Abstract
This article reports on the realtions between the activities of enzymes, such as phenylalanine–ammonia lyase (PAL) and glutathione-s-transferase (GST), and the concentrations of total tannins, total glutathione (TGSH), total phytochelatins (TPC), and lipid peroxidation (MDA) in leaves and roots of sorghum cultivars 132 and 552 that are exposed to four concentrations (0, 10, 20, and 30 mg l−1) of AlCl3 in a mixture of river sand and peat. The Al3+ significantly increased the level of tannins, TGSH, and TPC in the roots and leaves of both cultivars. In the roots of both cultivars, the production of TPC was significantly coupled to decrease in the total TGSH. The concentration of TGSH in the leaves of cultivar 132 was found to be higher than in those of cultivar 552. In the plants treated with 10, 20, and 30 mg l−1 Al3+, the PAL activities in leaves of both cultivars increased (by 73, 44, and 18 %, respectively), the TGSH in the roots of cultivar 552 declined (by 50, 45, and 23 %, respectively), the GST activities in the leaves of both cultivars were higher (90, 98, and 100 %, respectively) than those of the control plants. Al3+ also enhanced levels of MDA in the leaves and roots. These results suggested that the increase in PAL and GST activities might be controlled by antioxidant potentials and different routes of carbon channeling in the leaves. In cultivar 552, antioxidant compounds such as TPC and TGSH with rapid turnover and high accumulation were more effective than tannins for leaves because tannin was low. In cultivar 132, the amount of tannins was high and stable; therefore they do not need high accumulation of TPC in leaves. The depletion of TGSH can be ascribed to the Al3+-induced TPC synthesis in the leaves and roots of cultivar 552. The syntheses of TPC and MDA can be related to changes in TGSH and tannins, suggesting that TGSH and tannins are normally involved in Al3+ sequestration under conditions of subtoxic exposure. The increased TPC in the roots could provide an effective means of restricting Al3+ to these organs by chelating. In cultivar 552, TGSH contents may have been consumed for two strategies: the maintenance of regular redox potential, and the precursor for TPC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most strategies of plant tolerance to Al3+ exposure are based on the reduction, by maintaining the cytosolic concentration of free Al3+. In this way, the plant cell avoids accumulation of Al3+ by compartmentalization, although this distribution is not clearly established yet [17]. Several investigations described that Al3+ triggered increase in organic acid anion release from root tips of Al3+-tolerant plant [7]. Root exudation of phenolic compounds has been described by many authors. Phenolics can reverse the toxic effects of Al3+ on hexokinase [33]. However, at equimolar concentrations, they are less efficient than citrates in developing complexes with Al3+. Also, simple tannins like {catechol} are effective at low pH, where H+ efficiently competes with Al3+ for the binding sites in 1:1 complexes [ 2, 22 ]. Therefore, tannins' sites in themselves are considered important for complex formation with Al3+ in acidic environments. Recent investigations found Al3+-induced exudation of the flavonoid-type tannins {catch in} and quercetin from 10-mm root tips in an Al3+-resistant maize variety [2, 6]. The Al3+-induced exudation of {catechin} at the rate of 100 μmol per tip h−1 in comparison with citrate, which did not exceed 1 ηmol per tip h−1, a rate reported for citrate exudation in maize by other authors [6].
The known proteins–SH compounds are small metal-binding peptides with the structure (γ-glu-cys)n-gly, in which “n” varies from 2 to 11. The synthesis from glutathione, homo-glutathione, hydroxymethyl-glutathione is catalyzed by a transpeptidase, and called phytochelatin (PC) synthesis [4], [30]. PC syntheses have been shown to be activated by a broad range of metals and metalloids, in particular Cd2+, Ag2+, Zn2+, Cu2+, and Au2+ [4].
In plants, total glutathione or TGSH (GSH + GSSG) is involved in cellular processes, including defense against reactive oxygen species (ROS). TGSH exists in two forms: reduced glutathione (GSH) and oxidized glutathione (GSSG). The reduction potential of glutathione depends on the intracellular GSH/GSSG ratio (Pekker et al. 2002). The conjugation of TGSH with such molecules is governed by glutathione S-transferase (GST). GST catalyzes the conjugation of TGSH with metal ions and helps them to sequester into vacuoles [16].
The control of oxidant levels is achieved by antioxidative systems. These defense systems are composed of metabolites, such as ascorbate, glutathione, tocopherol, tannin; and enzymatic scavengers of activated oxygen, such as superoxide dismutase, peroxidases, etc. [27].
Polyphenols are plant secondary metabolites consisting of condensed tannin, and are ∗OH radical scavengers because phenolic groups are excellent nucleophiles and are also able to quench lipid peroxidation, action as chain break antioxidant. Tannin chelates metal because of its ten galloyl groups, and it diminishes intestinal non-heme iron absorption [25].
In Sorghum’s cultivars, tannin is an abundant component with as high as 8–15 % of dry weight which prevents damage from Al3+ stress. In our previous chemical study, carried out on two cultivars, the results revealed that tannin in cultivar 132, was 10 % higher than that in cultivar 552, although the leaves' toughness in cultivar 552 was higher than that in cultivar 132 [19, 22]. Polyphenols compounds and specialized condensed tannins play an important role in plant defense by the oxidation of endogenous tannins compound into quinines [28]. PAL is considered the key enzyme in phenolic biosynthesis since it catalyzes the reductive deamination of l-phenylalanine to form trans-cinnamic acid, the first step in the biosynthesis of plant phenylpropanoid compounds. Tolerance to Al3+ in sorghum may be defined as the ability to survive in a soil that is toxic to other plants. The question is whether this means that only a single biochemical or molecular change is required to produce tolerance to Al3+. The main goal of this study is to test the hypothesis by comparing the activities of TPC, TGSH, and tannins, in the detoxification of Al3+. We compared the GST activities of the two cultivars and tried to develop the relation between TGSH accumulation and Al3+-induced TPC synthesis, oxidative stress, and involvement of the key enzyme PAL in the first step of plant phenylpropanoid i biosynthesis of in the leaves and roots of two cultivars of sorghum.
Materials and Methods
Plant Materials
The experiments were carried out between 21 April and 18 September 2010, under natural daylight in the University of Bu Ali Sian Hamadan. During growth season, the temperature ranged between 25 ± 5 °C. In this study, two sorghum cultivars differing in Al3+ accumulation in leaves and roots were selected based on previous study [22]. The cultivars were obtained from the seed research center of Isfahan. The seeds were sterilized for 20 min in a 10 % sodium hypochlorite solution. Seeds of two cultivars were cultivated in cycle pots with surface area of 1,015 cm² and depth of 60 cm.
The medium culture was river sand and peat in 3:1 ratio, respectively. The Hoagland’s nutrient solution was added to each pot once in 2 weeks [15]. Simultaneously, the AlCl3 was applied in four concentrations (0, 10, 20, and 30 mg l−1) as thresholds fixed by (Juan et al. 2002). The plant samples were harvested 50 days after sowing.
Sample Preparation
After the incubation period, all seedlings were submerged in cold 10 mM CaCl2 for 10 min to remove the adhering Al3+ in roots surfaces. Then, they were washed with distilled water. The plant samples were cut into roots and leaves, and each part was homogenated and subdivided into two parts. One was immediately weighed and frozen in liquid nitrogen and kept at −80 °C for analysis of total TPC, TGSH, tannin, and Al3+ concentrations. The fresh leaves and roots were sampled to determine GST, MDA, and PAL activities. Leaf areas per plant were determined (directed method) using a leaf area meter (LI 3100; Li-Cor, Lincoln, NB, USA). The samples were placed in the oven at 80 °C for 4 days; then the dry weight was measured separately.
Determination of Total Phytochelatins (TPC)
The TPC were extracted and assayed according to the method suggested previously [30]. In short, total TPC were extracted by homogenizing 0.5 g frozen plant material with 2 ml of a 5 %, 5-sulfosalicyclic acid with 6.3 mM diethylene triamine penta acetic (DTPA) at 0 °C (using mortar, pestle, and quartz sand). The homogenate was centrifuged at 12,000 g at 4 °C for 10 min. Clear supernatants were collected and immediately used for the assay of total TPC compound. The concentrations of total TPC compound were determined using Ellmans reagent (DTNB). 300 μl of supernatant was mixed with 630 μl of 0.5 M K2HPO4, final pH 7.5, and the absorbance was measured at 412 nm on a spectrophotometer (Perkin Elmer, Lambda 45, UV/vis D6484. USA). After addition of 25 μl of DTNB solution (6.3 mM DTNB in 0.143 M K2HPO4 and 6.3 mM DTPA, PH 7.5), the A412 was measured again after 2 min (€DTNB = 13,600 mol l−1 min−1 cm−1).
Determination of Total Glutathione (TGSH)
The TGSH (GSH + GSSG) was extracted and assayed according to the method reported previously [9]. Frozen plant materials were homogenized in 0.1 M sodium phosphate. 0.005 EDTA buffer (pH 8.0), and 25 % metaphosphoric acid (used for protein precipitation). The glutathione cycles were continuously monitored between its oxidized and reduced forms; reduction of GSSG was being affected by NADPH and glutathione reductase, while non-enzymic oxidation of GSH was being affected by DTNB. The homogenate was centrifuged at 12,000 g at 4 °C for 15 min to obtain supernatant for TGSH determination. The ion of 5-carboxy-4-nitrothiophenol thus produced absorbs at 420 nm (€12.9 × 103 m −1 cm−1) and the reaction rate becomes linear (as a dynamic equilibrium between the two forms of glutathione is established) within l min and remains linear for 15 min. Within a range of glutathione concentrations (70–350 nanomol-GSSG under the experimental conditions). The rate of increase of extinction at 350–420 nm is linearly related to the concentration of glutathione present. Total TGSH was determined fluorometrically and fluorescence intensity was recorded at 420 nm after excitation light at 350 nm on the Spectrophotometer. Linear regression analysis of the rates as a function of concentration at 420 nm after excitation at 350 nm was the following equation: Rate = 0.01464[TGSH] + 0.0091, R2 = 0.997.
Determination of Phenylalanine Ammonia-Lyase Activity (EC 4.3.1.5)
Activity of PAL was measured according to the method of [28], with slight modifications. To measure PAL, samples of 1 g of freshly weighed roots or leaves were ground in a chilled mortar in an ice bath with 0.5 g quartz sand, 0.5 g buffer saturated Polyclar AT, and 2 ml of 0.1 m borate buffer (pH 8.8), 15/am 2-mercaptoethanol. After centrifugation for 10 min at 20,000 g, the supernatant was layered on a Sephadex G-25 column buffered with 0.1 m borate buffer (pH 8.8) and centrifuged. 1 ml of enzyme extract was incubated with 2 ml of borate buffer (50 mM, pH = 8.8) and 1 ml of l-phenylalanine (20 mM) for 60 min at 37 °C. The reaction was stopped with 1 ml of 1 M HCl. The assay mixture was extracted with 3 ml of toluene by overtaxing for 30 s. The absorbance of toluene phase containing trans-cinnamic acid was measured at 290 nm. Enzyme activity was expressed as ηmol trans-cinnamic acid released, h−1g−1 FW.
Glutathione-s-Transferase (GST; EC 2.5.1.18)
The GST activity was determined spectrophotometrically according to the method of [8]. One gram of plant samples was extracted in 5 ml medium containing 50 mM phosphate buffer, pH 7.5, 1 mM EDTA, and 1 mM DTT. The enzyme activity was assayed in a reaction mixture containing 50 mM phosphate buffer, pH 7.5, 1 mM 1-chloro-2, 4-dinitrobenzene (CDNB). The reaction was initiated by the addition of 1 mM TGSH, and formation of S-(2, 4-di nitrophenyl) glutathione (DNP-GS) was monitored for increase in absorbance at 340 nm to calculate the GST-specific activity.
Determination of Tannins Compounds
Total condensed tannin was determined with acid butanol assay [10]. In a screw cap culture, 6 ml of the acid butanol reagent was added to a 1 ml aliquot of the sample, followed by addition of 0.2 ml of the iron reagent and vortexing of the sample. The tube was capped loosely, and put in a boiling water bath for 50 min. The tube was cooled and the absorbance was read at 550 nm; the absorbance of a blank containing only sample solvent, acid butanol and iron was subtracted from the sample absorbance. The purified tannin mg g−1 was used for standard curves.
Determination of Lipid Peroxidation (MDA)
The level of lipid peroxidation in plant tissues was determined as 2-thio barbituric acid (MDA) reactive metabolites chiefly malomdialdehyde as described previously [3]. Plant tissues (0.2 g) were extracted in 2 ml of 0.25 % MDA made in 10 % TCA. Extract was heated at 95 °C for 30 min and then quickly cooled on ice. After centrifugation at 10,000 g for 10 min, the absorbance of the supernatant was measured at 532 nm. Correction of non-specific turbidity was made by subtracting the absorbance value taken at 600 nm. The level of lipid peroxidation is expressed as nmol of MDA formed using an extinction coefficient of 155 mM cm−1.
Statistical Analyses
Two-factor factorial ANOVA was used to compare TGSH, nonprotein thiols. Leaves and root parameters in two cultivars were compared using SPSS software (version 13).
Results
Changes in the Levels of Al
The total Al3+ concentration in the seedlings of both cultivars increased with increasing Al3+ level in the medium culture (Tables 1 and 2; P < 0.005). However, the amount of Al3+ in leaves of cultivar 132 was higher than that in cultivar 552 (Table 2; P < 0.005). The Al3+ concentration in the roots was much higher than that in the leaves, indicating that the root is the main part for Al3+ accumulation in a sorghum plant. The cultivar 552 had the higher Al3+ accumulation when treated with 10, 20, and 30 mg−1Al3+, which may be attributed to the highest Al3+ concentration in roots and lower root DWduring the treatment (Table 2; P < 0.005).
Cultivars Leave DW and Leaves Expansion
A gradual decrease in total DW was observed with the increase in Al3+ concentration (Fig. 1; Table 1, P < 0.001). Al3+ in low concentration (10 mg l−1) leaf area (LA) in cultivar 132 and dry weight leaves (DWL) in cultivar 552 were significantly increased (Table 1; f (3, 3) = 12; P < 0.001; Fig 1a and b). Leaf expansion in cultivar 132 (3.08 m−2; Fig. 1b) was higher than that in cultivar 552 (2.67 m−2). In comparison with the control, a significant decrease in DW leaves and roots was observed at 30 mg l−1Al3+. The decrease in DW of roots was higher than leaves (P < 0.005). Compared with the controls, at 30 mg l−1 Al3+, the DW in leaves and roots of cultivar 132 decreased approximately by 17 and 23 %, respectively. In cultivar 552, the leaf DW decreased by 26 % when treated with 30 mg l−1 Al3+, whereas the equivalent reduction for roots was 32 % (Fig. 1a).
Effects of Al3+on dry weight (DW) leaves and roots (a) and leaves area per plant (b) in sorghum plants grown in medium solution containing different concentration of AlCl3. data are mean ± SD of four replicates. The treatment mean values followed by different letters (a, b, and c) are significantly different (P < 0.05)
Total Glutathione (TGSH)
The concentrations of TGSH in leaves and roots were significantly different depending upon cultivars and Al3+ treatment (Fig. 2c; Table 1). With increase of Al3+ concentration, there was decrease in the amount of TGSH: 5 % in leaves and 53 % in roots of cultivar 132 (Fig. 2c; P < 0.005). However, in cultivar 552, Al3+ stress caused a significant decrease (73 %) in TGSH in roots and 47 % in leaves. The amount of TGSH in cultivar 552 was higher than that in cultivar 132 (Fig 2c; P < 0.005).
Effects of Al3+ on TPC (€DTNB = 13,600 mol l−1 min−1 cm−1) (a), tannin (mg g−1 DW) (b) and TGSH (μmolg−1 FW) (c) in leaves and roots of sorghum plants grown in medium solution containing different concentration of AlCl3. Data are mean ± SD of four replicates. The treatment mean values followed by different letters (a, b, and c) are significantly different (P < 0.05)
Total Phytochelatins (TPC)
The TPC in roots and leaves of two cultivars was significantly increased with increasing Al3+ concentration (Fig. 2a; Table 1; P < 0.005). The levels of TPC were increased by 70 % in roots and about 36 % in leaves of cultivar 132, and those of TPC were increased by 98 % in roots and about 78 % in leaves of cultivar 552.
Changes in Tannin
Evidence indicates that, tannins' contents in cultivars significantly increased in all treatments under Al3+ concentration (Fig. 2b; Table 1; P < 0.005). With comparison of means the concentration of total tannins in leaves was about (cultivar 132, 60.22 mg g−1 and cultivar 552, 23.17 mg g−1) increased (Table 2).
Change in PAL Activity (EC 4.3.1.5)
The results showed that activity of PAL significantly increased in the leaves of two cultivars (Fig. 3a; Table 1; P < 0.005) in response to Al3+, with cultivar 132 having higher PAL activities than those of cultivar 552. There was an increase in the concentration of PAL in the leaves (when comparing the mean values) from 73.41 μmol g−1 Cinnamic acid F.W in cultivar 132; and 43.52 μmol g−1 Cinnamic acid FW in cultivar 552; Fig. 3a) Compared with the controls, at 30 mg l−1 Al3+, the PAL activities in the roots of two cultivars increased approximately by 50 % (Fig 3a; Table 1; P < 0.005). PAL activity was lower in cultivar 552 than that in cultivar 132.
Effects of Al3+ on activities of PAL (μmol cinnamic acid g−1 h−1 FW) (a), GST (μmol g−1min−1 FW) (b) in leaves and roots of sorghum plants grown in medium solution containing different concentration of AlCl3. Data are mean ± SD of four replicates. The treatment mean values followed by different letters (a, b, and c) are significantly different (P < 0.05)
Change in Glutathione-s-Transferees Activity (EC 2.5.1.18)
Results in Fig. 3b showed that the activity of GST significantly increased in the leaves of two cultivars (Fig. 3b; Table 1; P < 0.005) in response to Al3+. Cultivar 552 was found to have higher GST activity than cultivar 132. There was increase in the concentration of GST in leaves (when comparing the mean values) by 285.7 μmol/g−1min−1 protein t in (cultivar 552, and 243.1 μmol/g−1min−1 protein in cultivar 132; Fig. 3b). Compared with the controls, at 30 mg l−1 Al3+, the GST activities in roots of cultivars 552 and 132 increased by approximately 98 and 80 %, respectively (Fig 3b; Table 1; P < 0.005).
Change in Lipid Peroxidation (MDA)
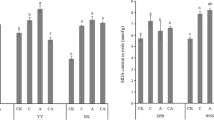
Results in Fig. 4 showed that the levels of MDA significantly increased in leaves of two cultivars (Fig. 4; Table 1 P < 0.005) in response to Al3+. MDA content in new leaves of Al3+-treated cultivar 552 was significantly higher than that in cultivar 132. The results showed a significant increase that was more pronounced in leaves than in roots of the Al3+-treated cultivars. In high concentrations of Al3+ (20 and 30 mg l−1), MDA in the leaves of cultivar 552 was increased and consequently influenced the glutathione's content in cultivars 552 (Fig. 4).
Effects of Al3+ on MDA (TBRS ηmol g−1min −1 FW) in leaves and roots of sorghum plants grown in medium solution containing different concentration of AlCl3. Data are mean ± SD of four replicates. The treatment mean values followed by different letters (a, b and c) are significantly different (P < 0.05)
Correlation Between TPC and TGSH
The TPC represent the all groups of sorghum leaves phytochelatins, and their concentrations have been shown to correlate positively with TGSH of the leaves and roots in cultivar 552 (Table 3, leaves; R2 = 97; y = 0.38x + .55 and roots; R2 = 87; y = 1.02x + .1.28, P < 0.005). The changes in correlation coefficients in the TPC and TGSH with increased concentrations of Al3+ in leaves in cultivars 132 were not significant. (Table 3, leaves; R2 = 16; y = 0.47x + .29 and roots; R2 = 97; y = −0.3x + .0.55, P < 0.005). No significant correlation was noted between TPC and TGSH of the leaves in cultivar 132 (Table 3; P < 0.005).
Correlation Between TPC and GST Activity
There was a direct correlation between the activities of GST and the concentration of TPC in the leaves and roots in cultivar 552 (Table 3, leaves; R2 = 85; y = −0.0097x + 1.26 and roots; R2 = 98; y = −.0.001x + 1.02, P < 0.005). Correlations between the changes in the amount of TPC per leaf and GST activity were mostly non-significant in the leaves of cultivar 132, but in the roots, they were significant (Table 3, leaves; R2 = 0.16; y = 0.47x + 0.29 and roots; R2 = 0.81; y = −0.38x + 0.55 + 1.02, P < 0.005).
Correlation Between TGSH and GST Activity
A positive relationship was found between GST activity and TGSH in the roots of two cultivars (Table 3, roots 132; R2 = 0.98; y = 0.0.0026x + 0.16 and roots 552; R2 = 0.8; y = −0.0021x + 0.21, P < 0.005). There was significant correlation between GST activity and TGSH of the leaves in two cultivars (Table 3, leaves 132; R2 = 0.8; y = 0.002x + 0.16 and leaves 552; R2 = 0.75; y = −0.001x + 0.21, P < 0.005).
Discussion
Sorghum (Sorghum bicolor L.) serves as staple food for the majority of world population and its productivity is drastically limited under environmental stress. The possible mechanisms of effectiveness and protection against the metal toxicity is by enzymatic action and extending our knowledge about the antioxidant process, especially in different organs, which could provide information regarding the regulation of this process [21]. In this study, two sorghum cultivars differing in leaves and roots Al3+ accumulation according to a previous study was selected. The cultivar 552 grown at 30 mg l−1 Al3+ was moderately chlorotic, exhibiting brown lesions on the leaves and appearing noticeably smaller than controls. Roots of Al3+-treated cultivar 552 generally appeared darker than control plants, perhaps Al3+ stimulated efflux of phenolic and organic acids, due to the fact that the surface of roots was darker, but the cultivar 132 grown at 30 mg l−1 was low chlorotic, appearing slightly smaller than controls [20], [21], [24] and [32]. Various phenotypic symptoms in response to heavy metal stress include reduced leaf expansion, chlorosis that may lead to necrosis. Despite the ability of the cultivars to reduce Al3+ toxicity, the concentration of Al3+ in the growth medium was almost higher than concentration of Al3+ in the roots, and in the leaves (Table 2). The Al3+ was accumulated mainly in old leaves in cultivars during the growth, and the amount in young leaves of cultivars was very low [19, 22]. The distribution of Al3+ between the roots fractions of cultivar 132 and cultivar 552 is shown in Table 2. Most of the Al3+ was retained in the roots, an average of 78.4 % of Al3+ in the cultivar 552 roots and 60.5 % of Al3+ in the cultivar 132 roots was found in the roots fraction. Al3+ decreases DW of the leaves, roots and leaf area in two cultivars, and the DW decrease in 30 mg l−1 Al3+ was higher than that other Al3+ concentrations (Fig. 1; Table 1). The results showed agreement with the previous study [17]. In Al3+ stress, the dry weight partitioning between roots and leaves was significantly changed, at high concentrations of Al3+ below ground biomass was high constrained rather than above ground and DW root decreased more than DW leaves (Fig. 1a).
In low concentrations of Al3+, it stimulates the growth in some plants [1]. With increasing concentrations Al3+ in medium cultural till 10 mg l−1, the DW of the leaves and leaf area in cultivar 132 were increased, but only DWL in cultivar 552 was increased (Fig. 1a, b). This experiment showed that low accumulation of Al3+ in leaves of two cultivars may be a strategy to protect photosynthetic function from the induced oxidative stress [24]. The cultivars can avoid Al3+ damage, which emerged in the presence of 30 mg l−1 of the Al3+, and can develop defense mechanisms to cope with the affinity of Al3+ for TPC and tannin. In the present study, in roots of cultivars, the amount of Al3+ in the roots was increased continuously, and the Al3+ concentration in leaves of cultivar 132 was higher than cultivar 552, but Al3+ concentration in the roots cultivar 132 was lower than cultivar 552 (Table 2). This agrees with previous findings in sorghum and in other plants [7], [17] and [22].
The TPC increased by 98 % from the first concentration to 30 mg l−1 Al3+ in the roots cultivar 552 (Fig. 2a). By contrast, TGSH decreased by 73 % in cultivar 552 from the first concentration to the 30 mg l−1 Al3+ (Fig. 2b). In cultivar 132, TPC increased by 70 % from the first concentration to the 30 mg l−1 Al3+ in roots. By contrast, TGSH decreased by 53 % in cultivar 132 from the first concentration to the 30 mg l−1 (Fig. 2a, c), agreement with the observed [8], [26]. In the present study, the threshold Al3+ exposure levels for root growth inhibition and TPC accumulation coincide in two cultivars (Fig. 2a, c; Table 2). Although Al3+ induces TPC accumulation, the capacities of the two cultivars in antioxidant and TPC accumulation in the roots and leaves were different (Fig. 2a). The amounts of TPC in the leaves and roots of cultivar 552 were higher than those in cultivar 132, but the amounts of tannins in the leaves and roots of cultivar 552 werelower than those of cultivar 132 (Fig. 2b). Following Al3+ stress, a deep alteration of the TGSH status occurred mainly in the leaves and roots of cultivar 552. The TGSH is used as substrate for TPC production. Therefore, TPC may be functionally important to cultivars 552 under conditions of Al3+ stress, in agreement with the previous results [32]. The threshold concentration levels of Al3+, for TPC accumulation appeared in the cultivar 552 were lower than the cultivar 132 (Table 2; Fig. 2a). In cultivar 132, the induced TPC accumulation was not apparent until the threshold concentration level of Al3+ for acute toxicity had been exceeded, suggesting that tannins are normally involved in Al3+ sequestration under conditions of sub toxic exposure [22], [32]. It is likely, as previously argued, that Al3+ bound to the cytosolic SH group dependent enzyme, reduces sucrose synthesis [4].
In two cultivars, Al3+ increased the TPC in roots which has been widely considered as a mechanism whereby these cultivars can acquire a degree of resistance to Al3+ toxicity (Table 2; Fig. 2a).
The Relations Between TPC, TGSH, and Al3+
In two cultivars, the roots concentration of TPC in 30 mg l−1 Al3+ was significantly higher than the controls (Fig. 2a). The localization of TPC in the roots could provide an effective means of restricting Al3+ to this organ by chelating in the form of Al-SH (TPC) complexes, and the transport to leaves was restricted [6]. A negative correlation was found between TPC and TGSH (Fig. 2a, c). The variation of TGSH in leaves may be influenced by leaf synthesis, as suggested by the significant correlation noted between TGSH and (TGSH + GSSG). However, the correlation between TGSH and (TGSH + GSSG) remained significant after subtracting TPC effect on Al3+ (Fig. 2c). This result was expected, since the relationship between TGSH and (TGSH + GSSG) was scarcely documented [5]. The TGSH is a central metabolic and is involved in the reaction forming TPC [12]. The statistical analysis shown indicated that both TGSH and TPC exhibited in two cultivars, a high degree of variability as shown by the (Fig. 2a, c). This variability has been caused by the differences due to cultivars variety in antioxidants and tannin in leaves and roots (Fig. 2b, Fig. 4). In cultivar 132 leaves, Al3+ treatment increased total tannin's content, mediated by enhanced activity of PAL (Figs. 2b, 3a), but in cultivar 552, the total tannin was low and consequently the activity of PAL was low (Fig. 3a). In cultivar 552, TGSH contents were higher than those in cultivar 132, and Al3+ treatment increased TGSH content, mediated by enhanced activity of GST (Fig. 2c, 3b; and Table 3). The TGSH contents may have been consumed for two strategies: the maintenance of regular redox potential, and the precursor for TPC synthesis, in agreement with the previously observed results [18].
The major effect observed in this research was that 30 mg l−1 of Al3+ in medium culture, the TGSH level by about 47 % in leaf cultivars 552 and by about 5 % in leaf cultivar 132 were decreased Fig. 2c, in agreement with the previousluy observed data [30]. Therefore, in cultivar 552 leaves parallel to the transient depletion of TGSH which is used as substrate for TPC production, the synthesis of TGSH seem accurse. The result in cultivar 132 has shown that the level of antioxidant regulated by TGSH content and parallel to the transient depletion of TGSH does not seem for TPC production (Fig. 2a, c; and Table 3). Also the distribution of TPC in the roots and leaves of cultivar 132 were different, in agreement with the previously observed behaviors [5], [18].
It also explains in cultivar 552 why, in Al3+-treated leaves the pool of TGSH decreased. In cultivar 552, the amount of tannin was very low, and this cultivar needed quantitatively to maintain as much in reduced forms. The high cost of this activity could be mitigated by using TPC in antioxidant activity against Al3+. TGSH can mobile to many organs in plants, and this characteristic is very useful for an antioxidant [26]. Furthermore, TGSH efficiently lowers the probability that Al3+ will bind to SH in the active sites of many photosynthetic enzymes, which would alter their functionality and inhibit photosynthesis [17]. This was a predictable result because it is known that Al3+ acts as a strong sink for SH grope, which increases the demand for sulfate absorption. The predominance of the TPC, this confirms the suggestion that SH grope can trap Al3+ only when they are in the reduced state [14].
The amount of TGSH in cultivar 552 was higher than cultivar 132, this difference in two cultivars, may be due to the synthesis of TGSH in the leaves and roots, or recycling of TGSH in the roots and leaves. It has been suggested that cultivar 552 with high TGSH concentration may improve their growth and antioxidant resistance under Al3+ excess, therefore TGSH regeneration by the Glutamin–Glutamat cycle is a key antioxidant mechanism against Al3+ stresses [8]. therefor the first toxicity of Al3+, in cultivar 552 antioxidant compounds such as TGSH with rapid turnover and high cumulative to be cost effective than tannin for leaves because tannin was low and stable. In cultivar 132, antioxidant compounds such as tannin with low turnover and high accumulation, seem to be more cost effective than TGSH for leaves, because the leaf toughness in cultivar 552 was higher than cultivar 132 [13], [22]. In cultivar 552, TGSH can mobile between roots and leaves, for this reason the amount of TGSH in the control leaves of cultivar 552 was high. In cultivar 132, the tannin can not mobile between roots and leaves [32].
In cultivar 552, when the Al3+ concentration was increased, the transformation of TGSH to TPC was high, but in cultivar 132, when the Al3+ concentration was increased, the synthesis of tannin was increased, and the transformation of TGSH to TPC was very low. The transformation of TGSH in cultivar 552 was much higher than the rate of synthesis, however, in cultivar 132 the rate of transformation was lower than the rate of synthesis. Therefore loss of TGSH due to Al3+ stress in cultivar 132 was lower than cultivar 552, agreement with the observed [18].
The Relation Between TPC, TGSH, MDA, and Tannin and GST and PAL Activities
Changes in chemical defenses including tannins and various activities MDA and GST in the presence of Al3+ in the leaves and roots of cultivars were investigated. MDA is the marker for lipid peroxidation increase in metal stress [27]. Al3+ increased MDA activity in two cultivars, which coincided with a high decrease of TGSH, in roots (Fig 2c, Fig.4). With the increase of the concentrations of Al3+, activities of GST were increased in the leaves of two cultivars, and increase in cultivar 552 was higher than that in cultivar 132 (Fig. 3b). With increasing Al3+ concentrations, the amount of tannins in the leaves of two cultivars increased, and the increase in cultivar 132 was higher than that in cultivar 552 (Fig. 2b). When cultivar 552 was exposed to Al3+, the equilibrium between production tannins and TGSH was broken, resulting in oxidative damage; increased allocation to TGSH which are potential antioxidant compounds markedly increased the activity of GST enzyme, while MDA activity greatly increased as compared with control (Fig. 4 and Table 3). Thus, balance between MDA and TGSH generation and scavenging compound tannins in leaves may reflect the defense strategy in two cultivars [33].
Tannins are derived from polyphenols, which are formed from phenylalanine by the activity of PAL. Thus, PAL is often speculated to be a key enzyme in tannins metabolism (Figs. 2b, 3a). The PAL enzyme can readily be induced by some environmental stresses [21]. Thus, we examined the possibility that PAL activity might be induced by concentration of Al3+. Figure 4 indicates that, with increased Al3+ concentrations in medium culture, in cultivar 552 the activity of PAL increased and reached to a maximum value. But in cultivar 132, the increase in the activity of PAL was higher than that in cultivar 552. On the other hand, the amount of tannin in cultivar 132 increased with increasing concentrations of Al3+. These results indicate that an increase in tannin in cultivar 132 is based on an increase in the activity of PAL (Figs. 2b, 3a). Also, in cultivar 552, the enhanced formation of TPC during TGSH depletion is preceded by an increase in Al3+ concentrations. The decrease in TGSH in cultivar 552 is dependent on an increase in the activity of GST enzyme and subsequent lowering of PAL activity (Fig. 3a and Table 3). The results are consistent with the previous observations [19], [20] and [34]. Cultivar 132 exhibited a maximum value of PAL activity, which is two times higher than that of cultivar 552 and minimum of TGSH. Upon treatment with 10 mg l−1 Al3+, the activity of PAL was not significantly altered in cultivar 552—rather it increased in cultivar 132 (Fig. 3a).
In the experiment, the increase in PAL activity might be controlled by either antioxidant potential of cells or activation by Al3+. One of the mechanisms for the tolerance in cultivar 132 seems to be the accumulation of antioxidant molecules such as tannin which inhibits the facilitation of the peroxidation of phospholipids [11], [19].
Regarding the result obtained in this experiment, two strategies for Al3+ resistance in two cultivars have been suggested. First, the increase in tannins is responsible for the formation of phenoxy radicals. These metabolites may participate in ROS scavenging through antioxidant activity or can be directly chelated Al3+. It has been well documented that Al3+ stresses are responsible for the increase in tannins which would be antioxidant and associated with decreased plant growth [23], [31].
Second, the changes that occurred, in the pathway of TGSH synthesis and TPC metabolism of the leaves and roots, indicated the establishment of the fact that TGSH metabolism was very active with high activation of MDA (Fig. 4). The data obtained from the Al3+ stresses suggest that the increased activities of GST and PAL are among the important factors of tolerance for Al3+ which permits preservation of membrane integrity and leaf growth (Fig. 3a, Fig. 4).
The observed increases in the activities of PAL and GST in two cultivars might indicate extensive lipid peroxidation of cell membrane. Significant decreases in DW occurred following the 30 mg l−1 Al3+ treatment, which coincided with the increases in MDA, tannin, and TPC (Fig. 2a, b and Table 3). The induction of total tannins and changes in the contents of TGSH in the leaves of two cultivars play respective important roles in resistance to Al3+, which are intimately connected with GST and PAL activities (Fig. 3a and Table 3). These results agree with the general theory that, when plants are exposed to metals, they switch from normal primary metabolism to the multitude of secondary metabolism pathways, and the activation processes of novel stresses in enzymes and genes take place [29]. Therefore, the potent antioxidant properties of tannins in cultivar 132 are a common response to Al3+ resistance, resulting in enhanced resistance to Al3+ by tannin (Fig. 2b) [32]. The Al3+-treated cultivars 552 caused the highest GST activity, while Al3+-treated cultivar 132 was having the highest PAL activity. It seems that intensive activity of GST coupled with the small changes in tannins during the Al3+ stresses. The production of tannins caused an increase in the antioxidant activity; therefore activation of GST in cultivar 132 was lower than that in cultivar 552 (Fig. 2b and Table 3).
Conclusion
Plants respond to Al3+ toxicity in a variety of ways. Al3+ increased allocation to tannins, TGSH, and TPC which are potential defensive compounds. When the two cultivars were analyzed, the behaviors of the compounds of TPC, TGSH, and tannin, and the PAL and GST activities were different in the roots and leaves. In cultivar 132, the involvement of tannins was more than of TPC and TGSH in the reactions triggered by Al3+; therefore, the TPC in the leaves remained low and activation of GST in cultivar 132 was lower than that in cultivar 552. There was a high TPC concentration and a decrease of TGSH in the leaves of cultivar 552. It is probably a combination of multiprocesses that were involved being responsible to cause Al3+ resistance. The TPC synthesis induces TGSH depletion in the leaves of cultivars. It was concluded that this decrease in cultivar 552 is caused by different routes of carbon channeling (TGSH) translocation from leaves to roots, to enable a larger amount of precursor to be available for TPC synthesis in roots, resulting in changes in TGSH metabolism in the leaves.
References
Ahn SJ, Sivaguru M, Chung GC, Rengel Z, Matsumoto H (2002) Aluminium-induced growth inhibition is associated with impaired efflux and influx of H across the plasma membrane in root apices of squash (Cucurbita pepo). J Environ Qual 53:1959–1966
Bores W, Heller C, Michel K (1996) Flavonoids and polyphenol: chemistry and biology, antioxidants. Marcel Dekker, New York, pp 409–466
Chien H-F, Wang J-W, Lin CC, Kao CH (2001) Cadmium toxicity of rice leaves is mediated through lipid peroxidation. Plant Growth Regul 33:205–213
Cobbett CS (2000) Phytochelatin biosynthesis and function in heavy-metal detoxification. Curr Opin Plant Biol 3:211–216
De Vos, Vonk MJ, Vooijs R, Schat H (2001) Glutathione depletion due to copper-induced phytochelatin synthesis causes oxidative stress in Silene cucubalus. Plant Physiol 98:853–858
Dejene E, Angelika S, Walter JH (2005) Localization of aluminium in the maize root apex: can morin detect cell wall-bound aluminium. J Exp Bot 56(415):1351–1357
Delhaize E, Ryan PR (1995) Aluminum toxicity and tolerance in plants. Plant Phsyiol 107:315–321
Ezaki B, Suzuki M, Motoda H, Kawamura M, Nakashima S, Matsumoto H (2004) Mechanism of gene expression of arabidopsis glutathione S-transferase, AtGST1, and AtGST11 in response to aluminum stress. Plant Physiol 134:1672–1682
Foyer Ch, Theodoulou FL, Delrot S (2001) The functions of inter-and intracellular glutathione transport systems in plants. Trends Plant Sci 6:486–487
Gebrehiwot L, Beuselinck RB, Robert CA (2002) Seasonal variations in condensed tannin concentration of three lotus species. Agron J 94:1059–1065
Gomez-Vasquez R, Day R, Buschmann H, Randles S, Beeching JR, Cooper RM (2004) Phenylpropanoids, phenylalanine ammonia lyase and peroxidases in elicitor-challenged Cassava (Manihot esculenta) suspension cells and leaves. Ann Bot 94:87–97
Grill E, Loeffler S, Winnacker EL, Zenk MH (1989) Phytochelatins, the heavy-metal-binding peptides of plants, are synthesized from glutathione by a specific γ-glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase). Proc Natl Acad Sci USA 86:6838–6842
Hagerman AE, Carlson DM (1998) Biological responses to tannins and other polyphenols. Recent Res Dev Agric Food Chem 1998(2):689–704
Heiss SA, Wachter J, Bogs C, Cobbett T (2003) Rausch, phytochelatin synthase (PCS) protein is induced in Brassica juncea leaves after pro-longed Cd exposure. J Exp Bot 54:1833–1839
Hoagland, Arnon DRDI (1950) The water-culture method for growing plants without soil. Calif Agric Exp Stn Circ 347:1–32
Hossain MZ, Hossain MD, Fujita M (2006) Induction of pumpkin glutathione s-transferases by different stresses and its possible mechanisms. Biol Plantarum 50:210–218
Juan B, Charlotte P (2002) Fast root growth responses, root exudates, and internal detoxification as clues to mechanisms of aluminum toxicity and resistance: a review. Environ Exp Bot 48:75–92
Maier EA, Matthews RD, McDowell JA, Walden RR, Ahner BA (2003) Environmental cadmium levels increase phytochelatin and glutathione in lettuce grown in a chelator-buffered nutrient solution. J Environ Qual 32:1356–1364
Malmir HA (2010) The relation between phenylalanine ammonia-lyase, polyphenol oxidase activities and Flavonoids, lignin’s and toughness in leaves of sorghum (sorghum bicolor) exposed to Zinc .3rd International congress of environmental research, University of Mauritius, Reduit, 16–18 Sept 2010
Malmir HA (2010) The relation between antioxidant enzyme, chlorophyll, flavonoids, lignin and toughness in sorghum (sorghum bicolor) exposed to zinc. The 1st annual international conference ibb environmental science & technology, which will take place at Ibb University, Ibb city, Yemen, 1–3 Aug 2010
Malmir HA (2011) Comparison of antioxidant enzyme activities in leaves, stem and roots of sorghum (Sorghum bicolor L.) exposed to chromium (VI). African J Plant Sci 5(5):436–444
Malmir HA, Mostajeran A, Almodares A, Asghari A, Afkhami A (2009) The effects of aluminum on fiber and protein bound condensed tannin, polyphenols and some growth index in two sorghum cultivars. Int J Bot 5(1):58–66
Mamoudou H, Dick, Riet H (2002) Comparison of content in phenolic compounds, polyphenol oxides, and peroxidase in grains of fifty sorghum varieties from Burkina Faso. J Agric Food Chem 50:3780–3788
Matsumoto H (2001) Cell biology of aluminum toxicity and tolerance in higher plants. Int Rev Cytol 200:1–46
Moyer RA, Hummer KE, Finn CE, Frei B, Wrolstad RE (2002) Anthocyanins, phenolics and Antioxidant capacity in diverse small fruits: vaccinum, Rubus, and Ribes. J Agric Food Chem 50:519–525
Noctor G, Arisi ACM, Jouanin L, Kunert KJ, Rennenberg H, Foyer ChH (1998) Glutathione: biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J Exp Bot 49:624–631
Olga Bk, Eija VL, Kurtav Fg (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91(2):179–194
Ortega-Garcıa F, Blanco S, Peinado MA, Peragon J (2009) Phenylalanine ammonia-lyase and phenolic compounds in leaves and fruits of Olea europaea L. cv. Picual during ripening. Sci Food Agric 89:398–406
Osone Yoko, Masakitateno (2005) Applicability and limitations of optimal biomass allocation models: a test of two species from fertile and infertile habitats. Ann Bot 95:1211–1220
Pagliari M, Sanita’ di Toppi L (2005) Oxidative stress and phytochelatin characterization in bread wheat exposed to cadmium excess. Plant Physiol Biochem 43:45–54
Pekker I, Elisha TO, Mittler R (2002) Reactive oxygen intermediates and glutathione regulate the expression of cytosolic ascorbate peroxidase during iron-mediated oxidative stress in bean. Plant Mol Biol 49:429–438
Peter A, Stoutjesdi JK (2001) Possible involvement of condensed tannins in aluminum tolerance of lotus Pendulatus. Aust J Plant Physiol 28(11):1063–1074
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol 299:152–177
Tomas-Barberan F, Espı′n JC (2001) Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J Sci Food Agric 81:853–876
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Malmir, H.A. The Relations Between Phenylalanine–Ammonia Lyase, Glutathione-s-Transferase Activities and the Concentrations of Total Tannins, Phytochelatins, Glutathione, and Peroxidation in two Cultivars of Sorghum (Sorghum bicolor (L.) Moench) Exposed to Aluminum. Agric Res 1, 240–250 (2012). https://doi.org/10.1007/s40003-012-0023-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40003-012-0023-9