randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2014;15:966–74.
van Rhee F, Fayad L, Voorhees P, et al. Siltuximab, a novel anti-interleukin-6 monoclonal antibody, for Castleman’s disease. J Clin Oncol. 2010;28:3701–8.
Sitenga J, Aird G, Ahmed A, Silberstein PT. Impact of siltuximab on patient-related outcomes in multicentric Castleman’s disease. Patient Relat Outcome Meas. 2018;9:35–41.
Finch DK, Sleeman MA, Moisan J, et al. Whole-molecule antibody engineering: generation of a high-affinity anti-IL-6 antibody with extended pharmacokinetics. J Mol Biol. 2011;411:791–807.
Study to assess the safety and tolerability of MEDI5117 in rheumatoid arthritis patients. https://clinicaltrials.gov/ct2/results?cond = &term = NCT01559103&cntry = &state = &city = &dist=. Retrieved 28 May 2018.
Mease P, Strand V, Shalamberidze L, et al. A phase II, double-blind, randomised, placebo-controlled study of BMS945429 (ALD518) in patients with rheumatoid arthritis with an inadequate response to methotrexate. Ann Rheum Dis. 2012;71:1183–9.
Zhao Q, Pang J, Shuster D, Hung C, Baglino S, Dodge R, et al. Anti-IL-6 antibody clazakizumab is more potent than tocilizumab in blocking in vitro and ex vivo IL-6-induced functions (abstract). Arthritis Rheum. 2013;65(Suppl):S1020.
Weinblatt ME, Mease P, Mysler E, et al. The efficacy and safety of subcutaneous clazakizumab in patients with moderate-to-severe rheumatoid arthritis and an inadequate response to methotrexate: results from a multinational, phase IIb, randomized, double-blind, placebo/active-controlled, dose-ranging study. Arthritis Rheumatol. 2015;67:2591–600.
Kretsos K, Golor G, Jullion A, et al. Safety and pharmacokinetics of olokizumab, an anti-IL-6 monoclonal antibody, administered to healthy male volunteers: a randomized phase I study. Clin Pharmacol Drug Dev. 2014;3:388–95.
Genovese MC, Fleischmann R, Furst D, et al. Efficacy and safety of olokizumab in patients with rheumatoid arthritis with an inadequate response to TNF inhibitor therapy: outcomes of a randomised Phase IIb study. Ann Rheum Dis. 2014;73:1607–15.
Genovese MC, Durez P, Fleischmann R, Tanaka Y, Furst DE, Yamanaka H, Vasyutin I, Kaviarasu T, Korneva E, Koloda D, Takeuchi T. Olokizumab treatment of both Western and Asian patients with rheumatoid arthritis who have failed anti-TNF treatment results in sustained improvements in patient-reported outcomes (abstract). Arthritis Rheumatol. 2016;68 :10.
Takeuchi T, Tanaka Y, Yamanaka H, et al. Efficacy and safety of olokizumab in Asian patients with moderate-to-severe rheumatoid arthritis, previously exposed to anti-TNF therapy: results from a randomized phase II trial. Mod Rheumatol. 2016;26:15–23.
Evaluation of the effectiveness and safety of two dosing regimens of olokizumab (OKZ), compared to placebo, in subjects with rheumatoid arthritis (RA) who are taking an existing medication called a tumour necrosis factor alpha inhibitor but have active disease. https://clinicaltrials.gov/ct2/results?cond=&term = NCT02760433&cntry = &state = &city = &dist=. Retrieved 28 May 2018.
Evaluation of the effectiveness and safety of two dosing regimens of olokizumab (OKZ), compared to placebo, in subjects with rheumatoid arthritis (RA) who are taking methotrexate but have active disease. from https://clinicaltrials.gov/ct2/results?cond=&term=NCT02760368&cntry=&state=&city=&dist=. Retrieved 28 May 2018.
Evaluation of the effectiveness and safety of two dosing regimens of olokizumab (OKZ), compared to placebo and adalimumab, in subjects with rheumatoid arthritis (RA) who are taking methotrexate but have active disease. https://clinicaltrials.gov/ct2/results?cond=&term=NCT02760407&cntry=&state=&city=&dist=. Retrieved 28 May 2018.
Evaluation of the long term safety, tolerability and efficacy of two dosing regimens of olokizumab (OKZ), in subjects with rheumatoid arthritis (RA) who previously completed 24 weeks of blinded treatment in one of the core studies—CREDO 1, 2 or 3. https://clinicaltrials.gov/ct2/results?cond=&term=NCT03120949&cntry=&state=&city=&dist=. Retrieved 28 May 2018.
Genovese MC, Fleischmann R, Tanaka Y, Furst DE, Yamanaka H, Joshi R, Zhu W, Shao J, Mashimo H, Takeuchi T. Long-term safety and efficacy of olokizumab in patients with moderate-to-severe rheumatoid arthritis who have previously failed anti-TNF treatment. ACR/ARHP annual meeting, 2015.
Open-label study to assess the safety and efficacy of CDP6038 in patients who completed RA0056. https://clinicaltrials.gov/ct2/show/NCT01296711?term=NCT01296711&rank=1. Retrieved 23 Oct 2018.
The long-term safety and efficacy of olokizumab (CDP6038) with active rheumatoid arthritis. from https://clinicaltrials.gov/ct2/show/study/NCT01533714?term=NCT01533714&rank=1. Retrieved 23 Oct 2018.
Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5:520.
Takeuchi T, Yamanaka H, Harigai M, et al. Sirukumab in rheumatoid arthritis refractory to sulfasalazine or methotrexate: a randomized phase 3 safety and efficacy study in Japanese patients. Arthritis Res Ther. 2018;20:42.
Takeuchi T, Thorne C, Karpouzas G, et al. Sirukumab for rheumatoid arthritis: the phase III SIRROUND-D study. Ann Rheum Dis. 2017;76:2001–8.
Aletaha D, Bingham CO 3rd, Tanaka Y, et al. Efficacy and safety of sirukumab in patients with active rheumatoid arthritis refractory to anti-TNF therapy (SIRROUND-T): a randomised, double-blind, placebo-controlled, parallel-group, multinational, phase 3 study. Lancet. 2017;389:1206–17.
Taylor PC, Schiff MH, Wang Q, et al. Efficacy and safety of monotherapy with sirukumab compared with adalimumab monotherapy in biologic-naive patients with active rheumatoid arthritis (SIRROUND-H): a randomised, double-blind, parallel-group, multinational, 52-week, phase 3 study. Ann Rheum Dis. 2018;77:658–66.
Long-term safety and efficacy of sirukumab in participants with RA completing studies CNTO136ARA3002 or CNTO136ARA3003. https://clinicaltrials.gov/ct2/results?cond=&term=NCT01856309&cntry=&state=&city=&dist=. Retrieved 29 May 2018.
Food and Drug Administration, Center for drug evaluation and research, summary minutes of the arthritis advisory committee meeting, 2 August 2017. https://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/arthritisadvisorycommittee/ucm575678.pdf. Retrieved 29 May 2018.
Arthritis Advisory Committee briefing document by Janssen Research & Development for Plivensia™ (sirukumab), 28 June 2017. https://www.fda.gov/downloads/advisorycommittees/committeesmeetingmaterials/drugs/arthritisadvisorycommittee/ucm569153.pdf. Retrieved 29 May 2018.
Aletaha D, Thorne C, Schiff M, Harigai M, Agarwal P, Rao R, Cohen C, Cheng B, Brown K, Hsu B. Integrated phase 3 safety results of sirukumab, an anti-IL-6 cytokine monoclonal antibody, in patients with active rheumatoid arthritis (abstract). Arthritis Rheumatol. 2017; 69 (suppl 10). http://acrabstracts.org/abstract/integrated-phase-3-safety-results-of-sirukumab-an-anti-il-6-cytokine-monoclonal-antibody-in-patients-with-active-rheumatoid-arthritis/. Retrieved 27 May 2018.
Withdrawal assessment report for Plivensia™ (sirukumab), 14 September 2017. http://www.ema.europa.eu/docs/en_GB/document_library/Application_withdrawal_assessment_report/2018/02/WC500243181.pdf. Retrieved 29 May 2018.
Ogdie A, Haynes K, Troxel AB, et al. Risk of mortality in patients with psoriatic arthritis, rheumatoid arthritis and psoriasis: a longitudinal cohort study. Ann Rheum Dis. 2014;73:149–53.
Sparks JA, Chang SC, Liao KP, et al. Rheumatoid arthritis and mortality among women during 36 years of prospective follow-up: results from the Nurses’ Health Study. Arthritis Care Res (Hoboken). 2016;68:753–62.
Zhang Y, Lu N, Peloquin C, et al. Improved survival in rheumatoid arthritis: a general population-based cohort study. Ann Rheum Dis. 2017;76:408–13.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
GRB has received grants from Roche, including travel support, and has received consulting fees/payment for lectures from Roche, Sanofi, and Janssen. EF has received grants, consulting/speaker fees from Roche and Sanofi. ABA has received honoraria for lecturing from MSD.
Funding
No sources of funding were used to support the writing of this manuscript.
Rights and permissions
About this article
Cite this article
Avci, A.B., Feist, E. & Burmester, G.R. Targeting IL-6 or IL-6 Receptor in Rheumatoid Arthritis: What’s the Difference?. BioDrugs 32, 531–546 (2018). https://doi.org/10.1007/s40259-018-0320-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40259-018-0320-3