Abstract
The fractionation of 10 metals (As, Co, Cr, Cu, Mn, Ni, Pb, Se, V, and Zn) within labile fractions in shallow marine sediments collected from the coasts of Sabah and Sarawak, Malaysia, was evaluated. Spatial distribution revealed that coastal sediments from Sabah were approximately 10% higher in metal content than sediments from Sarawak. Risk assessment code, enrichment factor, and pollution accumulation index calculations were used to investigate the environmental hazards of elements. For the risk assessment code, the modified Tessier sequential extraction procedure was applied. The risk assessment code values showed that metal V recorded the lowest environment risk (~ 10%) while As, Co, Cr, Cu, Mn, and Zn exhibited medium risk (Risk assessment code range of 11%–30%). The element Ni displayed no risk (0.67%) to the environment, whereas Se and Pb recorded the highest risk with values of 47% and 52%, respectively. For the enrichment factor calculation, the continental crust data presented by Taylor (Taylor, Geochim Cosmochim Acta 28:1273–1285, 1964) were used as background, with metal Al used as a reference element. Results illustrated that most of the metals show enrichment (enrichment factor > 1). However, Se was considered extremely severe to the environment (enrichment factor > 50). While the pollution accumulation index calculation demonstrated that all metals under study can be considered as non-contaminant elements except for Ni, V, and Co. These findings indicated that marine sediments in Sabah are more polluted with metal contaminants than the sediments in Sarawak, despite both states having numerous active oil- and gas-related production facilities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metal pollution in coastal areas is a common environmental problem that has been discussed by many researchers (Bubb and Lester 1991; Idris et al. 2007). There are numerous sources of metal contaminations at shorelines, such as shipping traffic close to the coast, different activities (industrial and agricultural), and vehicle emissions (Basheer 2018). Metal pollution of the aquatic environment and the subsequent uptake by organisms and humans are health risks that can lead to the genetic alteration of cells or morphological abnormalities. Therefore, it is crucial to consider the level of metals in water and sediments as sediments act as a sink for contaminants.
The content of a particular metal discharged from polluted sediment depends on the metal speciation (Szefer et al. 1995; Soto-Jiménez and Páez-Osuna 2001). Generally, metals may either be bound within particles and can thus include metal complexes into organic matter, or be positioned in the facade coating deposited on the particles (Raj, Kumar, et al. 2019). The existing form of the metals controls the mobility and ecotoxicity of metals, knowledge of which is required to investigate the toxic effect and biogeochemical pathways of the metal species (Ali I 2005, Al-Mur 2020). Therefore, chemical extractions (single or sequential) are applied to illustrate the environmental effect of metals. To assess the availability of heavy metals in soils, various routine single extraction methods, either acid or complexing solutions, have been long used (Lindsay & Norvell, 1978; Ure et al., 1993). These single-pass extractions are not likely to recover all the available metals because they are onetime point measurements and are not able to mimic the reactions taking place in the soil particles (Shirvani et al., 2007). On the other hand, in sequential extraction, the obtained fractions depend on the chemicals used to extract a chemically defined phase. For instance, five fractions are obtained with the Tessier method (Tessier et al. 1979): metals associated with exchangeable fraction, metals bound to carbonate, metals that connect to hydrous oxides of Fe, and Mn, metals associated with organic and sulfide compounds and residual fraction.
The association of the metals with the chemical fractions, followed by the required strength, shows their bioavailability and the risk coupled with their occurrence in the aquatic system (Tytła 2019). Several indexes have been developed to evaluate the environmental risk of metals in surface sediments, one of which was the risk assessment code (RAC) (2011). As proposed by (Perinetetal.1985), RAC mainly applies the sum of exchangeable and carbonate-associated fractions to assess the availability of metals in sediments. The RAC evaluates the accessibility of metals in solution by applying a scale to measure the percentage of these metals in sediments that can be extracted in the exchangeable fraction and bound to carbonate fractions. Metals in these fractions have weak bonds, which may lead them to be more labile and thus reflect the pollution history of the sediments (Mohan et al. 2012).
Recently, methodologies and models for evaluating hazards, setting priorities, establishing criteria for environmental quality, and evaluating risks have been developed (Dadar et al. 2017; Shahsavani et al. 2017; Ghasemidehkordi et al. 2018; Shen et al. 2019). Several metal risk assessment indicators of sediment and environmental quality have been established on different bases, such as geo-accumulation index (Igeo), which depends on the total constituents (Loska et al. 2004, Lu, Li et al. 2009), which modified by (Karbassi et al. 2007) to pollution accumulation index (\({I}_{POLL}).\)
Risk assessment code (RAC) depends on availability (Jain 2004; Passos, Alves, et al. 2010; Thanh-Nho et al. 2019); sediment quality guidelines based on biological toxicity (Desrosiers et al. 2010), (Leung et al. 2006); and multiple variants approach (Viguri et al. 2007). Another type of model for evaluating hazards in sediment is normalization to the reference element. Normalization to the reference element method is based on the postulation that a linear association exists between the conventional element and the metals in natural conditions. If the concentration of the conventional metal changes, then because of the change in natural phenomena, the concentration of other metals will change with the same factor as the conventional metal. (Rahn 1976; Veerle 1993) reported that the reference element should have several characteristics: (i) high concentration in rock or soil, (ii) minimal pollution sources, (iii) ease of determination by many analytical techniques, and (iv) free from contamination during sampling.
Understanding the behavior of metals at the shallower sediment of Sabah and Sarawak requires studying the content and geochemical distribution of heavy metals. This study aimed to explore the pollution status at Sabah and Sarawak bays as active oil and gas exploration and production areas and use RAC, enrichment factor (EF), and Ipoll for evaluating the environmental hazard of some metals in the shallow marine sediment at both sites.
This study was carried out in the Malaysian Nuclear Agency, Malaysia, and King Faisal University, Saudi Arabia, 2020.
Materials and methods
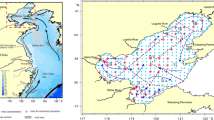
A total of 26 sediment sites were chosen at various distances (0.7–113 m) from the coasts of Sabah and Sarawak, Malaysia, within 1°45.93′–7°24.68′N latitude and 109°49.20′–119°03.78′E longitude (Fig. 1). Three samples were collected from a total depth range of 3.5–109 m and designated as surface, middle, and bottom for each site. The samples were collected using a sediment grab sampler.
Samples were stored in polyethylene containers at − 4 °C. They were then dried in a thermal oven at 50 °C and homogenized by powdering in an agate grinder. The homogenized samples were sieved through a 0.2 mm nylon grid sieve and heated at 50 °C until a constant weight was established. The moisture content of the prepared samples was measured using an AND MX-50 moisture analyzer and found to be ≤ 5%. The pH for the samples was also detected by immersing the sediment samples in deionized water with a sediment-to-water ratio of 1:5. The pH was measured after 10 min and found to range from 6.5 to 7.5(ROSSA et al. 2015).
All the reagents were of analytical grade. The glassware and plastic materials used were earlier treated for a week in 10% (v/v) HNO3and then rinsed with deionized water.
Sequential extraction procedures
For the extraction, 1.00 g of each dried sample was accurately weighed in a 100-ml pre-cleaned centrifuge tube, and then, the modified Tessier scheme was applied (Table 1). At the end of each step, the tube was centrifuged for 10 min at 4000 rpm and the solution was decanted into a 50-ml polyethylene container. Next, 10 ml of deionized water was added to the residue and centrifuged for 5 min, after which the solution was decanted to 50 ml and then stored at 4 °C (Rosado et al. 2016) until analysis. The residue was used to proceed to the next step. A blank sample was prepared for each set following the above procedure without the addition of a sample.
In the original Tessier scheme, the use of MgCl2 in the exchangeable fraction caused a matrix effect during the analysis of the extracted samples. Thus, a modification was carried out as shown in Table 1 (Filgueiras et al. 2002), where NH4CH3COOwas used instead. In the Fe–Mn oxide fraction, the concentration of NH2OH·HCl was increased from 0.04 M to 0.1 M to increase the extractability.
Digestion method
The digestion method was applied to achieve the total concentration of the metals under study. Currently, the most common method used to digest environmental samples is microwave-assisted total digestion (Sandroni and Smith 2002). For this method, 0.5 g of each dried sample was accurately weighed and mixed with 6 ml of 17 M HNO3, 2 ml of 8.8 M H2O2, and 2 ml of 0.02 M HF (Marin et al. 2008). The samples were digested in a microwave oven (MARS 5 from CEM) within the program shown in Table 2. A standard reference material, namely marine sediment (PACS-2) from the National Research Council of Canada, was treated as above. Blank samples were also prepared for each set by adding the digestion acid mixture used above without any sample. All the processes were repeated in triplicate.
The prepared sediment samples were analyzed using an ELAN 6000 PerkinElmer SCIEX ICP-MS Spectrometer. ICP multi-element calibration standard solution from ScharlauChemie was used to calibrate the system. The concentration of metals in the dry sample was calculated using Eq. (2):
where
C = digest concentration (µg /ml),
V = final volume in ml after sample preparation,
W = weight in g of wet sample and.
S = % solids/100.
One of the most effective methods for normalization to a reference element is the enrichment factor (EF) calculation (Kusin et al. 2017).
The EF can be calculated from Eq. (2):
where Cx nrichment is to be determined, Cn is the concentration of the n normalizing element assumed to be a unique characteristic of the background.
Another method that can give an effective way for measuring pollution was I geo(Loska et al. 2004), which was modified by (Karbassi et al. 2007) to pollution accumulation index (\({I}_{POLL})\) as follows:
(where Cn is the sediment metal concentration and Bn is the metal concentration in the shale) to:
where IPOLL, Bc, and Lp are indicative of pollution intensity, bulk concentration, and lithogenous portion, respectively.
Reliability of the results
Reliability of the obtained data was achieved by calculating the recovery of the fraction results of the sequential extraction procedures by the sum of the amounts of metals removed Ms in each step of the procedures with the results of the pseudo-total digestion Mt (Sect. 2.2), as shown in Eq. (5)(Hullebusch, Utomo, et al. 2005):
Results and discussion
Spatial distribution of the elements
The sample sites in Sabah and Sarawak are shown in Figs. 2 and 3, respectively. However, as shown in Fig. 2, the spatial distribution of the metals throughout Sabah’s coastal area shows a similar distribution of element As at all locations, except at sites SB05, SB13and SB15, which display about twice the As concentration of other locations. All other metals show similar distributions throughout Sabah sites, which likely indicate no different sources for metal emissions in the sample sites.
In Fig. 3, the spatial distribution of metals throughout Sarawak shows a similar pattern for elements with some exceptions. For instance, the distribution of element As is similar at all sites, except at site SR14 (~ 25%). However, all other metals display low concentrations. Similarly, about 25% of the metal content at one site, location SR13, was identified as metals Ni and Cr. This finding can be an indication of a limited variety of metal emission sources.
The comparison between the spatial distribution of the metals in Sabah and Sarawak coastal sediments indicates that, despite both states having numerous active oil- and gas-related production facilities, the metal concentrations are higher in Sabah than in Sarawak. This result indicates that marine sediments in Sabah might be exposed to external pollution sources, or the higher metal content could be linked to the geological features of the Sabah coast.
Table 3 shows the reliability of the modified Tessier procedure. When applying the modified Tessier procedure, good recovery (between 90 and 110%) for metals As, Co, Cr, Cu, Ni, Pb, V, and Zn is obtained. The recovery for Mn and Se (119% and 88%, respectively) can be accepted, though it is slightly distant from the accepted limit (Bashar Qasim 2014).
Summary of statistics of the mobile fractions
Tables 4 and 5 summarize the distribution of metals at the exchangeable and carbonate fractions using the modified Tessier sequential extraction procedure (mean, standard deviation, minimum value, maximum value, and median). The achieved minimum concentrations of the metals in the first two fractions were below the detection limit of the ICP-MS. This result agreed with the fact that Fe metal tends to associate with the Fe–Mn oxide fraction in sediments. As can be noted, the fractionation profile of elements varied between the first two labile fractions. The average concentration of metals showed that only Pb preferred to bound into the exchangeable fraction while higher concentrations of As, Cr, Co, Cu, Mn, V, and Zn were found at the carbonate fraction. It has been reported that the geochemical behavior of the Cu, Mn, and Zn ions enables be adsorbed onto the surfaces of carbonate minerals and further incorporated into the crystal lattice (Billon et al. 2002).
Quality control of the data
Quality control of the achieved results was conducted using marine sediment from the National Research Council of Canada, namely PACS-2 certified reference material (CRM). Table 6 shows the analytical and certified values, the error, and the recovery for the studied metals concerning the CRM. The table reveals that all metals understudy show good recovery (where we compare the measured concentration to actual spike concentration added) and low error (recovery = 100% ± 20%) (El Zokm et al. 2015).
Summary of statistics for total element content
Table 7 shows the summary of statistics applied to obtain the average, standard deviation, minimum value, maximum value, and median for the total concentration of samples using ICP-MS.
RAC calculations
The released metals from the fractions are classified as weakly bonded metals. If the RAC percentage is less than 1%, then the sediment is of no risk to the aquatic environment. When the percentage is in the range of 1%–10%, then it is associated with low risk. When the value of the RAC is in the range of 11%–30% and 31%–50%, then the sediment is linked with medium risk and high risk, respectively. The sediment considered to cause very high risk has a RAC value above 50% (Ghrefat and Yusuf 2006). Table 8 shows the calculated RAC percentages and the outcome from these values are discussed accordingly.
From Table 8 and the calculated RAC, metals As, Co, Cr, Cu, Mn, and Zn are considered as a medium risk to the environment as their RAC values are between 11 and 30%. The results are in agreement with the reported data for Cd, Cr, Cu, Co, Ni, Mn, Pb, and Zn on the river estuarine sediments from Mahanadi Basin, India (Sundaray et al. 2011). Moreover, metal V recorded the lowest environment risk (10%) while Ni displayed no risk (0.67%). By contrast, Se showed a high risk to the environment (47%) and Pb was considered the highest environment risk (52%). These results can be compared with those obtained by (Yu et al. 2011), who evaluated the environmental risk of heavy metals (i.e., Cu, Pb, Zn, Cd, and Cr) in surface sediments by using RAC. They found that the order of the elements according to their risk levels was Zn > Cu and Cd > Pb and Cr. (Ghrefat et al. 2011) used RAC in soil samples collected along Zerqa River, Jordan, and found that Cd came under the very high-risk category; Mn showed high risk; Cu, Ni, Pb and Zn showed the medium risk to the environment; and Cr posed a low risk to the environment.
We can conclude that the sediments of Sabah and Sarawak are at medium risk for most of the metals under investigation. However, regular environmental monitoring programs may offer data about to what extent the risk will affect the surrounding environment over a long period.
Enrichment factor
This method assumes a linear relationship between the conservative element and the heavy metals in natural conditions. If the concentration of the conservative metal changes, then due to the change in natural phenomena, the concentration of other metals will change with the same factor as the conservative element (Veerle 1993).
The EF was calculated using Eq. (2), and the results are summarized in Table 9.
The data of the continental crust were used as background data (Taylor 1964), with Al as the reference element (Pourkhabbaz 2014, Tahir 2017); the results of Al concentration are listed in Table 7. As in the literature, EF values < 1 point to no enrichment, 1–3 are slightly enriched, 3–5 are moderate, 5–10 are moderately severe, 10–25 are severe, 25–50 are very severe, and > 50 are extremely severe.
From Table 9, all metals show enrichment (EF > 1), except Co and Mn, which show no enrichment (EF < 1). Metals Cr, Cu, Ni, and V have minor enrichment (1–3), Pb and Zn are moderately severe (5–10) and As is severe (18.9). However, Se is extremely severe to the environment (EF > 50). In a reported study, Al was used as the normalizing element to study the EF of trace elements in the sediments of Italian harbors (Renzi et al. 2011). They found that only Pb and Cu were enriched in the harbors while the values of the other elements were lower than expected compared to the natural levels. In addition, Al was used earlier (Léopold et al. 2008), in which EF calculations displayed a significant enrichment of toxic metals in the area, except for Ni with 0.72–1.39. In particular, the area was strongly polluted and moderately polluted with Cd and Pb, respectively.
Pollution accumulation index
Another measurement for pollution level in sediment was taken using Eq. 4 in which IPOLL was calculated for elements under study, and the results are tabulated in Table 10. As proposed by (Karbassi et al. 2007), the lithogenous portion of metals can be calculated by summation of metal concentration in exchangeable, carbonate, bound to Fe–Mn, and organic fractions using a modified Tessier sequential extraction scheme. According to (Li et al. 2014), if the result of IPOLL ≤ mean, sediments or soils are uncontaminated to moderately contaminated, while it will be moderately contaminated if the value 1 ≤ IPOLL ≥ 2. On the other hand, the soil or sediment will be considered as heavily contaminated when 2 ≤ IPOLL ≥ 3 and it will be heavily to extremely contaminated when 3 ≤ IPOLL ≥ 4. Table 10 shows that sediment of Sabah and Sarawak is uncontaminated with all metals under study except for Ni, V (moderately contaminated), and Co (heavily to extremely contaminated).
Conclusion
The present study indicated that the metal content of shallow marine sediments is higher at Sabah coast than at Sarawak coast, despite the numerous active oil- and gas-related production facilities at both states. The spatial distribution of metals in Sabah and Sarawak coastal sediments also illustrates that marine sediments in Sabah received more metal pollution than those in Sarawak. According to the calculated RAC, which deals with the metals' concentrations in mobile fractions, most of the investigated elements in Sabah and Sarawak were at medium risk to the local environment. In terms of the EF, Co and Mn displayed no enrichment while Cr, Cu, Ni, and V showed minor enrichment. Pb and Zn were moderately severe while As was severe. However, only Se enrichment was considered extremely severe to the environment. For IPOLL, all metals under study can be considered as non-contaminant elements except for Ni, V, and Co.
A data availability statement
Data are available on request from the authors.
Code availability
Not Applicable.
References
Ali I, Gupta VK, Aboul-Enein HY (2005) Metal ion speciation and capillary electrophoresis: application in the new millennium. Electrophoresis 26:3988–4002
Al-Mur BA (2020) Geochemical fractionation of heavy metals in sediments of the Red Sea. Saudi Arabia Oceanologia 62(1):31–44
Bashar Qasim MM-H (2014) Potentially toxic element fractionation in technosoils using two sequential extraction schemes. Environ Sci Pollut Res 21(7):054–5065
Basheer AA (2018) Chemical chiral pollution: Impact on the society and science and need of the regulations in the 21st century. Chirality 30:402–406
Billon G, Ouddane B, Gengembre L, Boughriet A (2002) On the chemical properties of sedimentary sulfur in estuarine environments. Phys Chemi Chem Phys 4:751–756
Bubb JM, Lester JN (1991) The impact of heavy metals on lowland rivers and the implications for man and the environment. Sci Total Environ 100:207–233
Dadar M, Adel M, Nasrollahzadeh Saravi H, Fakhri Y (2017) Trace element concentration and its risk assessment in common kilka (Clupeonella cultriventris caspia Bordin, 1904) from southern basin of Caspian Sea. Toxin Rev 36(3):222–227
Desrosiers M, Babut M, Pelletier M, Bélanger C, Thibodeau S, Martel L (2010) Efficiency of sediment quality guidelines for predicting toxicity: the case of the st. Lawrence River Integr Environ Assess Manag 6:225–239
El Zokm GM, Okbah MA, Younis AM (2015) Assessment of heavy metals pollution using AVS-SEM and fractionation techniques in Edku Lagoon sediments, Mediterranean Sea. Egypt J Environ Sci Health, Part A 50(6):571–584
Elbehiry F, Elbasiouny H-R, Hassan B, Eric C (2019) Mobility, distribution, and potential risk assessment of selected trace elements in soils of the Nile Delta. Egypt Environ Monit Assess 191(12):713
Filgueiras AV, Lavilla I, Bendicho C (2002) Chemical sequential extraction for metal partitioning in environmental solid samples. J Environ Monit 4:823–857
Ghasemidehkordi B, Malekirad AA, Nazem H, Fazilati M, Salavati H, Shariatifar N, Rezaei M, Fakhri Y, Mousavi Khaneghah A (2018) Concentration of lead and mercury in collected vegetables and herbs from Markazi province, Iran: a non-carcinogenic risk assessment. Food Chem Toxicol 113:204–210
Ghrefat H, Yusuf N (2006) Assessing Mn, Fe, Cu, Zn, and Cd pollution in bottom sediments of Wadi Al-Arab Dam Jordan. Chemosphere 65(11):2114–2121
Ghrefat HA, Yusuf N, Jamarh A, Nazzal J (2011) fractionation and risk assessment of heavy metals in soil samples collected along Zerqa River, Jordan. Environ Earth Sci 66:199–208
Hullebusch EDV, Utomo S, Zandvoort MH, Lens PNL (2005) Comparison of three sequential extraction procedures to describe metal fractionation in anaerobic granular sludges. Talanta 65(2):549–558
Idris AM, Eltayeb MAH, Potgieter-Vermaak SS, Grieken RV, Potgieter JH (2007) Assessment of heavy metals pollution in Sudanese harbours along the Red Sea Coast. Microchem J 87:104–112
Jain CK (2004) metal fractionation study on bed sediments of River Yamuna. India Water Res 38:569–578
Karbassi AR, Monavari SM, Nabi Bidhendi GhR, Nouri J, Nematpour K (2007) Metal pollution assessment of sediment and water in the Shur River. Environ Monit Assess 147(1):107
Kusin FM, Rahman MS, Madzin Z, Jusop S, Mohamat Yusuff F, Ariffin M (2017) The occurrence and potential ecological risk assessment of bauxite mine-impacted water and sediments in Kuantan, Pahang Malaysia. Environ Sci Pollut Res 24(2):1306–1321
Léopold EN, Jung MC, Auguste O, Ngatcha N, Georges E, Lape M (2008) Metals pollution in freshly deposited sediments from river Mingoa, main tributary to the Municipal lake of Yaounde. Cameroon Geosci J 12:337
Leung A, Cai ZW, Wong MH (2006) Environmental contamination from electronic waste recycling at Guiyu, Southeast China. J Mater Cycles Waste Manag 8:21–33
Li P, Qian H, Howard KW, Wu J, Lyu X (2014) Anthropogenic pollution and variability of manganese in alluvial sediments of the Yellow River, Ningxia, northwest China. Environ Monit Assess 186(3):1385–1398
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci Soc Am J 42:421–428
Loska K, Wiechua D, Korus I (2004) Metal Contamination of farming soils affected by industry. Environ Int 30:159–165
Lu X, Li L, Wang L, Lei K, Huang J, Zhai Y (2009) Contamination assessment of mercury and arsenic in roadway dust from Baoji China. Atmos. Environ 43(15):2489–2496
Marin B, Chopin EIB, Jupinet B, Gauthier D (2008) Comparison of microwave-assisted digestion procedures for total trace element content determination in calcareous soils. Talanta 77(1):282–288
Mohan M, Augustine T, Jayasooryan KK, Shylesh Chandran MS, Ramasamy EV (2012) Fractionation of selected metals in the sediments of Cochin estuary and Periyar River, southwest coast of India. Environmentalist 32(4):383–393
Passos E, Alves J, Santos ID, Alves J, Garcia C, Costa AS (2010) Assessment of trace metals contamination in estuarine sediments using a sequential extraction technique and principal component analysis. Microchem J 96:50–57
Perin G, Craboledda L, Lucchese M, Cirillo R, Dotta L, Zanetta M, Oro A, (1985) Heavy metal speciation inthe sediments of northern adriatic sea. a new approach for environmental toxicity determination. In: T.D. Lekkas (Ed.), Heavy Metal in the Environment 2
Pourkhabbaz MNAA (2014) Application of geoaccumulation index and enrichment factor for assessing metal contamination in the sediments of Hara Biosphere Reserve Iran. Chem Spec Bioavailab 26(2):99–105
Rahn KA (1976) The chemical composition of the atmospheric aerosol. University of Rhode Island, USA
Raj D, Kumar A, Maiti S (2019) Evaluation of toxic metal(loid)s concentration in soils around an open-cast coal mine (Eastern India). Environ Earth Sci 78:1–9
Renzi M, Tozzi A, Baroni D, Focardi S (2011) Factors affecting the distribution of trace elements in harbour sediments. Chem Ecol 27(3):235–250
Rosado D, Usero J, Morillo J (2016) Ability of 3 extraction methods (BCR, Tessier and protease K) to estimate bioavailable metals in sediments from Huelva estuary (Southwestern Spain). Mar Pollut Bull 102(1):65–71
Rossa CG, Fernandes PM, Pinto A (2015) Measuring foliar moisture content with a moisture analyzer. Can J for Res 45:776–781
Sandroni V, Smith CMM (2002) Microwave digestion of sludge, soil and sediment samples for metal analysis by inductively coupled plasma-atomic emission spectrometry. Anal Chim Acta 468(2):335–344
Shahsavani A, Fakhri Y, Ferrante M, Keramati H, Zandsalimi Y, Bay A, Hosseini Pouya SR, Moradi B, Bahmani Z, Mousavi Khaneghah A (2017) Risk assessment of heavy metals bioaccumulation: fished shrimps from the Persian Gulf. Toxin Rev 36(4):322–330
Shen F, Mao L, Sun R, Du J, Tan Z, Ding M (2019) Contamination evaluation and source identification of heavy metals in the sediments from the Lishui River Watershed, Southern China. Int J Environ Res Public Health 16(3):336
Soto-Jiménez MF, Páez-Osuna F (2001) Distribution and normalization of heavy metal concentrations in mangrove and lagoonal sediments from Mazatlán Harbor (SE Gulf of California). Estuar Coast Shelf Sci 53(3):259–274
Sundaray S, Nayak B, Lin S, Bhatta D (2011) Geochemical Speciation and risk assessment of heavy metals in the river estuarine sediments—a case study: Mahanadi basin, India. J Hazard Mater 186:1837–1846
Szefer P, Kusak A, Szefer K, Jankowska H, Lowicz M, Ali AA (1995) Distribution of selected metals in sediment cores of Puck Bay. Baltic Sea Marine Pollut Bull 30(9):615–618
Tahir S-CPANM (2017) The common pitfall of using enrichment factor in assessing soil heavy metal pollution. Malays J Anal Sci 21(1):52–59
Taylor SR (1964) Abundance of chemical elements in the continental crust: a new table. Geochim Cosmochim Acta 28(8):1273–1285
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51(7):844–851
Tham TT, Lap BQ, Mai NT, Trung NT, Thao PP, Huong NTL (2021) Ecological risk assessment of heavy metals in sediments of Duyen hai seaport area in Tra Vinh Province Vietnam. Water, Air, & Soil Pollut 232(2):49
Thanh-Nho N, Marchand C, Strady E, Vinh T-V, Nhu-Trang T-T (2019) Metals geochemistry and ecological risk assessment in a tropical mangrove (Can Gio, Vietnam). Chemosphere 219:365–382
Tytła M (2019) Assessment of heavy metal pollution and potential ecological risk in sewage sludge from municipal wastewater treatment plant located in the most industrialized region in poland—case study. Int J Environ Res Public Health 16(13):2430
Ure M, Thomas R, Littlejohn D (1993) Ammonium acetate extracts and their analysis for the speciation of metal ions in soils and sediments. Int J Environ Anal Chem 51:65–84
Veerle VA (1993) Concentration and partitioning of heavy metals In: The Sheldt Estuary. phd, Antwerpen University
Viguri JR, Irabien MJ, Yusta I, Soto J, Gómez J, Rodriguez P, Martinez-Madrid M, Irabien JA, Coz A (2007) Physico-chemical and toxicological characterization of the historic estuarine sediments: a multidisciplinary approach. Environ Int 33:436–444
Yu GB, Liu Y, Yua S, Wu SC, Leung AOW, Luo XS, Xu B, Li HB, Wong MH (2011) Inconsistency and comprehensiveness of risk assessments for heavy metals in urban surface sediments. Chemosphere 85:1080–1087
Acknowledgements
The authors acknowledge the Deanship of Scientific Research at King Faisal University, for the financial support under Ambitious Researcher [GRANT 496].
The authors also acknowledge the Malaysian nuclear Agency for their support in samples collection and sample preparation.
Funding
Funding for this study was received from the Deanship of Scientific Research at King Faisal University, Ambitious Researcher [GRANT 496].
Author information
Authors and Affiliations
Contributions
AYA was involved in conceptualization, investigation, visualization, methodology, and writing the original draft preparation. MPA carried out supervision. SMS was responsible for statistical analysis, and writing, reviewing, and editing.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing interests.
Ethics approval
Not Applicable.
Consent to participate
Not Applicable.
Consent for publication
Not Applicable.
Additional information
Editorial responsibility: S. R. Sabbagh-Yazdi.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ahmed, A.Y., Abdullah, M.P. & Siddeeg, S.M. Environmental hazard assessment of metals in marine sediments of Sabah and Sarawak, Malaysia. Int. J. Environ. Sci. Technol. 20, 7877–7886 (2023). https://doi.org/10.1007/s13762-022-04514-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-022-04514-z