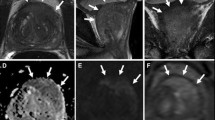
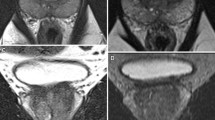
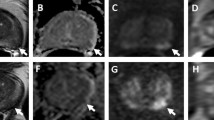
Abstract
Purpose of Review
To highlight the recent evidence about the use of mp-MRI in the local staging of prostate cancer, enhancing the potential benefits compared to the known and more invasive canonical staging methods.
Recent Findings
Multiparametric magnetic resonance (mp-MRI) plays a key role not only in diagnosis, but also in local staging of prostate cancer, as it can assess with high accuracy the extracapsular extension of the tumor, invasion of seminal vesicles, the involvement of lymph nodes and the presence of possible bone metastases.
Summary
Prostate cancer is currently the most common male cancer in Europe and it is associated with a high mortality rate, which is why it is necessary to make an early and accurate diagnosis. Firstly, it is important to distinguish between a disease confined to the gland and a disease with a loco regional or remote extension, since neoplastic staging is the starting point for risk stratification, prognosis and therapeutic planning. According to the PI-RADS guidelines v 2.1 the main mp-MRI sequences necessary to obtain a complete and accurate resonance imaging are the T2-weighted sequences (T2WI), which represent the pivotal morphological sequences for local staging, together with the diffusion-weighted imaging (DWI) and dynamic sequences acquired after intravenous contrast medium administration (DCE). However, due to some limitations, mp-MRI does not have the same diagnostic power in the evaluation of lymph nodes, for which the gold standard remains the extended pelvic nodal dissection (ePLND), nor in the detection of distant metastases. For this last aspect, the next generation imaging foresees the use of whole-body MRI (WB-MRI), especially for the high accuracy in the early detection of local and remote bone metastases.






Similar content being viewed by others
References
Recently published papers of particular interest have been highlighted as: • Of importance •• Of major importance
Mottet N, et al. EAU–ESTRO–SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. EurUrol. 2016. https://doi.org/10.1016/j.eururo.2016.08.003.
Arnold M, Karim-Kos HE, Coebergh JW, et al. Recent trends in incidence of five common cancers in 26 European countries since 1988: analysis of the European Cancer Observatory. Eur J Cancer. 2015;51:1164–87.
Leitzmann MF, Rohrmann S. Risk factors for the onset of prostatic cancer: age, location, and behavioural correlates. ClinEpidemiol. 2012;4:1–11.
Esposito K, Chiodini P, Capuano A, et al. Effect of metabolic syndrome and its components on prostate cancer risk: meta-analysis. J Endocrinol Invest. 2013;36:132–9.
Albright F, Stephenson RA, Agarwal N, et al. Prostate cancer risk prediction based on complete prostate cancer family history. Prostate. 2015;75:390–8.
Hemminki K. Familial risk and familial survival in prostate cancer. World J Urol. 2012;30:143–8.
Castro E, Goh C, Leongamorniert D, et al. Effect of BRCA mutations on metastatic relapse and cause-specific survival after radical treatment for localised prostate cancer. EurUrol. 2015;68:186–93.
Castro E, Goh C, Olmos D, et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol. 2013;31:1748–57.
Bancroft EK, Page EC, Castro E, et al. Targeted prostate cancer screening in BRCA1 and BRCA2 mutation carriers: results from the initial screening round of the IMPACT study. EurUrol. 2014;66:489–99.
Litwin MS, Tan HJ. The diagnosis and treatment of prostate cancer: a review. JAMA. 2017;317:2532e42. https://doi.org/10.1001/jama.2017.7248.
Caglic I, Barrett T. Optimising prostate mpMRI: prepare for success. ClinRadiol. 2019;74(11):831–40. https://doi.org/10.1016/j.crad.2018.12.003.
Ahmed HU, El-ShaterBosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389:815e22. https://doi.org/10.1016/S0140-6736(16)32401-1.
Kasivisvanathan V, Rannikko AS, Borghi M, et al. Collaborators MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. 2018;378:1767e77. https://doi.org/10.1056/NEJMoa1801993.
Moldovan PC, Van den Broeck T, Sylvester R, et al. What is the negative predictive value of multiparametric magnetic resonance imaging in excluding prostate cancer at biopsy? A systematic review and meta- analysis from the European Association of Urology Prostate Cancer Guidelines Panel. EurUrol. 2017;72:250e66. https://doi.org/10.1016/j.eururo.2017.02.026.
Weinreb JC, Barentsz JO, Choyke PL, et al. PI-RADS prostate imaging—reporting and data system: 2015, version 2. Eur Urol 2016;69:16e40. https://doi.org/10.1016/j.eururo.2015.08.052
•• Caglic I, Viljem K, Barrett T. Multiparametric MRI-local staging of prostate cancer and beyond. Radiol Oncol. 2019;53(2):159-70.
BrizmohunAppayya M, Adshead J, Ahmed HU, Allen C, Bainbridge A, Barrett T, et al. National implementation of multi-parametric magnetic resonance imaging for prostate cancer detection—recommendations from a UK consensus meeting. BJU Int. 2018;122:13–25. https://doi.org/10.1111/bju.14361.
Cagiannos I, Karakiewicz P, Eastham JA, Ohori M, Rabbani F, Gerigk C, et al. A preoperative nomogram identifying decreased risk of positive pelvic lymph nodes in patients with prostate cancer. J Urol. 2003;170:1798–803. https://doi.org/10.1097/01.ju.0000091805.98960.13.
Gervasi LA, Mata J, Easley JD, Wilbanks JH, Seale-Hawkins C, Carlton CE, et al. Prognostic significance of lymph nodal metastases in prostate cancer. J Urol. 1989;142(21):332–6. https://doi.org/10.1016/S0022-5347(17)38748-7.
Epstein JI, Partin AW, Potter SR, Walsh PC. Adenocarcinoma of the prostate invading the seminal vesicle: prognostic stratification based on pathologic parameters. Urology. 2000;56:283–8. https://doi.org/10.1016/S0090-4295(00)00640-3.
Rayn KN, Bloom JB, Gold SA, Hale GR, Baiocco JA, Mehralivand S, et al. Added value of multiparametric magnetic resonance imaging to clinical nomograms for predicting adverse pathology in prostate cancer. J Urol. 2018;200:1041–7. https://doi.org/10.1016/j.juro.2018.05.094.
Godoy G, Tareen BU, Lepor H. Site of positive surgical margins influences biochemical recurrence after radical prostatectomy. BJU Int. 2009;104:1610–4. https://doi.org/10.1111/j.1464-410X.2009.08688.x.
Morlacco A, Sharma V, Viers BR, Rangel LJ, Carlson RE, Froemming AT, et al. The incremental role of magnetic resonance imaging for prostate cancer staging before radical prostatectomy. EurUrol. 2017;71:701–4. https://doi.org/10.1016/j.eururo.2016.08.015.
Boehmer D, Maingon P, Poortmans P, Baron M-H, Miralbell R, Remouchamps V, et al. Guidelines for primary radiotherapy of patients with prostate cancer. Radiother Oncol. 2006;79:259–69. https://doi.org/10.1016/j.radonc.2006.05.012.
EAU Guidelines. Edn. presented at the EAU Annual Congress Copenhagen 2018. ISBN 978-94-92671-01-1.
Brierley, J.D., et al. TNM classification of malignant tumors. UICC International Union Against Cancer. 8th edn. 2017. https://www.uicc.org/resources/tnm/publications-resources
Epstein, J.I., et al. The 2005 International Society of Urological Pathology (ISUP) consensus conference on gleason grading of prostatic carcinoma. Am J Surg Pathol. 2005;29:1228. https://www.ncbi.nlm.nih.gov/pubmed/16096414
Epstein, J.I., et al. The 2014 International Society of Urological Pathology (ISUP) consensus conference on gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016;40:244. https://www.ncbi.nlm.nih.gov/pubmed/26492179
Epstein, J.I., et al. A Contemporary Prostate Cancer Grading System: A Validated Alternative to the Gleason Score. Eur Urol, 2016. 69: 428. https://www.ncbi.nlm.nih.gov/pubmed/26166626
Barchetti F, Panebianco V. Multiparametric MRI for recurrent prostate cancer post radical prostatectomy and post-radiation therapy. BioMed Res Int. 2014. https://doi.org/10.1155/2014/316272.
Ventrella E, Eusebi L, Carpagnano FA, et al. Multiparametric MRI of prostate cancer: recent advances. CurrRadiol Rep. 2020;8:19. https://doi.org/10.1007/s40134-020-00363-1.
•• Turkbey B, Rosenkrantz AB, Haider MA, et al. Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur Urol. 2019;76:340–51. Very important because it refers to the last recommendation of PI-RADS, very useful in the daily clinical practice and for standardized reports, especially referring to the local staging of prostate cancer.
Barrett T, Turkbey B, Choyke PL. PI-RADS version 2: what you need to know. ClinRadiol. 2015;70:1165–76. https://doi.org/10.1016/j.crad.2015.06.093.
Turkbey B, Merino MJ, Gallardo EC, Shah V, Aras O, Bernardo M, et al. Comparison of endorectal coil and non endorectal coil T2W and diffusion- weighted MRI at 3 Tesla for localizing prostate cancer: correlation with whole-mounthistopathology. J MagnReson Imaging. 2014;39:1443–8. https://doi.org/10.1002/jmri.24317.
• Engels RRM, Istrael B, Padhani AR, et al. Multiparametric magnetic resonance imaging for the detection of clinically significant prostate cancer: what urologists needs to know. Part 1: acquisition. Eur Urol. 2019. https://doi.org/10.1016/j.eururo.2019.09.021. Exhaustive correlation between clinical, therapeutic and radiological aspects.
de Rooij M, Hamoen EHJ, Witjes JA, Barentsz JO, Rovers MM. Accuracy of magnetic resonance imaging for local staging of prostate cancer: a diagnostic meta-analysis. EurUrol. 2016;70:233–45. https://doi.org/10.1016/j.eururo.2015.07.029.
Rosenkrantz AB, Shanbhogue AK, Wang A, Kong MX, Babb JS, Taneja SS. Length of capsular contact for diagnosing extra-prostatic extension on prostate MRI: assessment at an optimal threshold. J MagnReson Imaging. 2016;43:990–7. https://doi.org/10.1002/jmri.25040.
Kim CK, Park SY, Park JJ, Park BK. Diffusion-weighted MRI as a predictor of extracapsular extension in prostate cancer. AJR Am J Roentgenol. 2014;202:W270–6. https://doi.org/10.2214/AJR.13.11333.
Giganti F, Coppola A, Ambrosi A, Ravelli S, Esposito A, Freschi M, et al. Apparent diffusion coefficient in the evaluation of side-specific extracapsular extension in prostate cancer: development and external validation of a nomogram of clinical use. Urol Oncol Semin Orig Investig. 2016;34(291):e9-17. https://doi.org/10.1016/j.urolonc.2016.02.015.
Barrett T, Priest AN, Lawrence EM, Goldman DA, Warren AY, Gnanapragasam VJ, et al. Ratio of tumor to normal prostate tissue apparent diffusion coefficient as a method for quantifying DWI of the prostate. AJR Am J Roentgenol. 2015;205:W585–93. https://doi.org/10.2214/AJR.15.14338.
Caglic I, PovalejBrzan P, Warren AY, Bratt O, Shah N, Barrett T. Defining the incremental value of 3D T2-weighted imaging in the assessment of prostate cancer extracapsular extension. EurRadiol. 2019. https://doi.org/10.1007/s00330-019-06070-6.
Peng Y, Schmid-Tannwald C, Wang S, Antic T, Jiang Y, Eggener S, et al. Seminal vesicle invasion in prostate cancer: evaluation by using multiparametricendorectal MR imaging. Radiology. 2013;267:797–806. https://doi.org/10.1148/radiol.13121319.
Grivas N, Hinnen K, de Jong J, Heemsbergen W, Moonen L, Witteveen T, et al. Seminal vesicle invasion on multi-parametric magnetic resonance imaging: correlation with histopathology. Eur J Radiol. 2018;98:107–12. https://doi.org/10.1016/j.ejrad.2017.11.013.
Jung DC, Lee HJ, Kim SH, Choe GY, Lee SE. Preoperative MR imaging in the evaluation of seminal vesicle invasion in prostate cancer: pattern analysis of seminal vesicle lesions. J MagnResonce Imaging. 2008;28:144–50. https://doi.org/10.1002/jmri.21422.
Kwee TC, Takahara T, Luijten PR, Nievelstein RAJ. ADC measurements of lymph nodes: inter- and intra-observer reproducibility study and an overview of the literature. Eur J Radiol. 2010;75:215–20. https://doi.org/10.1016/j.ejrad.2009.03.026.
Sadinski M, Medved M, Karademir I, Wang S, Peng Y, Jiang Y, et al. Short- term reproducibility of apparent diffusion coefficient estimated from diffusion-weighted MRI of the prostate. Abdom Imaging. 2015;40:2523–8. https://doi.org/10.1007/s00261-015-0396-x.
Shen G, Deng H, Hu S, Jia Z. Comparison of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: a meta-analysis. Skeletal Radiol. 2014;43:1503–13. https://doi.org/10.1007/s00256-014-1903-9.
Padhani AR, Lecouvet FE, Tunariu N, Koh D-M, De Keyzer F, Collins DJ, et al. Metastasis Reporting and Data System for Prostate Cancer: practical guidelines for acquisition, interpretation, and reporting of whole-body magnetic resonance imaging-based evaluations of multiorgan involvement in advanced prostate cancer. EurUrol. 2017;71:81–92. https://doi.org/10.1016/j.eururo.2016.05.033.
Larbi A, Pasoglou V, Triqueneaux P, Cyteval C, Tombal B, Omoumi P, et al. Whole-body MRI to assess bone involvement in prostate cancer and multiple myeloma: comparison of the diagnostic accuracies of the T1, short tau inversion recovery (STIR), and high b-values diffusion-weighted imaging (DWI) sequences. EurRadiol. 2018. https://doi.org/10.1007/s00330-018-5796-1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Each Author declares that they have no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with submitted article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Geriatrics.
Rights and permissions
About this article
Cite this article
Carpagnano, F.A., Eusebi, L., Tupputi, U. et al. Multiparametric MRI: Local Staging of Prostate Cancer. Curr Radiol Rep 8, 27 (2020). https://doi.org/10.1007/s40134-020-00374-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s40134-020-00374-y