Abstract
Postural change during anesthesia has a complex effect on the systemic and cerebral circulations which can potentially decrease cerebral blood flow and oxygenation. Cerebral oximetry is emerging as a monitor of cerebral perfusion with widespread application in many types of surgery. The technology is based on the differential absorption of oxygenated and deoxygenated hemoglobin to near-infrared light. However, the dynamic coupling that exists between cerebral arterial, venous and cerebrospinal fluid volumes may influence oximetric readings during postural change. Interpretation of cerebral oxygen saturation measurement must account for these changes in cerebral physiology if monitoring is to predict neurological outcome.
Similar content being viewed by others
Introduction
Optimal hypotension can be attained by a postural drainage of blood into the lower limbs. This postural hypotension is safe if carefully controlled to a pressure not lower than 55–65 mmHg. at heart level… (Hale Enderby, Royal College of Surgeons, 1952).
Surgery is usually performed with the patient supine, though specific operations require the patient to be placed prone, lateral, head-down or into a sitting position, each of which carry specific risk [1]. The neurosurgical sitting position, in particular, presents a major physiological challenge for the anesthetist, and in recent years it has diminished in use due to the risk of complication [2, 3]. However, this position is widely used by orthopedic surgeons for routine shoulder arthroscopy and cuff repair without any form of cerebral monitoring [4]. Of concern is that the sitting position can dramatically affect the circulation with arterial hypotension increasing the risk of cerebral hypoperfusion and ischemia [5, 6]. Several case reports from Pohl and Cullen [6] raised serious concerns about the safety of the sitting position and focused awareness on its physiological effects and management [7]. Even though the incidence of severe neurological injury in sitting patients is reportedly low [8], an awareness of its physiological effects is necessary to ensure patient safety during general anesthesia [9]. With each sitting patient, the primary intraoperative concern is whether arterial pressure remains adequate for cerebral perfusion.
In 1954 Hale Enderby described the technique of postural ischemia, where surgical bleeding was reduced by placing the patient into a steep reverse Trendelenburg tilt [10]. This head-up tilt, in association with volatile anesthesia and ganglionic blocking drugs, caused significant hypotension with systolic arterial pressure often decreasing to below 70 (and in some cases below 50) mmHg [11]. Importantly, Enderby carefully monitored these low levels of arterial pressure with the use of an oscillometer. Today, hemodynamic monitoring remains a fundamental aspect of anesthetic practice and the technique of induced hypotension remains an established practice though its use in the sitting position has been questioned [12].
Near-infrared spectroscopy (NIRS), a non-invasive optical technology, has emerged as a monitor of cerebral perfusion, and been recently used for surgery performed in the sitting position. Regional cerebral oxygen saturation (ScO2) of the frontal cortex is determined by comparing the specific absorbance patterns of oxygenated and non-oxygenated hemoglobin to near-infrared light [13]. As cerebral blood flow (CBF) decreases, tissue oxygen extraction will increase to maintain cerebral metabolism with an eventual decrease in hemoglobin saturation. In the presence of a stable metabolic rate, ScO2 is therefore an indirect measure of CBF and provides an “index” of organ ischemia [14]. Monitoring ScO2 allows clinically silent episodes of cerebral ischemia to be detected, and the technology has been used extensively, ranging from neonatal intensive care to adult surgery. However, many factors may influence oxygen transport and cerebral oxygen saturation including hematocrit, hemoglobin-O2 binding affinity (P50), inspiratory oxygenation and ventilation. In addition, CBF can be influenced by posture, head positioning and anatomical variation [15, 16].
Neurological monitoring with cerebral oximetry can potentially redefine the management of intraoperative hypoxia and hypotension [17•]. Intraoperative ScO2 measurement provides a therapeutic endpoint for clinical intervention, such as the use of vasopressor therapy to increase cerebral perfusion pressure (CPP). The concept of CPP is central to cerebral protection, and states that CBF is determined primarily by the pressure gradient across the cerebral vascular bed. The driving force for flow equates to the difference between arterial mean pressure and intracranial pressure (ICP) or central venous pressure (CVP). The head elevation debate continues as to whether CBF is more reliant on the perfusion pressure at head level, conceptually viewed as a declining or waterfall gradient; or alternatively, whether flow is best viewed as a continuous column of blood, siphoned into the low-pressured superior vena cava. In clinical practice these views may influence the threshold for intervention when managing hypotension. However without direct measurement of cerebral perfusion, the perception that blood pressure control alone is sufficient to maintain cerebral perfusion in all patients is limited. The clinical assumption that CBF simultaneously increases with arterial pressure elevation cannot be made.
In cardiac surgery, ScO2 monitoring has proven to be a valuable tool in the management of cerebral perfusion during cardiopulmonary bypass, and is predictive of clinical outcome [18]. However in non-cardiac surgery, the role of ScO2 monitoring remains to be validated, especially in surgical patients positioned upright. Upright positioning is known to cause reflex changes in ICP, cerebrovascular resistance (CVR), intracranial compliance (ICC), as well as global and regional changes in CBF. The interpretation of ScO2 measurement must, therefore, account for any physiological change that may alter photon penetration, absorbance or scatter.
Physiological Effects of Posture
In awake individuals, a change from supine to standing rapidly activates neuroendocrine reflexes that regulate blood pressure and maintain adequate cerebral perfusion [19, 20]. The arterial baroreflex responds rapidly—within seconds—to postural change inducing cardiac acceleration and peripheral vasoconstriction. Cerebral autoregulation responds simultaneously with cerebral vasodilation regulating CBF [21]. The coordination between these two responses is not fully understood. With aging, the homeostatic response to postural change declines, and in cardiovascular disease such as hypertension and possibly diabetes, regulation is impaired [22]. Cerebral blood vessels are also richly innervated with adrenergic nerve fibres [23] and recent evidence indicates that extracranial sympathetic nerve activity, intrinsically related to baroreceptor function [24], may influence CBF [25]. Hypotensive-bradycardic episodes (HBE), known to occur in upright shoulder surgery [26], are mediated by autonomic activation via a common mechanism responsible for vasovagal syncope, carotid sinus hypersensitivity and orthostatic syncope [27]. Importantly patients with orthostatic hypotension respond to postural change with larger swings in arterial pressure.
Posture and the Systemic Circulation
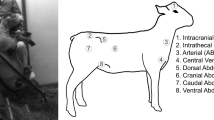
Marked physiological change occurs when an anesthetised patient is tilted from a supine to upright position (Fig. 1). Gravity causes blood to pool into the lower extremities and a shift in intrathoracic blood volume to the extrathoracic space occurs. Venous return is decreased resulting in a significant decrease in cardiac output, systemic vascular resistance and arterial pressure [28]. Echocardiography has demonstrated a significant reduction in left ventricular end-diastolic dimension and middle cerebral artery velocity [29]. Central venous and left-atrial pressure both decrease though anesthetic technique may influence this [30, 31]. Volume loading with crystalloid or colloid fluids do not fully prevent the decrease in cardiac output [31]. Correct positioning, with elevation of the knees to heart level and use of compression stockings [32], can facilitate venous return. Sequential compression of lower limbs significantly reduces hypotension and can support the mean arterial pressure (MAP) and the stroke volume index [33]. Inflation of medical anti-shock trousers (MAST) is a strategy used to increase CVP and jugular venous bulb pressure to reduce the risk of venous air embolism [34]. Anesthetic induction dose, volatile agents and intermittent positive pressure ventilation (IPPV) all aggravate this effect on preload [32]. In sitting patients, systolic blood pressure is reported to decrease by 47 % [3, 35]. In his series of upright positioning, Enderby reported that for each 1.25 cm above the level of the head, local arterial pressure is reduced by ~1 mmHg, resulting in a decrease in CPP approximating 25 mmHg [10].
Hemodynamic and intracranial changes associated with the sitting beachchair position during anesthesia. Source: AO Surgery Reference, www.aosurgery.org. Copyright and permission granted by AO Foundation, Switzerland
Posture and the Cerebral Circulation
The brain is critically dependent on a continuous substrate and oxygen supply, requiring 15 % of cardiac output, and accounting for 20 % of total body oxygen consumption and 25 % of total body glucose utilization [36]. In their classic study, Kety and Schmidt measured CBF as 54 ml min−1 100 g−1, equating to a total CBF of 702 ml min−1. Though subsequent studies have confirmed this value, significant individual variation occurs [37, 38]. With age CBF decreases by 3 ml min−1 per year [39]. The cerebral circulation is controlled by several mechanisms, with autoregulation maintaining a constant blood flow when arterial pressure and CPP are changing [40, 41]. Regional CBF is primarily coupled to metabolic rate, with vascular tone influenced by changes in neural activity, associated substrate production and pH. An increase in arterial carbon dioxide partial pressure (PaCO2) can induce vasodilatation, increasing CBF by 6 % per mmHg change in PaCO2, whilst hypocapnia decreases CBF 3 % per mmHg change in PaCO2 [42]. Control of CBF by sympathetic innervation has been demonstrated but its importance remains debated [43]. Activation is believed to protect the cerebral microcirculation from excessive increases in perfusion pressure and flow and influence cerebral capacitance vessels to regulate ICP and cerebral blood volume [44]. Postural change, including the 30° head-up tilt is commonly used in critical care, to reduce ICP and facilitate cerebral perfusion [45], often in combination with hyperventilation [46].
The brain is supplied by the internal carotid (ICA) and vertebral arteries (VA), which anastomose to form the ring-like arterial Circle of Willis. The anterior ICA circulation contributes 76 % of total flow, and the posterior vertebral system 24 % [37]. Anatomical variation of the cerebral circulation is common, with an incomplete circle of Willis reported in about 50 % of the population, usually involving at least one absent or hypoplastic communicating artery [47]. Congenital variations in the VA have also been described [48]. These variations are associated with asymmetrical flow [49] and in patients with severe ICA stenosis, a risk of stroke and transient ischemic attack is also present [50]. Delayed awakening and hemiparesis following shoulder surgery in the sitting position has been attributed to such variant anatomy [51].
Cerebral Blood Volume
The cerebral blood volume comprises three components: arterial, capillary and venous blood volumes. The arterial: venous blood volume ratio is ~0.3 [52] and capillary blood volume is considered negligible [53]. In healthy subjects, the effect of posture on intracranial physiology has been studied using magnetic resonance imaging (MRI) [54•]. With head elevation there is hydrostatic displacement of cerebrospinal fluid (CSF) from the cranial cavity to the spinal subarachnoid space. A dynamic reduction in cerebral venous blood volume occurs, with a resultant increase in ICC, a decrease in venous outflow pulsatility and an increase in cerebral transit time. These postural changes lower ICP and facilitate cerebral perfusion [55]. Further, hypercapnia can induce vasodilatation and increase arterial blood volume without necessarily a change in venous blood volume [56]. Hypercapnia also affects the circulation regionally with the temporal-occipital cortices tending to vasoconstrict and frontal cortices tending to vasodilate [57].
In supine subjects, venous outflow is primarily via the internal jugular veins. In the sitting position, flow shifts to the vertebral, epidural and deep cervical venous pathways, possibly as a result of the decrease in CSF volume. Postural change results in complete or partial collapse of the internal jugular vein. Venous outflow, in conjunction with CBF, decreases by 12 %. Spontaneous ventilation with inspiratory subatmospheric pressure facilitates cerebral venous return, but this mechanism is attenuated by positive pressure ventilation.
Cerebral Perfusion Pressure
Autoregulation maintains CBF at a constant level in response to changes in CPP. At the lower limit of autoregulation (LLA), maximal cerebral vasodilatation occurs below which CBF becomes pressure passive, decreasing as pressure falls. The threshold for LLA is debated, primarily because significant individual variation exists. In hypertensive patients, LLA is shifted to the right, with higher a CPP required to ensure adequate cerebral perfusion. With ageing, the vascular responsiveness of cerebral arterioles to PaCO2 diminishes [58] and the autoregulatory range also becomes narrowed. Studies in conscious patients have demonstrated ischemic symptoms to occur when arterial pressure decreases to 48 mmHg, but significant individual variation shows the onset of symptoms ranging from 29 to 89 mmHg [59]. Drummond has emphasized that the LLA is significantly higher than a conventional value of 50 mmHg in most patients and should be modified upward to reflect a range of values from 70 to 93 mmHg with a mean value of 80 mmHg [60]. Estimation of LLA should be based on individual resting MAP, with a 25 % decrease proposed as threshold, and a 40–50 % decrease indicative of ischemia [59].
The effective cerebral perfusion pressure (eCPP) is a concept that relates cerebral perfusion to the downstream resistance to flow within the cerebral vasculature [61]. Cerebral resistance vessels collapse when transmural pressure is affected by changes in both ICP and arterial pressure occurring with upright positioning. With small vessel closure, the critical closing pressure (CCP) is defined as the arterial pressure at which CBF becomes zero, and can be can be determined using transcranial Doppler. Simultaneous measurement of middle cerebral artery blood flow velocity (MCAv) and arterial waveforms provides an estimate of eCCP (eCPP = MAP−CCP). Estimates of CCP have been shown to be influenced by arterial PaCO2, ICP, cerebral autoregulation, intra-thoracic pressure, and MAP [62]. In patients undergoing shoulder surgery, McCulloch [35] found the sitting positioning to decrease MAP by 47 % and MCAv by 22 %. Cerebral vasodilatation was associated with a decrease in both the resistance area product (RAP), a measure of cerebrovascular resistance, and the apparent zero flow pressure (AZFP), an estimate of the critical closing pressure. They hypothesized that the decrease in MCAv was due to the dual effect of autoregulatory cerebro-vasodilatation, and a decrease in transmural wall pressure, caused by a lower ICP during beachchair positioning. Such small vessel collapse is considered to partly undermine the “siphon” hypothesis of CBF during upright positioning.
Principles of Cerebral Oximetry
Most biological tissues including bone are transparent to infrared light, and as light penetrates tissue, absorption will occur primarily by hemoglobin and cytochrome molecules. Within the NIR wavelength range 700–1,000 nm, the absorption spectra of oxyhemoglobin and deoxyhemoglobin differ, causing an alteration in the reflected NIRS signal. The ratio of oxygenated to nonoxygenated hemoglobin forms the basis of estimating brain oxygen saturation [63], with measurement derived using an algorithm based on the Beer-Lambert law [64]. Optodes placed on the forehead above the brow, transmit infra-red light, through the skull, to penetrate several centimetres into the frontal cortex [65]. The beam follows a curvilinear path, is subject to absorbance and scatter, and reflected photons are then received by surface detectors. Most devices are continuous wave (CW) monitors with a transmitter-detector distance (usually 4 cm) allowing deeper cortical signals to be separated from superficial extracranial signals [66]. The CW devices measure hemoglobin saturation from the red: infrared ratio, without measuring absolute values of oxy- and deoxy hemoglobin. Increased spatial resolution is achieved by optimising emitter-to-detector separation, using multiple wavelengths and detectors, or by determining absolute chromophore values. Frequency domain (FW) and time-domain approaches estimate ScO2 based on absolute values of oxygenated, deoxygenated and total hemoglobin, and are thought to account for variation in cerebral blood volume [67, 68]. The FW-NIRS devices account for individual scatter using a mathematical model based on photon diffusion theory to calculate tissue oxygen index (TOI) and total hemoglobin index (THI).
Multiple investigations have shown ScO2 to increase with hyperemia, and decrease with ischemia. Indocyanine green, a dye with similar absorbance spectra to hemoglobin, injected into the ICA during angiography increases ScO2 whilst ScO2 was unchanged with external carotid injection indicating detectors received predominately cortical signals [69]. Acute vessel occlusion results in temporal ScO2 desaturation in human and animal studies [70, 71]. The ScO2 desaturation correlates with jugular venous O2 saturation (SjvO2) under conditions of hypoxia and hypercapnia [32, 72, 73]. Functional MRI has shown NIRS measurement to be consistent with changes in deoxyhemoglobin concentration and regional cerebral blood volume [74]. The CBF autoregulation can be assessed using NIRS [75] and an NIRS-based autoregulation index, derived from ICP and blood pressure waveforms, has been developed [76]. Despite these validation studies, NIRS remains fundamentally limited in its application, since a small sample volume of the frontal cortex (1.5 cm3 at a depth of 1.5 cm) is used to represent global cerebral perfusion. Most cerebral regions including the vertebrobasilar arterial system remain unmonitored.
Clinical Experience
Cerebral oximetry has widespread application as a functional monitor of cerebral oxygen saturation during surgery and in the intensive care unit [14]. It has been used in carotid [77], abdominal [78], thoracic [79] and orthopedic surgery (Table 1). In paediatric patients ScO2 has been used to monitor sleep apnea [80] and cerebral autoregulation in preterm infants [81]. The ScO2 monitoring correlates with other monitors of cerebral ischemia, such as transcranial Doppler, somatosensory evoked potentials, and stump pressure during carotid cross-clamp [77, 82–84]. During cardiopulmonary bypass, ScO2 can provide a more rational means for individualizing MAP, since cerebral autoregulation is impaired in 20 % of patients predisposing them to perioperative stroke [85]. The ScO2 can identify the threshold for autoregulation, observed as 66 mmHg in a study of 225 patients (range 43–90 mmHg) [86]. Monitoring cerebral ScO2 during coronary artery bypass can avoid profound cerebral desaturation, and have significantly fewer incidences of major organ dysfunction [87]. In neonates undergoing arterial switch operations, a decreased preoperative ScO2 is associated with decreased Developmental Quotient scores at 30–36 months [88]. In children undergoing congenital heart surgery, perioperative death was associated with baseline cerebral saturation of <50 % [89]. Similarly in adult patients undergoing on-pump cardiac surgery, baseline ScO2 is an independent risk factor for 30-day and 1-year mortality [90]. Preoperatively ScO2 measurement has been used to identify patients with increased risk of cardiac morbidity and autonomic failure [90, 91].
Cerebral ischemia is likely to occur with a regional CBF < 220 ml min−1 (<15 ml 100 g−1 min−1 and CMRO2 < 1.3 ml 100 g−1 min−1) [38, 92]. In conscious patients, the normal ScO2 range is 60–75 %, with a coefficient of variation being 10% and values lower in the elderly [93, 94]. In awake humans, clinical symptoms of pre-syncope occur when cerebral desaturation exceeds 20 % [82, 95] and during carotid endarterectomy, ischemic symptoms reportedly occur with desaturation 13 % from baseline [96]. In cardiac surgery, a prolonged desaturation episode >20 % decline from baseline, or value of <50 %, is associated with a greater risk of postoperative cognitive decline, increased hospital stay [87, 97, 98], and adverse neurological outcome [99]. In animal models of deep hypothermic circulatory arrest, similar thresholds are associated with neurologic outcome. Brain energy depletion is characterized by increased cerebral lactate, and associated ScO2 values of 40–45 %, with levels below 35 % associated with cellular injury. [84, 100–102]. The threshold for cerebral ischemia is often taken as >20 % desaturation from baseline [77].
Validity of ScO2 with Postural Change
The effect of posture on cerebral blood volume and venous composition calls into question the accuracy of ScO2, since readings are primarily a measure of venous hemoglobin saturation (Fig. 2). Positive emission tomography has demonstrated the venous: arterial blood volume ratio, approximating 70 %, to correlate with ScO2 measurement [103]. However this absolute ratio may vary between individuals [43, 104], with hypoxia [105], hemodilution [106] and with changes in PaCO2 [107•]. Importantly head elevation causes a dynamic reduction in cerebral blood volume, with compensatory changes in vascular tone and arterial inflow. This relationship was demonstrated in patients placed in a head up tilt, where CBV and ScO2 both simultaneously decreased (0.07 ml 100 g−1 and 3.5 % respectively). Hyperventilation had a similar effect (0.06 ml 100 g−1 and 2.6 % respectively), presumably from a reduction in arterial inflow. This effect on ScO2 and CBV had no correlation to changes in MAP or cardiac output resulting from intravenous (not volatile inhalational) anesthesia. Similarly, the head down position with pneumoperitoneum during laparoscopy is associated with cerebral desaturation, likely due to changes in ICP, CBF and volume. This effect was more pronounced with propofol compared to sevoflurane [108].
The CW-NIRS devices incorporate algorithms that may not account for changes in venous content or optical pathlength, with resultant overestimation of desaturation values [109]. However CW-ScO2 monitoring has been demonstrated to remain constant with minor head elevation (20°) [110]. The FD-NIRS uses a quantitative approach where resolution of photon scattering enables “absolute” values of TOI and THI to be derived. In head injured patients, TOI and THI both respond to changes in MAP and ICP, and correlate with changes in brain tissue oxygenation [111]. This could indicate that elevation of MAP causes dilatation of arterial vessels, increasing both oxygenated Hb and CBV. However these values are also reliant on relative saturation ratios and whether these remain unaffected by significant changes in cerebral blood volume with posture is unknown.
Vasopressor therapy, often used to maintain CPP, also influences ScO2 measurement. Phenylephrine decreases ScO2 by 7 % in conscious subjects [112] despite an increase in MAP and MCAv. This discordance between CPP and ScO2 was abolished by increasing cerebral metabolic rate with exercise. Cerebral desaturation occurs during anesthesia with phenylephrine [29] and noradrenaline [113•] but not ephedrine [114]. The degree of desaturation with phenylephrine is also modulated by PaCO2 [115]. Since sympathomimetics are unlikely to cross the blood brain barrier, activation of cerebral sympathetic nerves has been postulated as a mechanism of action. In a separate study, where noradrenaline decreased ScO2 by 6 %, and whole body heating increased ScO2, a mechanism of noradrenaline-induced skin vasoconstriction was proposed [116]. The authors suggested that crosstalk between cutaneous and cerebral circulations is an inherent limitation of optode design, amplified by vasopressor therapy. Despite multiple studies showing that extracranial contamination is negligible, discussion remains concerning this phenomenon. This was evidenced in awake patients where desaturation occurred (6.8–18.8 %) from sequential forehead constriction using a pneumatic cuff [117]. Posture and vasopressor therapy together appear to be confounding influences on ScO2 measurement.
The Orthopedic Sitting Position
First reported in 1988 by Skyhar [118], the beachchair position has been adopted as a standard approach for shoulder surgery by many orthopaedic surgeons [119]. The position is deemed to provide superior anatomical orientation, improved surgical access, a lower incidence of brachial plexus neuropraxia from traction, and improved outcome compared to the lateral decubitus position [120]. In its original description, the beachchair position placed the patient in 45° of head elevation, however many surgeons use a more upright position, termed the “steep beachchair” or “barberchair” position [121]. Complications with beachchair positioning were soon reported, including neurological injury [6], HBE [122], and rarer venous air embolism [121]. Concern that the beachchair was an independent risk factor for neurological injury initiated a survey of practice by the American Shoulder and Elbow Society [119]. The results indicated orthopaedic surgeons used the beachchair twice as frequently as the lateral decubitus position, and the reported incidence of neurological injury was 0.0038–0.0046 %. A second survey[4] demonstrated a beachchair incidence of 65 % and a diversity of practice including : induced hypotension 28 %, non-invasive blood pressure correction for height 48 %, and the use of an arterial line in 14 % of patients.
It is apparent that beachchair positioning is practiced widely and safely in many centres [8, 123]. A recent review of 15,014 cases of shoulder arthroscopy over an 11-year period reported the incidence of perioperative neurologic complications to be rare [8]. In this review, patients underwent beachchair surgery in conjunction with regional anesthesia, propofol sedation, and spontaneous respiration via oxygen mask. The MAP was measured non-invasively and maintained at not <80 % of the preoperative value or >60 mmHg. The MAP was not corrected for height. These authors demonstrate the benefit of sedation in minimizing the hypotensive effect of the upright posture and a low incidence of morbidity. The technique of interscalene regional anesthesia with sedation is associated with less hypotension during upright positioning compared to volatile inhalational anaesthesia [124]. However, it may not be appropriate to extrapolate these results to patients receiving general inhalational anaesthesia.
General inhalational anesthesia, with spontaneous or IPPV, is associated with more hypotension in the sitting position compared to sedation, with the response dependant on drug choice and dosage. The increased intrathoracic pressure associated with IPPV is an important contributor to impaired CBF in the seated position [125]. In neurosurgical sitting patients [32] the effect of different anesthetic techniques (nitrous oxide with either enflurane, halothane, fentanyl-droperidol or morphine) on haemodynamic variables were compared [32]. Volatile anesthesia resulted in significant hypotension, and was associated with a reduction in cardiac index and systemic vascular resistance index but likely depends on the volatile agent used in each case. With general anesthesia, hyperventilation can decrease CBF, and volatile agents act to vasodilate cerebral vessels to increase CBF. Compared to isoflurane, sevoflurane preserves the dynamic cerebral pressure autoregulation in humans [126]. Further measures, such as fluid loading and the use of lower limb compression devices, can reduce the incidence of hypotension [127].
Monitoring ScO2 in Beachchair
A number of studies, including case reports, observational studies and a randomized trial have used ScO2 measurement to monitor cerebral oxygenation during beachchair surgery (Table 1) [29, 125, 128–135]. These studies confirm that beachchair positioning is associated with hypotension and ScO2 desaturation, ranging from 0 % to over 80 %. Importantly, anesthetic technique and definition of ScO2 baseline account for the variable incidence. The magnitude of desaturation is very dependent on how a relative change from baseline is measured. Desaturation will vary depending on whether the ScO2 baseline is measured as ScO2 inspiring room air, pre-induction ScO2 with oxygen supplementation or post induction ScO2 with assisted ventilation. Ventilation can significantly affect ScO2 values with 100 % inspiratory oxygen increasing ScO2 by 8 % and further with hypercapnia [136]. The ScO2 levels are elevated post-induction conferring a margin of safety before upright positioning. In the study reporting a desaturation incidence of 80 %, patients received positive pressure ventilation and the relative change was measured from a post-induction oxygenated ScO2 baseline [133]. In contrast, with a sevoflurane-interscalene technique, ScO2 desaturation was present but with ScO2 values returning to physiological levels on air, indicating adequate oxygenation during surgery [29]. Spontaneous ventilation will likely affect CPP differently than PPV, with augmentation of venous return and the effect of permissive hypercapnia on CBF both playing a role. The ScO2 levels are also influenced by choice of induction agent, with propofol and thiopentone increasing ScO2 and etomidate decreasing ScO2 [137]. Interscalene anaesthesia with sedation has significantly less hypotension and episodes of cerebral desaturation. In addition, there is speculation that interscalene anesthesia may influence ScO2 via sympathetic neural blockade.
Ultimately the relevance of desaturation depends on whether there is a significant change from basal levels. No studies were able to detect post-operative neurocognitive impairment despite reported episodes of desaturation. Neurological and neurocognitive outcome relating to ScO2 desaturation is a difficult area to study because the incidence of stroke in non-cardiac surgery is very low [138] and secondly, the reported cases with severe neurological injury related to beachchair surgery is extremely small [6, 119, 139]. However, post operative neurocognitive dysfunction (POCD) is prevalent in surgical patients, and appears to be related to episodes of cerebral desaturation [79, 140, 141] rather than to type of surgery [142]. Many of these studies in Table 1 focused on POCD as a primary endpoint in order to define a critical threshold for ScO2 desaturation.
Clinical Recommendations for Upright Positioning
Preoperative assessment aims to identify patients who may be susceptible to postural hypotension and increased risk of cerebral ischemia. Significant cardiovascular and cerebrovascular disease including carotid artery stenosis, may make such patients unsuitable for upright positioning. In these cases alternative surgical positioning should be sought or a regional technique with minimal sedation considered. Obesity, identified as a risk factor for cerebral desaturation [128] and patients with autonomic failure are more susceptible to postural hypotension [91]. During awake tilt-testing patients with autonomic dysfunction exhibit a critical reduction in cerebral oxygen delivery and ScO2 [143]. Similarly, patients with symptoms suggestive of orthostatic hypotension may be at risk of developing HBE, whilst patients with symptoms of vertebrobasilar insufficiency, termed “salon parlour syncope” require careful assessment. Preoperative ScO2 measurement with tilt-testing may be a valuable screen in such patients [91]. Baseline measurement of MAP, and ScO2 on room air will define individual endpoints for blood pressure management. Pre-induction hydration, leg stockings and intermittent calf compression can minimise venous pooling [33]. Attention to drug dosage, limitation of airway pressure with positive pressure ventilation, avoidance of hyperventilation, and correct positioning with graduated elevation, are recommended.
Blood Pressure Management
Arterial blood pressure management during upright surgery has been a focus of debate since the time of Enderby. In the case series described by Pohl and Cullen, neurological injury was attributed to a decrease in arterial pressure of 28–42 %. Vasopressor therapy is clinically appropriate to treat hypotension when arterial pressure is considered below the LLA. However, determination of this arterial threshold remains difficult since autoregulation exhibits wide individual variation, and the correlation between hypotension and neurological injury is poorly defined. A 30 % decrease in MAP from baseline has been associated with an increased risk of perioperative stroke [144], and during controlled hypotension with electroencephalographic monitoring, a target systolic blood pressure 90–100 mmHg was reported as “safe” [145]. Monitoring requires vigilance, particularly during the process of positioning. Some patients may be more susceptible to developing HBE with positioning and, in these cases, significant hypotension requires immediate intervention. A cautious approach is to target MAP close to baseline levels [7]. Prophylactic administration of vasopresssor by bolus or infusion, before positioning may attenuate the hemodynamic disturbance of the upright posture [29]. Measurement of arterial pressure via the calf is best avoided, and non-invasive arm measurement requires correction for head elevation. Intra-arterial measurement transduced at the tragus, has the advantage of being continuous and indicative of cerebral arterial pressure.
Significant ScO2 desaturation indicates reduced cerebral perfusion, with desaturation >20 % from baseline or an absolute value <50 %, being triggers for intervention. Desaturation can be managed systematically [18] by increasing CPP (with vasopressor or atropine), increasing venous return (leg elevation/compression, fluids), inducing cerebral vasodilatation with hypercapnia, facilitating cerebral venous drainage (adjusting head position, lowering airway pressure) or increasing blood oxygen content (FiO2 and haemoglobin). Asymmetrical desaturation between frontal cortices may indicate atypical cerebrovascular anatomy or vertebrobasilar compression.
Conclusion
Clinical experience has demonstrated cerebral oximetry to be a valid neurological monitor in supine patients. However upright positioning induces a dynamic change in intracranial blood volume and venous content, which artificially affects ScO2 measurement. The evidence indicates this artifact is small, but, nevertheless, requires awareness when interpreting ScO2 measurements during postural change, and with concomitant vasopressor use. Once the upright posture is attained, ScO2 monitoring remains valid to trend changes in cerebral perfusion, provided posture and PaCO2 remain constant. The ScO2 can similarly provide an endpoint for intervention when individual desaturation thresholds are reached. The question remains whether future developments in NIRS technology may account for these dynamic changes related to the sitting posture. Whether cerebral oximetry can identify and improve neurological outcome in noncardiac patients is currently unclear, particularly when the incidence of severe neurological injury is rare. Future studies of ScO2 monitoring may define more subtle injury, such as POCD, arising from episodes of silent ischemia during surgery.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Schubert A. Positioning injuries in anesthesia: an update. Advances in anesthesia. Maryland Heights: Elsevier; 2008. p. 31–65.
Gale T, Leslie K. Anaesthesia for neurosurgery in the sitting position. J Clin Neurosci. 2004;11(7):693–6.
Black S, Ockert DB, Oliver WC Jr, Cucchiara RF. Outcome following posterior fossa craniectomy in patients in the sitting or horizontal positions. Anesthesiology. 1988;69(1):49–56.
Lee L, Bruchas, R, Posner, KL, Caplan, RC, Domino, KB. Proceedings of the 2010 Annual Meeting of the American Society Anesthesiologists. A1133 Blood Pressure Management in the Beachchair Position: National Survey Results.
Rains DD, Rooke GA, Wahl CJ. Pathomechanisms and complications related to patient positioning and anesthesia during shoulder arthroscopy. Arthroscopy. 2010;27(4):532–41.
Pohl A, Cullen DJ. Cerebral ischemia during shoulder surgery in the upright position: a case series. J Clin Anesth. 2005;17(6):463–9.
Lanier W. Special editorial—cerebral perfusion: err on the side of caution. APSF Newsl. 2009;24(1):2–3.
Rohrbaugh M, Kentor ML, Orebaugh SL, Williams B. Outcomes of shoulder surgery in the sitting position with interscalene nerve block: a single-center series. Reg Anesth Pain Med. 2013;38(1):28–33.
Papadonikolakis A, Wiesler ER, Olympio MA, Poehling GG. Avoiding catastrophic complications of stroke and death related to shoulder surgery in the sitting position. Arthroscopy. 2008;24(4):481–2.
Enderby GE. Postural ischaemia and blood-pressure. Lancet. 1954;266(6804):185–7.
Eckenhoff JE, Enderby GE, Larson A, Davies R, Judevine DE. Human cerebral circulation during deliberate hypotension and head-up tilt. J Appl Physiol. 1963;18:1130–8.
Mazzon D, Danelli G, Poole D, Marchini C, Bianchin C. Beach chair position, general anesthesia and deliberated hypotension during shoulder surgery: a dangerous combination! Minerva Anestesiol. 2009;75(5):281–2.
Fox E, Jobsis-Vander Vliet FF, Mitnick MH. Monitoring cerebral oxygen sufficiency in anesthesia and surgery. Adv Exp Med Biol. 1985;191:849–54.
Murkin JM, Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth. 2009;103(Suppl 1):i3–13.
Fuchs G, Schwarz G, Kulier A, Litscher G. The influence of positioning on spectroscopic measurements of brain oxygenation. J Neurosurg Anesthesiol. 2000;12(2):75–80.
Picton P, Chambers J, Shanks A, Dorje P. The influence of inspired oxygen fraction and end-tidal carbon dioxide on post-cross-clamp cerebral oxygenation during carotid endarterectomy under general anesthesia. Anesth Analg. 2010;110(2):581–7.
• Grocott HP. Avoid hypotension and hypoxia: an old anesthetic adage with renewed relevance from cerebral oximetry monitoring. Can J Anaesth 2011 Aug;58(8):697–702. This paper discusses how cerebral oximetry monitoring may optimise arterial pressure management in patients, rather than relying on a generalised approach to blood pressure management.
Denault A, Deschamps A, Murkin JM. A proposed algorithm for the intraoperative use of cerebral near-infrared spectroscopy. Semin Cardiothorac Vasc Anesth. 2007;11(4):274–81.
Wieling W, Van Lieshout JJ, Ten Harkel AD. Dynamics of circulatory adjustments to head-up tilt and tilt-back in healthy and sympathetically denervated subjects. Clin Sci (Lond). 1998;94(4):347–52.
Carey BJ, Manktelow BN, Panerai RB, Potter JF. Cerebral autoregulatory responses to head-up tilt in normal subjects and patients with recurrent vasovagal syncope. Circulation. 2001;104(8):898–902.
Shamsuzzaman AS, Sugiyama Y, Mano T. A comparison of sympathetic vasomotor and cardiovascular responses to head-up tilt and to head-up suspension in humans. Environ Med. 1997;41(2):148–50.
Traon AP, Costes-Salon MC, Galinier M, Fourcade J, Larrue V. Dynamics of cerebral blood flow autoregulation in hypertensive patients. J Neurol Sci. 2002;195(2):139–44.
Edvinsson L, Hamel E. Perivascular nerves in brain vessels. In: Edvinsson LKD, editor. Cerebral blood flow and metabolism. Philidelphia: Lippincott Williams and Wilkins; 2002. p. 43–67.
Cassaglia PA, Griffiths RI, Walker AM. Sympathetic nerve activity in the superior cervical ganglia increases in response to imposed increases in arterial pressure. Am J Physiol Regul Integr Comp Physiol. 2008;294(4):R1255–61.
Sandor P. Nervous control of the cerebrovascular system: doubts and facts. Neurochem Int. 1999;35(3):237–59.
D’Alessio JG, Weller RS, Rosenblum M. Activation of the Bezold-Jarisch reflex in the sitting position for shoulder arthroscopy using interscalene block. Anesth Analg. 1995;80(6):1158–62.
Brignole M, Menozzi C, Del Rosso A, Costa S, Gaggioli G, Bottoni N, et al. New classification of haemodynamics of vasovagal syncope: beyond the VASIS classification. Analysis of the pre-syncopal phase of the tilt test without and with nitroglycerin challenge. Vasovagal Syncope International Study. Europace. 2000;2(1):66–76.
Coonan TJ, Hope CE. Cardio-respiratory effects of change of body position. Can Anaesth Soc J. 1983;30(4):424–38.
Soeding PF, Hoy S, Hoy G, Evans M, Royse CF. Effect of phenylephrine on the haemodynamic state and cerebral oxygen saturation during anaesthesia in the upright position. Br J Anaesth. 2013. doi:10.1093/bja/aet024.
Perkins-Pearson NA, Marshall WK, Bedford RF. Atrial pressures in the seated position: implication for paradoxical air embolism. Anesthesiology. 1982;57(6):493–7.
Buhre W, Weyland A, Buhre K, Kazmaier S, Mursch K, Schmidt M, et al. Effects of the sitting position on the distribution of blood volume in patients undergoing neurosurgical procedures. Br J Anaesth. 2000;84(3):354–7.
Marshall WK, Bedford RF, Miller ED. Cardiovascular responses in the seated position–impact of four anesthetic techniques. Anesth Analg. 1983;62(7):648–53.
Kwak HJ, Lee JS, Lee DC, Kim HS, Kim JY. The effect of a sequential compression device on hemodynamics in arthroscopic shoulder surgery using beach-chair position. Arthroscopy. 2010;26(6):729–33.
Meyer PG, Cuttaree H, Charron B, Jarreau MM, Perie AC, Sainte-Rose C. Prevention of venous air embolism in paediatric neurosurgical procedures performed in the sitting position by combined use of MAST suit and PEEP. Br J Anaesth. 1994;73(6):795–800.
McCulloch TJ, Liyanagama K, Petchell J. Relative hypotension in the beach-chair position: effects on middle cerebral artery blood velocity. Anaesth Intensive Care. 2010;38(3):486–91.
Magistretti PJ, Pellerin L. Cellular mechanisms of brain energy metabolism. Relevance to functional brain imaging and to neurodegenerative disorders. Ann N Y Acad Sci. 1996;777:380–7.
Schoning M, Walter J, Scheel P. Estimation of cerebral blood flow through color duplex sonography of the carotid and vertebral arteries in healthy adults. Stroke. 1994;25(1):17–22.
Soustiel JF, Glenn TC, Vespa P, Rinsky B, Hanuscin C, Martin NA. Assessment of cerebral blood flow by means of blood-flow-volume measurement in the internal carotid artery: comparative study with a 133xenon clearance technique. Stroke. 2003;34(8):1876–80.
Scheel P, Ruge C, Petruch UR, Schoning M. Color duplex measurement of cerebral blood flow volume in healthy adults. Stroke. 2000;31(1):147–50.
Diehl RR. Cerebral autoregulation studies in clinical practice. Eur J Ultrasound. 2002;16(1–2):31–6.
Rozet I, Vavilala MS, Lindley AM, Visco E, Treggiari M, Lam AM. Cerebral autoregulation and CO2 reactivity in anterior and posterior cerebral circulation during sevoflurane anesthesia. Anesth Analg. 2006;102(2):560–4.
Shimosegawa E, Kanno I, Hatazawa J, Fujita H, Iida H, Miura S, et al. Photic stimulation study of changing the arterial partial pressure level of carbon dioxide. J Cereb Blood Flow Metab. 1995;15(1):111–4.
Ito H, Kanno I, Fukuda H. Human cerebral circulation: positron emission tomography studies. Ann Nucl Med. 2005;19(2):65–74.
Auer LM, Edvinsson L, Johansson BB. Effect of sympathetic nerve stimulation and adrenoceptor blockade on pial arterial and venous calibre and on intracranial pressure in the cat. Acta Physiol Scand. 1983;119(3):213–7.
Ng I, Lim J, Wong HB. Effects of head posture on cerebral hemodynamics: its influences on intracranial pressure, cerebral perfusion pressure, and cerebral oxygenation. Neurosurgery. 2004;54(3):593–7.
Gelb AW, Craen RA, Rao GS, Reddy KR, Megyesi J, Mohanty B, et al. Does hyperventilation improve operating condition during supratentorial craniotomy? A multicenter randomized crossover trial. Anesth Analg. 2008;106(2):585–94.
Lippert H, Pabst R. Arterial variations in man: classification and frequency. Munich: J F Bergmann; 1985.
Wang Y, Cai A, Liu L. Sonographic diagnosis of congenital variations of the extracranial vertebral artery and assessment of its circulation. J Ultrasound Med. 2009;28(11):1481–6.
Hendrikse J, van Raamt AF, van der Graaf Y, Mali WP, van der Grond J. Distribution of cerebral blood flow in the circle of Willis. Radiology. 2005;235(1):184–9.
Henderson RD, Eliasziw M, Fox AJ, Rothwell PM, Barnett HJ. Angiographically defined collateral circulation and risk of stroke in patients with severe carotid artery stenosis. North American Symptomatic Carotid Endarterectomy Trial (NASCET) Group. Stroke. 2000;31(1):128–32.
Drummond JC, Lee RR, Howell JP Jr. Focal cerebral ischemia after surgery in the “beach chair” position: the role of a congenital variation of circle of Willis anatomy. Anesth Analg. 2011;114(6):1301–3.
Ito H, Kanno I, Iida H, Hatazawa J, Shimosegawa E, Tamura H, et al. Arterial fraction of cerebral blood volume in humans measured by positron emission tomography. Ann Nucl Med. 2001;15(2):111–6.
Mintun MA, Raichle ME, Martin WR, Herscovitch P. Brain oxygen utilization measured with O-15 radiotracers and positron emission tomography. J Nucl Med. 1984;25(2):177–87.
• Alperin N, Lee SH, Sivaramakrishnan A, Hushek SG. Quantifying the effect of posture on intracranial physiology in humans by MRI flow studies. J Magn Reson Imaging 2005 Nov;22(5):591–6. A remarkable paper investigating the effect of posture on cerebral physiology, by placing volunteers within a vertical gap MRI.
Kenning JA, Toutant SM, Saunders RL. Upright patient positioning in the management of intracranial hypertension. Surg Neurol. 1981;15(2):148–52.
Ito H, Ibaraki M, Kanno I, Fukuda H, Miura S. Changes in the arterial fraction of human cerebral blood volume during hypercapnia and hypocapnia measured by positron emission tomography. J Cereb Blood Flow Metab. 2005;25(7):852–7.
Ito H, Yokoyama I, Iida H, Kinoshita T, Hatazawa J, Shimosegawa E, et al. Regional differences in cerebral vascular response to PaCO2 changes in humans measured by positron emission tomography. J Cereb Blood Flow Metab. 2000;20(8):1264–70.
Ito H, Kanno I, Ibaraki M, Hatazawa J. Effect of aging on cerebral vascular response to Paco2 changes in humans as measured by positron emission tomography. J Cereb Blood Flow Metab. 2002;22(8):997–1003.
Finnerty FA Jr, Witkin L, Fazekas JF. Cerebral hemodynamics during cerebral ischemia induced by acute hypotension. J Clin Invest. 1954;33(9):1227–32.
Drummond JC. The lower limit of autoregulation: time to revise our thinking? Anesthesiology. 1997;86(6):1431–3.
Jagersberg M, Schaller C, Bostrom J, Schatlo B, Kotowski M, Thees C. Simultaneous bedside assessment of global cerebral blood flow and effective cerebral perfusion pressure in patients with intracranial hypertension. Neurocrit Care. 2010;12(2):225–33.
Panerai RB. The critical closing pressure of the cerebral circulation. Med Eng Phys. 2003;25(8):621–32.
Jobsis FF. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 1977;198(4323):1264–7.
Owen-Reece H, Smith M, Elwell CE, Goldstone JC. Near infrared spectroscopy. Br J Anaesth. 1999;82(3):418–26.
McCormick PW, Stewart M, Goetting MG, Dujovny M, Lewis G, Ausman JI. Noninvasive cerebral optical spectroscopy for monitoring cerebral oxygen delivery and hemodynamics. Crit Care Med. 1991;19(1):89–97.
Choi J, Wolf M, Toronov V, Wolf U, Polzonetti C, Hueber D, et al. Noninvasive determination of the optical properties of adult brain: near-infrared spectroscopy approach. J Biomed Opt. 2004;9(1):221–9.
Kurth CD, Thayer WS. A multiwavelength frequency-domain near-infrared cerebral oximeter. Phys Med Biol. 1999;44(3):727–40.
Ijichi S, Kusaka T, Isobe K, Okubo K, Kawada K, Namba M, et al. Developmental changes of optical properties in neonates determined by near-infrared time-resolved spectroscopy. Pediatr Res. 2005;58(3):568–73.
Hongo K, Kobayashi S, Okudera H, Hokama M, Nakagawa F. Noninvasive cerebral optical spectroscopy: depth-resolved measurements of cerebral haemodynamics using indocyanine green. Neurol Res. 1995;17(2):89–93.
Kashiwazaki D, Kuroda S, Terasaka S, Iwasaki Y. Detection of hemodynamic transient ischemic attack during hemodialysis with near-infrared monitoring in a patient with internal carotid artery occlusion. Surg Neurol. 2007;68(3):292–4.
LeMaire SA, Ochoa LN, Conklin LD, Widman RA, Clubb FJ Jr, Undar A, et al. Transcutaneous near-infrared spectroscopy for detection of regional spinal ischemia during intercostal artery ligation: preliminary experimental results. J Thorac Cardiovasc Surg. 2006;132(5):1150–5.
Kim MB, Ward DS, Cartwright CR, Kolano J, Chlebowski S, Henson LC. Estimation of jugular venous O2 saturation from cerebral oximetry or arterial O2 saturation during isocapnic hypoxia. J Clin Monit Comput. 2000;16(3):191–9.
MacLeod DB, Ikeda K, Vacchiano C, Lobbestael A, Wahr JA, Shaw AD. Development and validation of a cerebral oximeter capable of absolute accuracy. J Cardiothorac Vasc Anesth. 2012;26(6):1007–14.
Toronov V, Walker S, Gupta R, Choi JH, Gratton E, Hueber D, et al. The roles of changes in deoxyhemoglobin concentration and regional cerebral blood volume in the fMRI BOLD signal. Neuroimage. 2003;19(4):1521–31.
Steiner LA, Pfister D, Strebel SP, Radolovich D, Smielewski P, Czosnyka M. Near-infrared spectroscopy can monitor dynamic cerebral autoregulation in adults. Neurocrit Care. 2009;10(1):122–8.
Zweifel C, Castellani G, Czosnyka M, Helmy A, Manktelow A, Carrera E, et al. Noninvasive monitoring of cerebrovascular reactivity with near infrared spectroscopy in head-injured patients. J Neurotrauma. 2010;27(11):1951–8.
Samra SK, Dy EA, Welch K, Dorje P, Zelenock GB, Stanley JC. Evaluation of a cerebral oximeter as a monitor of cerebral ischemia during carotid endarterectomy. Anesthesiology. 2000;93(4):964–70.
Casati A, Fanelli G, Pietropaoli P, Proietti R, Tufano R, Montanini S, et al. Monitoring cerebral oxygen saturation in elderly patients undergoing general abdominal surgery: a prospective cohort study. Eur J Anaesthesiol. 2007;24(1):59–65.
Tang L, Kazan R, Taddei R, Zaouter C, Cyr S, Hemmerling TM. Reduced cerebral oxygen saturation during thoracic surgery predicts early postoperative cognitive dysfunction. Br J Anaesth. 2012;108(4):623–9.
Tobias JD. Cerebral oximetry monitoring with near infrared spectroscopy detects alterations in oxygenation before pulse oximetry. J Intensive Care Med. 2008;23(6):384–8.
Wong FY, Leung TS, Austin T, Wilkinson M, Meek JH, Wyatt JS, et al. Impaired autoregulation in preterm infants identified by using spatially resolved spectroscopy. Pediatrics. 2008;121(3):e604–11.
Moritz S, Kasprzak P, Arlt M, Taeger K, Metz C. Accuracy of cerebral monitoring in detecting cerebral ischemia during carotid endarterectomy: a comparison of transcranial Doppler sonography, near-infrared spectroscopy, stump pressure, and somatosensory evoked potentials. Anesthesiology. 2007;107(4):563–9.
Duffy CM, Manninen PH, Chan A, Kearns CF. Comparison of cerebral oximeter and evoked potential monitoring in carotid endarterectomy. Can J Anaesth. 1997;44(10):1077–81.
Pennekamp CW, Bots ML, Kappelle LJ, Moll FL, de Borst GJ. The value of near-infrared spectroscopy measured cerebral oximetry during carotid endarterectomy in perioperative stroke prevention. A review. Eur J Vasc Endovasc Surg. 2009;38(5):539–45.
Ono M, Joshi B, Brady K, Easley RB, Zheng Y, Brown C, et al. Risks for impaired cerebral autoregulation during cardiopulmonary bypass and postoperative stroke. Br J Anaesth. 2012;109(3):391–8.
Joshi B, Ono M, Brown C, Brady K, Easley RB, Yenokyan G, et al. Predicting the limits of cerebral autoregulation during cardiopulmonary bypass. Anesth Analg. 2012;114(3):503–10.
Murkin JM, Adams SJ, Novick RJ, Quantz M, Bainbridge D, Iglesias I, et al. Monitoring brain oxygen saturation during coronary bypass surgery: a randomized, prospective study. Anesth Analg. 2007;104(1):51–8.
Toet MC, Flinterman A, Laar I, Vries JW, Bennink GB, Uiterwaal CS, et al. Cerebral oxygen saturation and electrical brain activity before, during, and up to 36 h after arterial switch procedure in neonates without pre-existing brain damage: its relationship to neurodevelopmental outcome. Exp Brain Res. 2005;165(3):343–50.
Fenton KN, Freeman K, Glogowski K, Fogg S, Duncan KF. The significance of baseline cerebral oxygen saturation in children undergoing congenital heart surgery. Am J Surg. 2005;190(2):260–3.
Heringlake M, Garbers C, Kabler JH, Anderson I, Heinze H, Schon J, et al. Preoperative cerebral oxygen saturation and clinical outcomes in cardiac surgery. Anesthesiology. 2011;114(1):58–69.
Harms MP, Colier WN, Wieling W, Lenders JW, Secher NH, van Lieshout JJ. Orthostatic tolerance, cerebral oxygenation, and blood velocity in humans with sympathetic failure. Stroke. 2000;31(7):1608–14.
Powers WJ, Grubb RL Jr, Darriet D, Raichle ME. Cerebral blood flow and cerebral metabolic rate of oxygen requirements for cerebral function and viability in humans. J Cereb Blood Flow Metab. 1985;5(4):600–8.
Kishi K, Kawaguchi M, Yoshitani K, Nagahata T, Furuya H. Influence of patient variables and sensor location on regional cerebral oxygen saturation measured by INVOS 4100 near-infrared spectrophotometers. J Neurosurg Anesthesiol. 2003;15(4):302–6.
Thavasothy M, Broadhead M, Elwell C, Peters M, Smith M. A comparison of cerebral oxygenation as measured by the NIRO 300 and the INVOS 5100 near-infrared spectrophotometers. Anaesthesia. 2002;57(10):999–1006.
Szufladowicz E, Maniewski R, Kozluk E, Zbiec A, Nosek A, Walczak F. Near-infrared spectroscopy in evaluation of cerebral oxygenation during vasovagal syncope. Physiol Meas. 2004;25(4):823–36.
Al-Rawi PG, Kirkpatrick PJ. Tissue oxygen index: thresholds for cerebral ischemia using near-infrared spectroscopy. Stroke. 2006;37(11):2720–5.
Slater JP, Guarino T, Stack J, Vinod K, Bustami RT, Brown JM III, et al. Cerebral oxygen desaturation predicts cognitive decline and longer hospital stay after cardiac surgery. Ann Thorac Surg. 2009;87(1):36–44.
Hong SW, Shim JK, Choi YS, Kim DH, Chang BC, Kwak YL. Prediction of cognitive dysfunction and patients’ outcome following valvular heart surgery and the role of cerebral oximetry. Eur J Cardiothorac Surg. 2008;33(4):560–5.
Fischer GW, Lin HM, Krol M, Galati MF, Di Luozzo G, Griepp RB, et al. Noninvasive cerebral oxygenation may predict outcome in patients undergoing aortic arch surgery. J Thorac Cardiovasc Surg. 2011;141(3):815–21.
Sakamoto T, Hatsuoka S, Stock UA, Duebener LF, Lidov HG, Holmes GL, et al. Prediction of safe duration of hypothermic circulatory arrest by near-infrared spectroscopy. J Thorac Cardiovasc Surg. 2001;122(2):339–50.
Sakamoto T, Zurakowski D, Duebener LF, Lidov HG, Holmes GL, Hurley RJ, et al. Interaction of temperature with hematocrit level and pH determines safe duration of hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 2004;128(2):220–32.
Kurth CD, Levy WJ, McCann J. Near-infrared spectroscopy cerebral oxygen saturation thresholds for hypoxia-ischemia in piglets. J Cereb Blood Flow Metab. 2002;22(3):335–41.
Ohmae E, Ouchi Y, Oda M, Suzuki T, Nobesawa S, Kanno T, et al. Cerebral hemodynamics evaluation by near-infrared time-resolved spectroscopy: correlation with simultaneous positron emission tomography measurements. Neuroimage. 2006;29(3):697–705.
Watzman HM, Kurth CD, Montenegro LM, Rome J, Steven JM, Nicolson SC. Arterial and venous contributions to near-infrared cerebral oximetry. Anesthesiology. 2000;93(4):947–53.
Wolff CB, Imray CH. Partitioning of arterial and venous volumes in the brain under hypoxic conditions. Adv Exp Med Biol. 2003;540:19–23.
Yoshitani K, Kawaguchi M, Iwata M, Sasaoka N, Inoue S, Kurumatani N, et al. Comparison of changes in jugular venous bulb oxygen saturation and cerebral oxygen saturation during variations of haemoglobin concentration under propofol and sevoflurane anaesthesia. Br J Anaesth. 2005;94(3):341–6.
• Meng L, Mantulin WW, Alexander BS, Cerussi AE, Tromberg BJ, Yu Z, et al. Head-up tilt and hyperventilation produce similar changes in cerebral oxygenation and blood volume: an observational comparison study using frequency-domain near-infrared spectroscopy. Can J Anaesth 2012;59(4):357–65. This paper demonstrates cerebral desaturation occurring in association with changes in cerebral blood volume during head elevation.
Kim SJ, Kwon JY, Cho AR, Kim HK, Kim TK. The effects of sevoflurane and propofol anesthesia on cerebral oxygenation in gynecological laparoscopic surgery. Korean J Anesthesiol. 2010;61(3):225–32.
Pattinson KT, Imray CH, Wright AD. What does cerebral oximetry measure? Br J Anaesth. 2005;94(6):863 author reply 63–4.
Pollard V, Prough DS, DeMelo AE, Deyo DJ, Uchida T, Widman R. The influence of carbon dioxide and body position on near-infrared spectroscopic assessment of cerebral hemoglobin oxygen saturation. Anesth Analg. 1996;82(2):278–87.
Budohoski KP, Zweifel C, Kasprowicz M, Sorrentino E, Diedler J, Brady KM, et al. What comes first? The dynamics of cerebral oxygenation and blood flow in response to changes in arterial pressure and intracranial pressure after head injury. Br J Anaesth. 2011;108(1):89–99.
Brassard P, Seifert T, Wissenberg M, Jensen PM, Hansen CK, Secher NH. Phenylephrine decreases frontal lobe oxygenation at rest but not during moderately intense exercise. J Appl Physiol. 2010;108(6):1472–8.
• Meng L, Cannesson M, Alexander BS, Yu Z, Kain ZN, Cerussi AE, et al. Effect of phenylephrine and ephedrine bolus treatment on cerebral oxygenation in anaesthetized patients. Br J Anaesth 2011;107(2):209–17. These authors highlight the influence of vasopressor therapy on ScO 2 measurement, an area of current investigation.
Nissen P, Brassard P, Jorgensen TB, Secher NH. Phenylephrine but not ephedrine reduces frontal lobe oxygenation following anesthesia-induced hypotension. Neurocrit Care. 2010;12(1):17–23.
Meng L, Gelb AW, Alexander BS, Cerussi AE, Tromberg BJ, Yu Z, et al. Impact of phenylephrine administration on cerebral tissue oxygen saturation and blood volume is modulated by carbon dioxide in anaesthetized patients. Br J Anaesth. 2012;108(5):815–22.
Sorensen H, Secher NH, Siebenmann C, Nielsen HB, Kohl-Bareis M, Lundby C, et al. Cutaneous vasoconstriction affects near-infrared spectroscopy determined cerebral oxygen saturation during administration of norepinephrine. Anesthesiology. 2012;117(2):263–70.
Davie SN, Grocott HP. Impact of extracranial contamination on regional cerebral oxygen saturation: a comparison of three cerebral oximetry technologies. Anesthesiology. 2012;116(4):834–40.
Skyhar MJ, Altchek DW, Warren RF, Wickiewicz TL, O’Brien SJ. Shoulder arthroscopy with the patient in the beach-chair position. Arthroscopy. 1988;4(4):256–9.
Friedman DJ, Parnes NZ, Zimmer Z, Higgins LD, Warner JJ. Prevalence of cerebrovascular events during shoulder surgery and association with patient position. Orthopedics. 2009;32(4):256.
Wakim E, Beaufils P. Arthroscopy of the shoulder with the patient in beach-chair position. Rev Chir Orthop Reparatrice Appar Mot. 1991;77(8):577–80.
Peruto CM, Ciccotti MG, Cohen SB. Shoulder arthroscopy positioning: lateral decubitus versus beach chair. Arthroscopy. 2009;25(8):891–6.
Liguori GA, Kahn RL, Gordon J, Gordon MA, Urban MK. The use of metoprolol and glycopyrrolate to prevent hypotensive/bradycardic events during shoulder arthroscopy in the sitting position under interscalene block. Anesth Analg. 1998;87(6):1320–5.
Yadeau JT, Casciano M, Liu SS, Edmonds CR, Gordon M, Stanton J, et al. Stroke, regional anesthesia in the sitting position, and hypotension: a review of 4,169 ambulatory surgery patients. Reg Anesth Pain Med. 2011;36(5):430–5.
Soeding PF, Wang J, Hoy G, Jarman P, Phillips H, Marks P, et al. The effect of the sitting upright or ‘beachchair’ position on cerebral blood flow during anaesthesia for shoulder surgery. Anaesth Intensive Care. 2011;39(3):440–8.
Yadeau JT, Liu SS, Bang H, Shaw PM, Wilfred SE, Shetty T, et al. Cerebral oximetry desaturation during shoulder surgery performed in a sitting position under regional anesthesia. Can J Anaesth. 2011;58(11):986–92.
Summors AC, Gupta AK, Matta BF. Dynamic cerebral autoregulation during sevoflurane anesthesia: a comparison with isoflurane. Anesth Analg. 1999;88(2):341–5.
Kwak HJ, Lee JS, Lee DC, Kim HS, Kim JY. The effect of a sequential compression device on hemodynamics in arthroscopic shoulder surgery using beach-chair position. Arthroscopy. 2010;26(6):729–33.
Salazar D, Sears B, Aghdasi B, Only A, Francois A, Tonino P, et al. Cerebral desaturation events during shoulder arthroscopy in the beach chair position: patient risk factors and neurocognitive effects. J Shoulder Elbow Surg. 2013. doi:10.1016/j.jse.2012.12.036.
Ko SH, Cho YW, Park SH, Jeong JG, Shin SM, Kang G. Cerebral oxygenation monitoring of patients during arthroscopic shoulder surgery in the sitting position. Korean J Anesthesiol. 2012;63(4):297–301.
Moerman AT, De Hert SG, Jacobs TF, De Wilde LF, Wouters PF. Cerebral oxygen desaturation during beach chair position. Eur J Anaesthesiol. 2012;29(2):82–7.
Jeong H, Lee SH, Jang EA, Chung SS, Lee J, Yoo KY. Haemodynamics and cerebral oxygenation during arthroscopic shoulder surgery in beach chair position under general anaesthesia. Acta Anaesthesiol Scand. 2012;56(7):872–9.
Lee JH, Min KT, Chun YM, Kim EJ, Choi SH. Effects of beach-chair position and induced hypotension on cerebral oxygen saturation in patients undergoing arthroscopic shoulder surgery. Arthroscopy. 2011;27(7):889–94.
Murphy GS, Szokol JW, Marymont JH, Greenberg SB, Avram MJ, Vender JS, et al. Cerebral oxygen desaturation events assessed by near-infrared spectroscopy during shoulder arthroscopy in the beach chair and lateral decubitus positions. Anesth Analg. 2010;111(2):496–505.
Tange K, Kinoshita H, Minonishi T, Hatakeyama N, Matsuda N, Yamazaki M, et al. Cerebral oxygenation in the beach chair position before and during general anesthesia. Minerva Anestesiol. 2010;76(7):485–90.
Koh JL, Levin SD, Chehab EL, Murphy GS. Cerebral oxygenation in the beach chair position: a prospective study on the effect of general anesthesia compared with regional anesthesia and sedation. J Shoulder Elbow Surg. 2013. doi:10.1016/j.jse.2013.01.035.
Picton P, Shanks A, Dorje P, Mashour GA. The influence of basic ventilation strategies on cerebral oxygenation in anesthetized patients without vascular disease. J Clin Monit Comput. 2010. doi:10.1213/ANE.0b013e3181c5f160.
Lovell AT, Owen-Reece H, Elwell CE, Smith M, Goldstone JC. Continuous measurement of cerebral oxygenation by near infrared spectroscopy during induction of anesthesia. Anesth Analg. 1999;88(3):554–8.
Mashour GA, Shanks AM, Kheterpal S. Perioperative stroke and associated mortality after noncardiac, nonneurologic surgery. Anesthesiology. 2011;114(6):1289–96.
Bhatti MT, Enneking FK. Visual loss and ophthalmoplegia after shoulder surgery. Anesth Analg. 2003;96(3):899–902.
Yao FS, Tseng CC, Ho CY, Levin SK, Illner P. Cerebral oxygen desaturation is associated with early postoperative neuropsychological dysfunction in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2004;18(5):552–8.
Papadopoulos G, Karanikolas M, Liarmakopoulou A, Papathanakos G, Korre M, Beris A. Cerebral oximetry and cognitive dysfunction in elderly patients undergoing surgery for hip fractures: a prospective observational study. Open Orthop J. 2012;6:400–5.
Evered L, Scott DA, Silbert B, Maruff P. Postoperative cognitive dysfunction is independent of type of surgery and anesthetic. Anesth Analg. 2011;112(5):1179–85.
Hunt K, Tachtsidis I, Bleasdale-Barr K, Elwell C, Mathias C, Smith M. Changes in cerebral oxygenation and haemodynamics during postural blood pressure changes in patients with autonomic failure. Physiol Meas. 2006;27(9):777–85.
Bijker JB, Persoon S, Peelen LM, Moons KG, Kalkman CJ, Kappelle LJ, et al. Intraoperative hypotension and perioperative ischemic stroke after general surgery: a nested case-control study. Anesthesiology. 2012;116(3):658–64.
Gillespie R, Shishani Y, Streit J, Wanner JP, McCrum C, Syed T, et al. The safety of controlled hypotension for shoulder arthroscopy in the beach-chair position. J Bone Joint Surg Am. 2012;94(14):1284–90.
Acknowledgments
Paul Soeding is supported by a research Grant from the Australian and New Zealand College of Anesthetists (ANZCA) for pulmonary hypertension research and a Jessica Cooper Grant for the use of laboratory equipment for the former.
Ethical Standards
Human and Animal Rights and Informed Consent. This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soeding, P. Effects of Positioning on Cerebral Oxygenation. Curr Anesthesiol Rep 3, 184–196 (2013). https://doi.org/10.1007/s40140-013-0020-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40140-013-0020-y