Abstract
Background:
Tissue engineering represents a promising approach for the production of bone substitutes. The use of perfusion bioreactors for the culture of bone-forming cells on a three-dimensional porous scaffold resolves mass transport limitations and provides mechanical stimuli. Despite the recent and important development of bioreactors for tissue engineering, the underlying mechanisms leading to the production of bone substitutes remain poorly understood.
Methods:
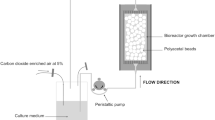
In order to study cell proliferation in a perfusion bioreactor, we propose a simplified experimental set-up using an impermeable scaffold model made of 2 mm diameter glass beads on which mechanosensitive cells, NIH-3T3 fibroblasts are cultured for up to 3 weeks under 10 mL/min culture medium flow. A methodology combining histological procedure, image analysis and analytical calculations allows the description and quantification of cell proliferation and tissue production in relation to the mean wall shear stress within the bioreactor.
Results:
Results show a massive expansion of the cell phase after 3 weeks in bioreactor compared to static control. A scenario of cell proliferation within the three-dimensional bioreactor porosity over the 3 weeks of culture is proposed pointing out the essential role of the contact points between adjacent beads. Calculations indicate that the mean wall shear stress experienced by the cells changes with culture time, from about 50 mPa at the beginning of the experiment to about 100 mPa after 3 weeks.
Conclusion:
We anticipate that our results will help the development and calibration of predictive models, which rely on estimates and morphological description of cell proliferation under shear stress.






Similar content being viewed by others
References
Vunjak-Novakovic G, Obradovic B, Martin I, Bursac PM, Langer R, Freed LE. Dynamic cell seeding of polymer scaffolds for cartilage tissue engineering. Biotechnol Prog. 1998;14:193–202.
Freed LE, Vunjak-Novakovic G. Cultivation of cell–polymer tissue constructs in simulated microgravity. Biotechnol Bioeng. 1995;46:306–13.
Mueller SM, Mizuno S, Gerstenfeld LC, Glowacki J. Medium perfusion enhances osteogenesis by murine osteosarcoma cells in three-dimensional collagen sponges. J Bone Miner Res. 1999;14:2118–26.
David B, Bonnefont-Rousselot D, Oudina K, Degat MC, Deschepper M, Viateau V, et al. A perfusion bioreactor for engineering bone constructs: an in vitro and in vivo study. Tissue Eng Part C Methods. 2011;17:505–16.
Meyer U, Wiesmann HP, Kruse-Lösler B, Handschel J, Stratmann U, Joos U. Strain-related bone remodeling in distraction osteogenesis of the mandible. Plast Reconstr Surg. 1999;103:800–7.
Bancroft GN, Sikavitsas VI, van den Dolder J, Sheffield TL, Ambrose CG, Jansen JA, et al. Fluid flow increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteoblasts in a dose-dependent manner. Proc Natl Acad Sci U S A. 2002;99:12600–5.
Cartmell SH, Porter BD, García AJ, Guldberg RE. Effects of medium perfusion rate on cell-seeded three-dimensional bone constructs in vitro. Tissue Eng. 2003;9:1197–203.
Glowacki J, Mizuno S, Greenberger JS. Perfusion enhances functions of bone marrow stromal cells in three-dimensional culture. Cell Transplant. 1998;7:319–26.
Grayson WL, Bhumiratana S, Grace Chao PH, Hung CT, Vunjak-Novakovic G. Spatial regulation of human mesenchymal stem cell differentiation in engineered osteochondral constructs: effects of pre-differentiation, soluble factors and medium perfusion. Osteoarthritis Cartilage. 2010;18:714–23.
McCoy RJ, O’Brien FJ. Influence of shear stress in perfusion bioreactor cultures for the development of three-dimensional bone tissue constructs: a review. Tissue Eng Part B Rev. 2010;16:587–601.
Raimondi MT, Moretti M, Cioffi M, Giordano C, Boschetti F, Laganà K, et al. The effect of hydrodynamic shear on 3D engineered chondrocyte systems subject to direct perfusion. Biorheology. 2006;43:215–22.
Goldstein AS, Juarez TM, Helmke CD, Gustin MC, Mikos AG. Effect of convection on osteoblastic cell growth and function in biodegradable polymer foam scaffolds. Biomaterials. 2001;22:1279–88.
Grayson WL, Marolt D, Bhumiratana S, Fröhlich M, Guo EX, Vunjak-Novakovic G. Optimizing the medium perfusion rate in bone tissue engineering bioreactors. Biotechnol Bioeng. 2011;108:1159-70.
Hung CT, Pollack SR, Reilly TM, Brighton CT. Real-time calcium response of cultured bone cells to fluid flow. Clin Orthop Relat Res. 1995;313:256–69.
Jungreuthmayer C, Jaasma MJ, Al-Munajjed AA, Zanghellini J, Kelly DJ, O’Brien FJ. Deformation simulation of cells seeded on a collagen-GAG scaffold in a flow perfusion bioreactor using a sequential 3D CFD-elastostatics model. Med Eng Phys. 2009;31:420–7.
Leclerc E, David B, Griscom L, Lepioufle B, Fujii T, Layrolle P, et al. Study of osteoblastic cells in a microfluidic environment. Biomaterials. 2006;27:586–95.
Park JY, Yoo SJ, Patel L, Lee SH, Lee SH. Cell morphological response to low shear stress in a two-dimensional culture microsystem with magnitudes comparable to interstitial shear stress. Biorheology. 2010;47:165–78.
Pedersen JA, Boschetti F, Swartz MA. Effects of extracellular fiber architecture on cell membrane shear stress in a 3D fibrous matrix. J Biomech. 2007;40:1484–92.
Sikavitsas VI, Bancroft GN, Holtorf HL, Jansen JA, Mikos AG. Mineralized matrix deposition by marrow stromal osteoblasts in 3D perfusion culture increases with increasing fluid shear forces. Proc Natl Acad Sci U S A. 2003;100:14683–8.
Horikawa A, Okada K, Sato K, Sato M. Morphological changes in osteoblastic cells (MC3T3-E1) due to fluid shear stress: cellular damage by prolonged application of fluid shear stress. Tohoku J Exp Med. 2000;191:127–37.
McGarry JG, Klein-Nulend J, Mullender MG, Prendergast PJ. A comparison of strain and fluid shear stress in stimulating bone cell responses–a computational and experimental study. FASEB J. 2005;19:482–4.
Jacobs CR, Temiyasathit S, Castillo AB. Osteocyte mechanobiology and pericellular mechanics. Annu Rev Biomed Eng. 2010;12:369–400.
Delaine-Smith RM, Sittichokechaiwut A, Reilly GC. Primary cilia respond to fluid shear stress and mediate flow-induced calcium deposition in osteoblasts. FASEB J. 2014;28:430–9.
Hoey DA, Downs ME, Jacobs CR. The mechanics of the primary cilium: an intricate structure with complex function. J Biomech. 2012;45:17–26.
Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J. 2006;20:811–27.
Porter BD, Lin AS, Peister A, Hutmacher D, Guldberg RE. Noninvasive image analysis of 3D construct mineralization in a perfusion bioreactor. Biomaterials. 2007;28:2525–33.
Voronov RS, VanGordon SB, Shambaugh RL, Papavassiliou DV, Sikavitsas VI. 3D tissue-engineered construct analysis via conventional high-resolution microcomputed tomography without X-ray contrast. Tissue Eng Part C Methods. 2013;19:327–35.
Wittkowske C, Reilly GC, Lacroix D, Perrault CM. In vitro bone cell models: impact of fluid shear stress on bone formation. Front Bioeng Biotechnol. 2016;4:87.
Zhao F, Vaughan TJ, McNamara LM. Quantification of fluid shear stress in bone tissue engineering scaffolds with spherical and cubical pore architectures. Biomech Model Mechanobiol. 2016;15:561–77.
Zhao F, Mc Garrigle MJ, Vaughan TJ, McNamara LM. In silico study of bone tissue regeneration in an idealised porous hydrogel scaffold using a mechano-regulation algorithm. Biomech Model Mechanobiol. 2018;17:5–18.
Williams C, Kadri OE, Voronov RS, Sikavitsas VI. Time-dependent shear stress distributions during extended flow perfusion culture of bone tissue engineered constructs. Fluids. 2018;3:25.
Voronov R, Vangordon S, Sikavitsas VI, Papavassiliou DV. Computational modeling of flow-induced shear stresses within 3D salt-leached porous scaffolds imaged via micro-CT. J Biomech. 2010;43:1279–86.
Alam TA, Pham QL, Sikavitsas VI, Papavassiliou DV, Shambaugh RL, Voronov RS. Image-based modeling: a novel tool for realistic simulations of artificial bone cultures. Technology (Singap World Sci). 2016;4:229–33.
Maniatopoulos C, Rodriguez A, Deporter DA, Melcher AH. An improved method for preparing histological sections of metallic implants. Int J Oral Maxillofac Implants. 1986;1:31–7.
Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5.
Chabanon M. Multiscale study of a perfusion bioreactor for bone tissue engineering [Doctoral dissertation]. Ecole centrale de Paris: Châtenay-Malabry; 2015.
Carman PC. Fluid flow through granular beds. Trans Inst Chem Eng. 1937;15:150–66.
Happel J, Brenner H. Low Reynolds number hydrodynamics: with special applications to particulate media. New York: Springer; 2012.
Bağcı Ö, Dukhan N, Özdemir M. Flow regimes in packed beds of spheres from pre-Darcy to turbulent. Transp Porous Media. 2014;104:501–20.
Fand RM, Kim BYK, Lam ACC, Phan RT. Resistance to the flow of fluids through simple and complex porous media whose matrices are composed of randomly packed spheres. J Fluids Eng. 1987;109:268–73.
Kececioglu I, Jiang Y. Flow through porous media of packed spheres saturated with water. J Fluids Eng. 1994;116:164–70.
Nield DA, Bejan A. Convection in porous media. New York: Springer; 2013.
Warren PB, Stepanek F. Wall shear rate distribution for flow in random sphere packings. Phys Rev Lett. 2008;100:084501.
Cruel M, Bensidhoum M, Nouguier-Lehon C, Dessombz O, Becquart P, Petite H, et al. Numerical study of granular scaffold efficiency to convert fluid flow into mechanical stimulation in bone tissue engineering. Tissue Eng Part C Methods. 2015;21:863–71.
Carslaw HS, Jaeger JC. Conduction of heat in solids. Oxford: Oxford Science Publications; 1959.
Cremer T, Werdan K, Stevenson AF, Lehner K, Messerschmidt O. Aging in vitro and d-glucose uptake kinetics of diploid human fibroblasts. J Cell Physiol. 1981;106:99–108.
Cho CH, Park J, Nagrath D, Tilles AW, Berthiaume F, Toner M, et al. Oxygen uptake rates and liver-specific functions of hepatocyte and 3T3 fibroblast co-cultures. Biotechnol Bioeng. 2007;97:188–99.
Sakar MS, Eyckmans J, Pieters R, Eberli D, Nelson BJ, Chen CS. Cellular forces and matrix assembly coordinate fibrous tissue repair. Nat Commun. 2016;7:11036.
Hillsley MV, Frangos JA. Alkaline phosphatase in osteoblasts is down-regulated by pulsatile fluid flow. Calcif Tissue Int. 1997;60:48–53.
Liegibel UM, Sommer U, Bundschuh B, Schweizer B, Hilscher U, Lieder A, et al. Fluid shear of low magnitude increases growth and expression of TGFbeta1 and adhesion molecules in human bone cells in vitro. Exp Clin Endocrinol Diabetes. 2004;112:356–63.
Ban Y, Wu YY, Yu T, Geng N, Wang YY, Liu XG, et al. Response of osteoblasts to low fluid shear stress is time dependent. Tissue Cell. 2011;43:311–7.
Hosseinkhani H, Hosseinkhani M, Khademhosseini A, Kobayashi H, Tabata Y. Enhanced angiogenesis through controlled release of basic fibroblast growth factor from peptide amphiphile for tissue regeneration. Biomaterials. 2006;27:5836–44.
Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech. 1994;27:339–60.
Mygind T, Stiehler M, Baatrup A, Li H, Zou X, Flyvbjerg A, et al. Mesenchymal stem cell ingrowth and differentiation on coralline hydroxyapatite scaffolds. Biomaterials. 2007;28:1036–47.
Brinkman HC. A calculation of the viscous force exerted by a flowing fluid on a dense swarm of particles. Flow Turbul Combust. 1949;1:27.
Zick AA, Homsy GM. Stokes flow through periodic arrays of spheres. J Fluid Mech. 1982;115:13–26.
Korin N, Bransky A, Dinnar U, Levenberg S. A parametric study of human fibroblasts culture in a microchannel bioreactor. Lab Chip. 2007;7:611–7.
Acknowledgements
The authors gratefully acknowledge the LabeX LaSIPS (No. ANR-10-LABX-0040-LaSIPS) managed by the French National Research Agency under the “Investissements d’avenir” programs (Nos. ANR-11-IDEX-0003-02 and ANR-10-EQPX-37 MATMECA Grant) for their financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare and all co-authors confirm agreement with the final statement. All authors have been appropriately disclosed according to the policy of the Journal.
Ethical statement
There are no animal experiments carried out for this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chabanon, M., Duval, H., Grenier, J. et al. Histological Method to Study the Effect of Shear Stress on Cell Proliferation and Tissue Morphology in a Bioreactor. Tissue Eng Regen Med 16, 225–235 (2019). https://doi.org/10.1007/s13770-019-00181-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-019-00181-3