Abstract
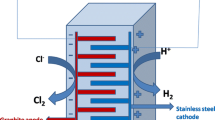
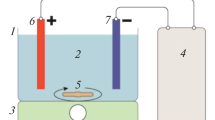
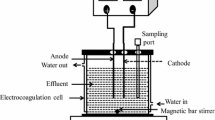
This study surveys the possibility to optimally produce active chlorine from synthetic saline solutions using electrolysis by Response Surface Methodology (RSM). Various operating parameters, such as sodium chloride concentration, electrical potential and electrolysis time were evaluated. Central composite design (CCD) was applied to determine the optimal experimental factors for chlorine production. The experimental design, statistical analysis of the data and optimization were performed using R 3.5.3 software. The results showed that the optimum value of electrical efficiency (42 mg Cl2/kj) was obtained at the electrical voltage of 15.73 V during 15.63 min in the presence of 63.42 g/l of sodium chloride. The optimum point for current efficiency was 38.40%, which was obtained at the electrical voltage of 10.76 V during 6.70 min in the presence of 34.65 g/l of sodium chloride. Moreover, generated active chlorine was optimized based on energy consumption, which was 77 mg/l for the energy consumption of 0.2 kWh/l at a current density of 2000 mA/cm2. The electrochemical production of the chlorine gas from saline or brine water can be extensively used for water disinfection.









Similar content being viewed by others
References
Miranzadeh M, Naderi M, Akbari H, Mahvi A, Past V. Adsorption of arsenic from aqueous solutions by Iron filings and the effect of magnetic field. Int Arch Health Sci. 2016;3:37–42.
Zaviska F, Drogui P, Pablo G. Statistical optimization of active chlorine production from a synthetic saline effluent by electrolysis. Desalination. 2012;296:16–23.
Kraft A. Electrochemical water disinfection: a short review. Platin Met Rev. 2008;52:177–85.
M. Naderi, V. Past, M.B. Miranzadeh, A.H. Mahvi, Survey of the magnetic field effect on arsenic removal from drinking water with and without iron filings, (2017).
M.E. Pulido, Evaluation of an electro-disinfection technology as an alternative to chlorination of municipal wastewater effluents, (2005).
J.D. Key, Development of a small-scale electro-chlorination system for rural water supplies, in, 2010.
Morita C, Sano K, Morimatsu S, Kiura H, Goto T, Kohno T, et al. Disinfection potential of electrolyzed solutions containing sodium chloride at low concentrations. J Virol Methods. 2000;85:163–74.
Spasojević M, Krstajić N, Spasojević P, Ribić-Zelenović L. Modelling current efficiency in an electrochemical hypochlorite reactor. Chem Eng Res Des. 2015;93:591–601.
Hsu G-SW, Lu Y-F, Hsu S-Y. Effects of electrolysis time and electric potential on chlorine generation of electrolyzed deep ocean water. J Food Drug Anal. 2017;25:759–65.
Myers RH, Montgomery DC, Anderson-Cook CM. Response surface methodology: process and product optimization using designed experiments. Hoboken: Wiley; 2016.
Gholami S, Naderi M, Yousefi M, Arjmand MM. The electrochemical removal of bacteria from drinking water. Desalin Water Treat. 2019;160:110–5.
Hsu G-SW, Hsia C-W, Hsu S-Y. Effects of electrode settings on chlorine generation efficiency of electrolyzing seawater. J Food Drug Anal. 2015;23:729–34.
Gholami S, Naderi M, Moghaddam AM. Investigation of the survival of bacteria under the influence of supporting electrolytes KCl, CuI and NaBr in the electrochemical method. Mental Health. 2018;4:104–11.
Lacasa E, Tsolaki E, Sbokou Z, Rodrigo MA, Mantzavinos D, Diamadopoulos E. Electrochemical disinfection of simulated ballast water on conductive diamond electrodes. Chem Eng J. 2013;223:516–23.
Saha J, Gupta SK. A novel electro-chlorinator using low cost graphite electrode for drinking water disinfection. Ionics. 2017:1–11.
Acknowledgments
This study has been funded by the Students’ Scientific Research Center (SSRC), Tehran University of Medical Sciences (Grant Number: 97-03-61-39600). The authors are grateful for the financial support provided by the mentioned center. Also, we appreciate the collaboration of the Department of Environmental Health Engineering Laboratories of the Tehran University of Medical Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Without lowering pH to below 2 and using low-cost and available graphite electrodes, active chlorine was formed in significant amounts
• The electrochemical production of free chlorine follows a first order model
• The results of this study led to the construction of two portable devices producing chlorine gas for water disinfection
Rights and permissions
About this article
Cite this article
Naderi, M., Nasseri, S. Optimization of free chlorine, electric and current efficiency in an electrochemical reactor for water disinfection purposes by RSM. J Environ Health Sci Engineer 18, 1343–1350 (2020). https://doi.org/10.1007/s40201-020-00551-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40201-020-00551-3