Abstract
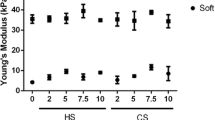
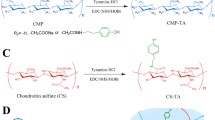
Novel hydrogel composed of both chondroitin sulfate (CS) and gelatin was developed for better cellular interaction through two step double crosslinking of N-(3-diethylpropyl)-N-ethylcarbodiimide hydrochloride (EDC) chemistries and then click chemistry. EDC chemistry was proceeded during grafting of amino acid dihydrazide (ADH) to carboxylic groups in CS and gelatin network in separate reactions, thus obtaining CS–ADH and gelatin–ADH, respectively. CS–acrylate and gelatin–TCEP was obtained through a second EDC chemistry of the unreacted free amines of CS–ADH and gelatin–ADH with acrylic acid and tri(carboxyethyl)phosphine (TCEP), respectively. In situ CS–gelatin hydrogel was obtained via click chemistry by simple mixing of aqueous solutions of both CS–acrylate and gelatin–TCEP. ATR-FTIR spectroscopy showed formation of the new chemical bonds between CS and gelatin in CS–gelatin hydrogel network. SEM demonstrated microporous structure of the hydrogel. Within serial precursor concentrations of the CS–gelatin hydrogels studied, they showed trends of the reaction rates of gelation, where the higher concentration, the quicker the gelation occurred. In vitro studies, including assessment of cell viability (live and dead assay), cytotoxicity, biocompatibility via direct contacts of the hydrogels with cells, as well as measurement of inflammatory responses, showed their excellent biocompatibility. Eventually, the test results verified a promising potency for further application of CS–gelatin hydrogel in many biomedical fields, including drug delivery and tissue engineering by mimicking extracellular matrix components of tissues such as collagen and CS in cartilage.











Similar content being viewed by others
References
Rahman RA, Radzi MAA, Sukri NM, Nazir NM, Sha’ban M. Tissue engineering of articular cartilage: from bench to bed-side. Tissue Eng Regen Med. 2014;12:1–11.
Kaushik SN, Kim B, Walma AMC, Choi SC, Wu H, Mao JJ, et al. Biomimetic microenvironments for regenerative endodontics. Biomater Res. 2016;20:14.
Gwak GH, Choi AJ, Bae YS, Choi HJ, Oh JM. Electrophoretically prepared hybrid materials for biopolymer hydrogel and layered ceramic nanoparticles. Biomater Res. 2016;20:1.
Castelletto V, Hamley IW, Stain C, Connon C. Slow-release RGD-peptide hydrogel monoliths. Langmuir. 2012;28:12575–80.
Bang S, Das D, Yu J, Noh I. Evaluation of MC3T3 cells proliferation and drug release study from sodium hyaluronate-BDDGE patterned gel. Nanomaterials (Basel). 2017;7:E328.
Lee TT, Garcia JR, Paez JI, Singh A, Phelps EA, Weis S, et al. Light-triggered in vivo activation of adhesive peptides regulates cell adhesion, inflammation and vascularization of biomaterials. Nat Mater. 2015;14:352–60.
Crescenzi V, Cornelio L, Di Meo C, Nardecchia S, Lamanna R. Novel hydrogels via click chemistry: synthesis and potential biomedical applications. Biomacromolecules. 2007;8:1844–50.
Gan Z, Zhang T, Liu Y, Wu D. Temperature-triggered enzyme immobilization and release based on cross-linked gelatin nanoparticles. PLoS One. 2012;7:e47154.
Sheikh Z, Hamdan N, Ikeda Y, Grynpas M, Ganss B, Glogauer M. Natural graft tissues and synthetic biomaterials for periodontal and alveolar bone reconstructive applications: a review. Biomater Res. 2017;21:9.
Zhu J, Marchant RE. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev Med Devices. 2011;8:607–26.
Wang SC, Chen BH, Wang LF, Chen JS. Characterization of chondroitin sulfate and its interpenetrating polymer network hydrogels for sustained-drug release. Int J Pharm. 2007;329:103–9.
Martel-Pelletier J, Kwan Tat S, Pelletier JP. Effects of chondroitin sulfate in the pathophysiology of the osteoarthritic joint: a narrative review. Osteoarthritis Cartilage. 2010;18:S7–11.
Lemaire PA, Huang L, Zhuo Y, Lu J, Bahnck C, Stachel SJ, et al. Chondroitin sulfate promotes activation of cathepsin K. J Biol Chem. 2014;289:21562–72.
Brühl H, Cihak J, Goebel N, Talke Y, Renner K, Hermann F, et al. Chondroitin sulfate activates B cells in vitro, expands CD138+ cells in vivo, and interferes with established humoral immune responses. J Leukoc Biol. 2014;96:65–72.
Muzzarelli RA, Greco F, Busilacchi A, Sollazzo V, Gigante A. Chitosan, hyaluronan and chondroitin sulfate in tissue engineering for cartilage regeneration: a review. Carbohydr Polym. 2012;89:723–39.
Little CJ, Kylyk WM, Chen X. The effect of chondroitin sulphate and hyaluronic acid on chondrocytes cultured within a fibrin–alginate hydrogel. J Funct Biomater. 2014;5:197–210.
Xing Q, Yates K, Vogt C, Qian Z, Frost MC, Zhao F. Increasing mechanical strength of gelatin hydrogels by divalent metal ion removal. Sci Rep. 2014;4:4706.
Benton JA, DeForest CA, Vivekanandan V, Anseth KS. Photocrosslinking of gelatin macromers to synthesize porous hydrogels that promote valvular interstitial cell function. Tissue Eng Part A. 2009;15:3221–30.
Van Vlierberghe S, Dubruel P, Schacht E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: a review. Biomacromolecules. 2011;12:1387–408.
Kimura Y, Ozeki M, Inamoto T, Tabata Y. Adipose tissue engineering based on human preadipocytes combined with gelatin microspheres containing basic fibroblast growth factor. Biomaterials. 2003;24:2513–21.
Chen S, Zhang Q, Nakamoto T, Kawazoe N, Chen G. Gelatin scaffolds with controlled pore structure and mechanical property for cartilage tissue. Tissue Eng Part C Methods. 2016;22:189–98.
Oka S, Matsumoto T, Kubo S, Matsushita T, Sasaki H, Nishizawa Y, et al. Local administration of low-dose simvastatin-conjugated gelatin hydrogel for tendon–bone healing in anterior cruciate ligament reconstruction. Tissue Eng Part A. 2013;19:1233–43.
Kwon HJ, Han Y. Chondroitin sulfate-based biomaterials for tissue engineering. Turk J Biol. 2016;40:290–9.
Jo S, Kim S, Noh I. Synthesis of in situ chondroitin sulfate hydrogel through phosphine-mediated Michael type addition reaction. Macromol Res. 2012;20:968–76.
Kim MJ, Shin YC, Lee JH, Jun SW, Kim CS, Lee Y, et al. Multiphoton imaging of myogenic differentiation in gelatin-based hydrogels as tissue engineering scaffolds. Biomater Res. 2016;20:2.
Wang LF, Shen SS, Lu SC. Synthesis and characterization of chondroitin sulfate–methacrylate hydrogels. Carbohydr Polym. 2003;52:389–96.
Jo S, Kim S, Hwang Y, Noh I. Induction and biological evaluations of self cross-linking chondroitin sulfate-poly(ethylene oxide) hydrogel. Macromol Res. 2011;19:1303–9.
Kim KS, Kim YS, Bao K, Wada H, Choi HS, Hahn SK. Bioimaging of botulinum toxin and hyaluronate hydrogels using zwitterionic near-infrared fluorophores. Biomater Res. 2017 21:15.
Lee SY, Bang S, Kim S, Jo SY, Kim BC, Hwang Y, et al. Synthesis and in vitro characterizations of porous carboxymethyl cellulose–poly(ethylene oxide) hydrogel film. Biomater Res. 2015;19:12.
Jo S, Kim D, Woo J, Yoon G, Park YD, Tae G, et al. Development and physicochemical evaluation of chondroitin sulfate–poly(ethylene oxide) hydrogel. Macromol Res. 2011;19:147–55.
Zheng Shu X, Liu Y, Palumbo FS, Luo Y, Prestwich GD. In situ crosslinkable hyaluronan hydrogels for tissue engineering. Biomaterials. 2004;25:1339–48.
Liu W, Li Z, Zheng L, Zhang X, Liu P, Yang T, et al. Electrospun fibrous silk fibroin/poly(L-lactic acid) scaffold for cartilage tissue engineering. Tissue Eng Regen Med. 2016;13:516–26.
Shimojo AAM, Galdames SEM, Perez AGM, Ito TH, Luzo ÂCM, Santana MHA. In vitro performance of injectable chitosan–tripolyphosphate scaffolds combined with platelet-rich plasma. Tissue Eng Regen Med. 2016;13:21–30.
Song WY, Liu GM, Li J, Luo YG. Bone morphogenetic protein-2 sustained delivery by hydrogels with microspheres repairs rabbit mandibular defects. Tissue Eng Regen Med. 2016;13:750–61.
Gupta KC, Haider A, Choi YR, Kang IK. Nanofibrous scaffolds in biomedical applications. Biomater Res. 2014;18:5.
Subbiah R, Suhaeri M, Hwang MP, Kim W, Park K. Investigation of the changes of biophysical/mechanical characteristics of differentiating preosteoblasts in vitro. Biomater Res. 2015;19:24.
Iovu M, Dumais G, du Souich P. Anti-inflammatory activity of chondroitin sulfate. Osteoarthritis Cartilage. 2008;16:S14–8.
Kim MH, Kim BS, Cho D, Kwon OH, Park WH. Silk fibroin/hydroxyapatite composite hydrogel induced by gamma-ray irradiation for bone tissue engineering. Biomater Res. 2017;21:12.
Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) Grant (2014K2A2A7060928 and 2015R1A2A1A10054592) and by Grants of the Korean Health Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A120822).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflict of interest was reported by the authors.
Ethical statement
There are no animal experiments carried out for this article.
Rights and permissions
About this article
Cite this article
Bang, S., Jung, UW. & Noh, I. Synthesis and Biocompatibility Characterizations of in Situ Chondroitin Sulfate–Gelatin Hydrogel for Tissue Engineering. Tissue Eng Regen Med 15, 25–35 (2018). https://doi.org/10.1007/s13770-017-0089-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13770-017-0089-3