Abstract
Carbon dioxide (CO2) is emitted into the atmosphere due to some anthropogenic activities, such as the combustion of fossil fuels and industrial output. As a result, fears about catastrophic global warming and climate change have intensified. In the face of these challenges, conventional CO2 capture technologies are typically ineffective, dangerous, and contribute to secondary pollution in the environment. Biological systems for CO2 conversion, on the other hand, provide a potential path forward owing to its high application selectivity and adaptability. Moreover, many bacteria can use CO2 as their only source of carbon and turn it into value-added products. The purpose of this review is to discuss recent significant breakthroughs in engineering bacteria to utilize CO2 and other one-carbon compounds as substrate. In the same token, the paper also summarizes and presents aspects such as microbial CO2 fixation pathways, engineered bacteria involved in CO2 fixation, up-to-date genetic and metabolic engineering approaches for CO2 fixation, and promising research directions for the production of value-added products from CO2. This review's findings imply that using biological systems like modified bacteria to manage CO2 has the added benefit of generating useful industrial byproducts like biofuels, pharmaceutical compounds, and bioplastics. The major downside, from an economic standpoint, thus far has been related to methods of cultivation. However, thanks to genetic engineering approaches, this can be addressed by large production yields. As a result, this review aids in the knowledge of various biological systems that can be used to construct a long-term CO2 mitigation technology at an industrial scale, in this instance bacteria-based CO2capture/utilization technology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
At the end of the 21st Conference of the Parties to the United Nations Framework Convention on Climate Change in December 2015, 195 countries signed the Paris Agreement. The agreement intends to “strengthen the global response to the threat of climate change” by limiting global average temperature rises to “well below 2 degrees Celsius above pre-industrial levels.” (United Nations 2015). Carbon dioxide (CO2) emissions have been continuously increasing in recent years, and these worrying trends are expected to continue. The rise in the earth’s temperature started just at the dawn of the industrial age, resulting in an increase in the amount of the so-called Greenhouse Gases (GHG) such as CO2, CH4, N2O, and chlorofluorocarbons (Ekwebelem et al. 2020). Over 80% of the global energy production is made from the burning of fossil fuels (Barati et al. 2021; Olivier and Peters 2020). These industrial processes release a significant amount of CO2 (Omoregbe et al. 2020; Wang et al. 2020), which makes up 68% of the total emissions (Olivier and Peters 2020). However, these GHGs have played a fundamental role in maintaining our planet’s temperature and life as we know it today (Barati et al. 2021; Senatore et al. 2020). On the other hand, an increase in food demand production is another global challenge linked to carbon fixation (Gleizer et al. 2020). Furthermore, a global effort to minimize Carbon footprint necessitates the decarbonization of several global major industries (de Blas et al. 2020; du Pont et al. 2016). In this light, the extensive production of carbon–neutral fuels (e.g., biodiesel, bioethanol, biomethanol, hydrogen, etc.) for transportation and energy storage has been identified as a sustainable approach (de Blas et al. 2020; du Pont et al. 2016; Ekwebelem et al. 2020; Gleizer et al. 2020; Kumar et al. 2018; Obileke et al. 2021).
To mitigate this global challenge (CO2 emission), more sustainable strategies have been proposed such as advancing the energy efficiency of the current technologies to improve CO2 fixation and improving natural CO2 capturing effectiveness (Kumar et al. 2018). Interestingly, efforts in the form of large-scale Carbon Capture and Storage (CCS) projects (about 39) are ongoing worldwide following these proposed sustainable approaches (Budinis et al. 2018). Unfortunately, only 29 (the number had increased from 17 in 2018) are fully operational (Jaganmoha 2021), while the starting financial demands have also greatly limited its global development (Budinis et al. 2018). However, state-of-the-art developments in genetic engineering and membrane biotechnologies have today made it possible to tackle these economic barriers using the same microorganisms that have carried out carbon sequestration and fixation in the carbon cycle for decades (Pattharaprachayakul et al. 2020; Schweitzer et al. 2021; Zahed et al. 2021). This microbial CO2 sequestration and fixation aid by ribulose 1,5-bisphosphate carboxylase/oxygenase (RuBisCO) and Carbonic Anhydrase (CA) are common in both archaeal and bacterial domains (Hu et al. 2019; Saini et al. 2011; Salehizadeh et al. 2020). Through these advances, CO2 can be efficiently converted into biomass and useful compounds such as CO, CH4, CH3OH, DME, olefins, and higher hydrocarbons that can contribute significantly in protecting the ecosystem (De Vietro et al. 2019; Tursi 2019; Tursi et al. 2019).
Due to the current environmental challenges, utilizing CO2 as a bio-feedstock for sustainable food and fuel production is attracting immense interest. Moreover, the effectiveness of abiotic solutions for CO2 utilization is limited by low product selectivity (generating unwanted products), extreme condition requirements for full functionality, and specificity to the composition of the feedstock (Gleizer et al. 2020). Biotic solutions, on the other hand, can overcome these limitations because they require certain climate conditions and are very specialized and resilient to environmental changes and suspended particles in chemicals (Gleizer et al. 2020; Senatore et al. 2020). Therefore, another approach—synthetic biology—has been leveraged as a promising way of overcoming these challenges. Through this approach, microorganisms and biosynthetic pathways can be modified by linking two pathways that do not co-exist naturally or localizing a pathway to an organelle to enable improvement over some of their limiting natural components. Furthermore, synthetic biologists and molecular biologists have developed engineering tools capable of modeling and engineering organisms with an enhanced potential to fast-track the pace of innovation and bioprocess optimization (Antoniewicz 2019; Yadav 2021). Not surprisingly, our knowledge base in molecular and synthetic biology is expanding simultaneously with our knowledge on how to mitigate global CO2 emissions for a clean environment. Undeniably, it is imperative to continue the efforts of developing sustainable strategies for minimizing carbon footprint. This review discusses various techniques that have been utilized to enhance the ability of bacteria to capture and utilize CO2.
Global CO2 emission trends
Prior to around 459,000 years ago, the CO2 concentration in the atmosphere was consistently less than 260 parts per million volume (ppmv) (Data 2019; Lüthi et al. 2008; Pisaric and Smol 2021). However, between 660 and 670,000 years ago, this value reached its lowest value of 170ppmv. This value quickly grew during the industrial age, reaching 386ppmv in 2010, with an annualized rate of roughly 2ppmv (Data 2019). The highest known value is 419 ppmv, which was reported in June 2021 (NOAA 2021). The global CO2 emissions have been 1.6% higher in June 2021 than June 2020 (NOAA 2021). GHG emissions are currently 57% greater than in 1990 and 43 percent higher than in 2000 (excluding land-use change) (NOAA 2021). As shown in Fig. 1., there was a dramatic and progressive rise in CO2 emissions after the industrial revolution, from 9.34 billion metric tons in 1960 to 36.44 billion metric tons in 2019. Studies show that this continuous increase has been the case since the start of the second industrial age (Hashimoto 2019). The only recorded reduction in CO2 emission globally was in 2020, which is due to the global lockdown caused by the COVID-19 pandemic (Global Carbon Project 2020). The global lockdown led to a decrease in global emissions of greenhouse gases as well as those resulting from non-combustion.
CO2 emissions from fossil fuels are the most significant contributor to GHG, with China (10.06GT), USA (5.42GT), India (2.65GT), Russia (1.16GT), and Japan (1.16GT) being the major contributors (Global Carbon Project 2020). Other major contributors making up the top ten list are Germany (0.75GT), Iran (0.72GT), South Korea (0.65GT), Saudi Arabia (0.62GT), and Indonesia (0.61GT) (Global Carbon Project 2020). Since 1880, when global average temperature increases were first recorded, 2020 was by a narrow margin one of the six hottest years (2015–2020), effectively tying 2016, the previous record (NASA 2021). Just like in 2019, when temperatures were warmer than average globally, temperatures throughout Europe, the Middle East, parts of Asia, and New Zealand have reached new highs. (Global Carbon Project 2020; Yoro and Daramola 2020). Fortunately, carbon capture, also known as sequestration, is an effective strategy to scavenge carbon dioxide from the atmosphere (Hart and Onyeaka 2020). It has become an effective approach to mitigate global warming through carbon footprint reduction. In this way, GHGs released through natural and anthropogenic activities and accumulated in the ecosystem are slowed down. In light of this, Fig. 1 shows an updated worldwide CO2 emission trajectory for 2020, gathered from earlier studies (Fraccascia and Giannoccaro 2019; Greer et al. 2019a, 2019b; Holz et al. 2018; Zhang et al. 2019).
Carbon capture and storage (CCS) and carbon capture and utilization (CCU) technologies
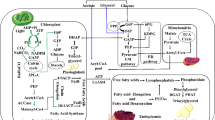
Carbon capture and storage (CCS) and carbon capture and utilization (CCU) are technologies that capture emissions of CO2 from point sources (e.g., industrial operations and power plants) for storage to prevent them from being released into the atmosphere (Markewitz et al. 2012). The distinction between CCS and CCU lies in where the captured CO2 ends up. CCS involves transferring collected CO2 to a suitable location for prolonged storage (Markewitz et al. 2012; Weisser 2007; Zapp et al. 2012), while CCU involves converting captured CO2 into value-added products (Markewitz et al. 2012). Figure 2 summarizes the various CCS and CCU choices. Post-conversion, pre-conversion, and oxy-fuel combustion are the three CO2 capture alternatives (Singh et al. 2011; Zaimes and Khanna 2013). It should be noted that the purpose of this article is not to offer a comprehensive technical review of CCS and CCU technologies; rather, it is to provide context and perspective for the article's main goal, which is to review and analyze recent significant breakthroughs in engineering bacteria utilize CO2 and other one-carbon compounds as substrate. Figure 3 summarizes these points. Another possibility is CO2 fixation by biomass. Because of the need for biofuels, microalgae are now being employed for this purpose. As a result, this is potentially a CCU option rather than a CCS approach. This is because microalgae would not be cultivated only to capture CO2 (Cuéllar-Franca and Azapagic 2015). From an economic standpoint, CCU promises to be a better alternative than CCS because CCS is a non-profitable operation. However, to maintain a positive economic and environmental balance, CCU's cost-effectiveness and environmental implications must be closely evaluated (Cuéllar-Franca and Azapagic 2015). As previously stated, instead of storing, the captured CO2 can be applied in the production of as a commercial product, either directly or after conversion. CO2 may be used directly in the food and beverage sector, as well as for enhanced oil recovery (EOR); it can also be processed into chemicals or fuels (Cuéllar-Franca and Azapagic 2015). Other solutions include increased oil and coal-bed methane recovery, CO2 conversion to chemicals and fuels, mineral carbonation, microalgae-based biofuels, liquid fuel production, urea production and yield boosting, and many other avenues that involves the utilization of CO2 as a chemical feedstock (Bains et al. 2017). The main challenge to CCU becoming a climate mitigation potential is that there are limited possibilities to have a meaningful effect by offsetting merely 1% of yearly CO2 emissions in the United States (Aresta and Dibenedetto 2007). The energy-intensive process of CO2 conversion, regulated by thermodynamics, is the fundamental challenge. As a result, in certain situations, the link to renewable energy may be an all-too-quick contrast to its disadvantageous carbon balance (von der Assen et al. 2014). Nonetheless, CO2-based processing (von der Assen and Bardow 2014) is a less carbon-intensive current method. To resolve this issue, a thorough examination of the CO2-emitting industry is required to determine the entire scope of its implications.
(Cuéllar-Franca and Azapagic 2015).
Bacteria growth for CO2 capture and utilization
It is well known that higher plants and microalgae possess the ability to fix CO2; however, bacteria have many benefits over these species, including a considerably quicker rapid growth rate and life cycle, the capacity to exist in a culture of high density, and the ability to be genetically engineered more readily (Bharti et al. 2014). Other than that, bacteria, just like other microbes, can produce a broad range of bio-alcohols and fatty acids for oil production which are essential industrial compounds (Bharti et al. 2014; Mohan et al. 2016).
The most popular industrial growing techniques for bacteria, like microalgae, are open ponds. Open ponds, comparatively, are a cost-effective culture system, however, it require a large surface area and are prone to contamination, which are two factors that are considered major limitations. Other more useful methods for bacteria growth for CCU are bioreactors and photobioreactors. Even for industrial applications, bioreactors and photobioreactors allow for the regulation of a broad variety of living conditions, but they are still highly costly equipment (Costa et al. 2006; Ketheesan and Nirmalakhandan 2012). Even though researchers have identified bacteria as a feasible option for CO2 capture from the atmosphere, the downside to their utilization involves the significant amount of work needed for their maintenance (Kumar et al. 2018; Saini et al. 2011). One of the most important qualities of this kind of system is advanced growth control settings, such as maintaining temperature and certain pH levels, controlling light, adding nutrients, and other environmental factors that prevent external contamination of pure cultures (Costa et al. 2006; Goli et al. 2016; Jajesniak et al. 2014). However, since bacterial cultures in open ponds systems are fragile, the most popular culture methods for these bacteria are flat panel photobioreactors and horizontal tube photobioreactors (Gebicki et al. 2009; Norsker et al. 2011). To effectively engineer a growth system for bacteria for CCU processes, a rectangular or square base frame is covered on both ends by a transparent panel in a flat panel photobioreactor. The level of aeration is maintained at 1L of air per liter of photobioreactor volume per minute (Sierra et al. 2008), and the photobioreactor is developed to take advantage of sunshine (Carvalho et al. 2006). Polyethylene, Polyvinyl Chloride (PVC or Vinyl), Poly(methyl methacrylate), or other acceptable materials with the correct thickness are used to ensure the gas tightness of the enclosed space, resist hydrostatic pressure, and decrease panel deflection (Gebicki et al. 2009; Norsker et al. 2011). A steel mesh frame binds the panels together to maintain the seal (Norsker et al. 2011). Flat panel photobioreactors are particularly attractive because when the thickness is maintained to a minimum, they have a high area-to-volume ratio which is favorable to bacteria growth (Carvalho et al. 2006). On the other hand, horizontal tubular photobioreactors are transparent tubular reactors with a certain inclination (less than 10 degrees). Interestingly, their orientation toward sunlight assists positively in a high light conversion efficiency (Dasgupta et al. 2010; Gebicki et al. 2009).
CO2 capture and utilization by bacteria
Admittedly, the rise in CO2 amount, attributable to anthropogenic activities, has a serious effect on the ecosystem and there is an acknowledged need to develop technologies for the sustainable capture and utilization CO2 (Chu et al. 2017; Jiang et al. 2019; Mustafa et al. 2020; Vidales et al. 2019). As a result, the generation and usage of renewable energy have piqued people's curiosity (HASSOUN and Hicham 2020; Mikhno et al. 2021). Primarily, to achieve a carbon–neutral environment with a sustainable paradigm, the amount of CO2 emission should be equal to the amount used (Mohan et al. 2016; Senftle and Carter 2017). Fortunately, various methods, such as biological CO2 conversion using microbes (Chiranjeevi et al. 2019; Ghosh and Kiran 2017; Molitor et al. 2019; Sultana et al. 2016), chemo-catalytic CO2 conversion via organic or inorganic catalysts (Aresta et al. 2014; Taheri Najafabadi 2013), light-induced or electrocatalytic CO2 conversion (Hu et al. 2013; Ma et al. 2014; Tu et al. 2014), and catalytic hydrogenation of CO2 (Ashley et al. 2009; Chang et al. 2017; Rodemerck et al. 2013; Saeidi et al. 2014; Wang et al. 2018), have shown the capacity to convert CO2 to bio-based products. However, from a large-scale point of view, none of these novel methods can solely resolve CO2 capture and usage problems.
Just as autotrophic bacteria are innately wired to utilize CO2 as the sole carbon using light energy or inorganic compounds (Fry and Peel 2016), methylotrophic bacteria, on the contrary, have the potential to utilize reduced one-carbon compounds containing no carbon–carbon bonds (example formate, methanol, and other methylated compounds) as sole carbon sources and energy (Kumar et al. 2019b, 2016). Interestingly, these microbes can be modified genetically to enhance their suitability for bioproduction processes, even in industrial settings. A US company LanzaTech currently produces simple substrates like acetone, ethanol, and lactate from waste syngas and flue gas using acetogens, and autotrophic bacteria (Liew et al. 2016). In this gas fermentation process, CO2, CO, and H2 served as the carbon and reducing energy sources. Furthermore, several high-profile projects on CO2 capture at pilot or industrial size have been carried out and developed in many industrialized nations Italy (EniTecnologie), Germany (E’ON Hanse AG, Vattenfall’s Senftenberg), New Zealand (LanzaTech), Netherlands (Algaelink), United Kingdom (AlgaeCAT), Canada (Carbon2Algae Solutions Inc. and the Natural Research Council (NRC), China (Hearol project), USA (Touchstone Research Laboratory, GreenFuel Technologies, Agcore Technologies’ COPAS™) (Salehizadeh et al. 2020). At the laboratory scale, Sakimoto (Sakimoto et al. 2016) investigated the solar-to-chemical potential of a biological-inorganic hybrid (Moorella thermoacetica with cadmium sulfide nanoparticles) to generate acetic acid from CO2 under visible radiation. These appreciable findings suggested a self-replicating approach toward solar-to-chemical CO2 reduction via bacteria. Subsequently, various inorganic-biological hybrid systems for CO2 capture and utilization were developed. In the studies, the metabolic flexibility of the bacteria was adapted by incorporating light-harvesting inorganic materials to initiate the transformation of CO2 into bio-based commodities (Ding et al. 2019; Kumar et al. 2019a; Wang et al. 2019; Ye et al. 2019).
Recent work has shown that bacterial isolates (Bacillus altitudinis) from mangrove sediments in India with positive carbonic anhydrase (CA) activity, showed significant sequestering ability with a reduction of 97% CO2 (Nathan and Ammini 2019). In an earlier study, researchers genetically modified a lithoautotrophic Gram-negative bacteria (Ralstonia eutropha) to generate isobutanol and 3-methyl-1-butanol utilizing CO2 as the sole source of carbon and electricity as the only energy input (Li et al. 2012). Liu et al. coupled the same bacterium (Ralstonia eutropha) with a cobalt-phosphorus water-splitting catalyst in subsequent research to convert CO2 straight into biomass, biofuels, or other value-added products (Liu et al. 2016). Furthermore, owing to the simple growth requirement of Heterotrophs like Escherichia coli, some studies have explored its potential for efficient capture and conversion of CO2 and other one-carbon compounds. Interestingly, these studies shared a considerable similarity in their sources of energy, which are one-carbon compounds (formate or methanol) that can be produced via electrochemical reduction of CO2 (Marlin et al. 2018; Yishai et al. 2016). For example, Chen et al. explored the ability of engineered E. coli to grow on methanol which is a renewable one-carbon (C1) feedstock for microorganisms (Chen et al. 2020). By using the reductive glycine pathway, Kim et al. explored the growth of reprogrammed E. coli on formate and methanol as a sustainable bioproduction rooted in CO2 and renewable energy (Kim et al. 2020). Also, Gleizer et al. studied the potential of E. coli to generate all biomass carbon from CO2 conversion (Gleizer et al. 2019).
Notwithstanding these revolutionary advances, some downsides such as poor multiplication rates, inadequate characterization, and incomplete validation at the industrial level have currently made these strains unsuitable for utilization at an industrial scale (Gleizer et al. 2020). Interestingly, Gleizer et al. (Gleizer et al. 2020) highlighted that the capacity of these modified microbes to generate energy from one-carbon compound, when combined with the electrochemical conversion of CO2 to one-carbon compound, increases the opportunities for a carbon–neutral economy. Another innovative approach is the use of hybrid systems. Hybrid systems are biotic-abiotic technologies that combine the best of both worlds and it is predicted that they will eventually outperform photosynthesis in terms of yields and energy effectiveness (Gleizer et al. 2020). For instance, a newly created hybrid microbe–metal interface integrates an inorganic, semiconducting light-harvester material with efficient and simple bacteria to create a revolutionary metal–microbe interface that aids microorganisms indirectly in capturing energy from the sun (Sahoo et al. 2020). Further, Su and colleagues studied the efficiency of nanowire-bacteria hybrids for Solar-powered CO2 Fixation (Su et al. 2020). They were able to enhance the CO2-reducing efficiency in a silicon nanowire/Sporomusa ovata system by looking into the microorganism-cathode interface. The rate of CO2 reduction at high voltage was inherently limited by a poor bacterium nanowire interface caused by an unfavorable alkaline environment (Su et al. 2020). In this study, the creation of a close-packed nanowire-bacteria cathode was aided by adjusting the bulk electrolyte pH and improving its buffering volume (Su et al. 2020).
It has been recommended that photovoltaic cells possess a greater energy conversion efficiency than photosynthesis in producing H2 and CO as feedstocks for archaea (Gleizer et al. 2020). These technologies are fundamentally modular, offering the selection of a biological host and a source of energy independent of one another (Gleizer et al. 2020). Similarly, the use of genetically tractable microorganisms is another appealing technology because it makes it easier to introduce new pathways (Gleizer et al. 2020). For instance, using H2 as a source of energy in E. coli, H2 is generated more effectively than formate and is suitable for the growth of microorganisms (Claassens et al. 2018). In the same token, genetically tractable hosts can also be used to introduce novel biosynthetic pathways to generate value-added products (Pontrelli et al. 2018). While these scientific achievements have been referred to as a “milestone,” there is still a long way to go, as it will be a few years before we can see this microorganism in action at industrial scale (Callaway 2019).
Engineering approach to improving CO2 capture by bacteria
Recent technological advances in comprehending microbial metabolic pathways, decoding genetic makeups, and much more have revolutionized the way we unravel the code of life, allowing us to make modifications that were seemingly unimaginable (Jiang et al. 2021; Lee et al. 2012; Majidian et al. 2018; Park et al. 2018b). Chemicals can be divided into four categories if they are found or reported to exist in nature “natural vs. non-natural,” and whether or not they can be manufactured by microbes' pathways “inherent vs. noninherent”: i) natural-inherent chemicals; (ii) natural-noninherent chemicals; (iii) nonnatural-noninherent chemicals; and (iv) nonnatural-created chemicals (Lee et al. 2012). Metabolic engineers analyze not just the effectiveness of a proposed metabolic pathway but also the most efficient means of constructing it in their efforts to obtain these many categories of molecules. Natural-inherent compounds, for example, can frequently be overproduced by directly altering the host strain to maximize native pathway fluxes at the system level (Lee et al. 2012). Consequently, microbial metabolic pathway engineering may concentrate on more intuitive approaches that employ standard metabolic and bioprocess engineering approaches to solve well-defined and well-known challenges (Lee et al. 2012).
Bacteria, namely E. coli, are one of the first genetically modified prokaryotic organisms (Li et al. 2015; Yang et al. 2020). This microorganism demonstrates a wide range of mutations as a result of the application of physical or chemical mutagens that will be chosen (Choi et al. 2016; Jajesniak et al. 2014; Li et al. 2015; Yang et al. 2020). This is because of their fast growth feature and the selective media on which they are cultured (Jajesniak et al. 2014). For example, after overnight growth, E. coli generates approximately 109 cells per milliliter (U/mL) (Senatore et al. 2020). Up to this point, only six CO2 fixation pathways have been suggested: (i) the Calvin–Benson–Bassham (CBB) cycle; (ii) the 3-hydroxypropionate/4-hydroxybutyrate cycle (3HP-4-HB); (iii) the dicarboxylate/4-hydroxybutyrate (DC/4-HB) cycle; (iv) the 3-Hydroxyproppionate bicycle (3-HP/malyl-CoA cycle); (v) the reductive tricarboxylic acid (rTCA) cycle and; (vi) the Wood–Ljungdahl (WL) (Saini et al. 2011; Salehizadeh et al. 2020). The aerobic pathways include the CBB, 3HP-4HB, and 3-HP/malyl-CoA, whereas the anaerobic pathways are the rTCA, WL, and DC/4HB (Saini et al. 2011; Salehizadeh et al. 2020).
A lot of work has recently gone into creating potential CO2 fixation pathways utilizing synthetic biology (Gong et al. 2016), and protein and metabolic engineering (Zhou et al. 2016). Synthetic biology concentrates on redesigning and repositioning innate pathways for CO2 fixation, modifying CO2-fixation pathways to increase CO2 delivery, and developing and optimizing the efficiency and durability of CO2 fixation enzymes to enable effective CO2 fixation (Gong et al. 2016, 2018). In a proof-of-concept experiment seeking to overhaul E. coli’s diet, Antonovsky and colleagues (Antonovsky et al. 2016) successfully introduced the ability to synthesize biomass from CO2 into E. coli, a heterotrophic organism. They developed a strain that absorbed CO2; however, it only represented a minute fraction of the organism's carbon intake; the remainder originated from an organic substance called pyruvate, which was supplied via a non-native Calvin–Benson–Bassham (CBB) cycle in evolved E. coli (Antonovsky et al. 2016).
In another latest work, metabolic rewiring and directed evolution generated E. coli strains that utilize CO2 as its primary source of carbon, with formate being oxidized to meet all of the reducing power and energy requirements via non-native CBB cycle (Gleizer et al. 2019). This led to the successful rewiring of obligate heterotrophs to full autotrophy over laboratory timescales (Gleizer et al. 2019). When compared to regular E. coli, which may grow exponentially every 20 min, autotrophic E. coli are slackers, multiplying every 18 h when cultivated in a 10% CO2 atmosphere (Gleizer et al. 2019). Hence, the emerging picture suggests that they cannot live without sugar at the current CO2 levels in the atmosphere, which are 0.041 percent. In trying to understand the genetic basis underlying this metabolic transition, Herz et al. suggest that five mutations are enough to permit robust growth when a non-native CBB cycle supplies all the metabolic building blocks derived sugar (Herz et al. 2017). These mutations can be discovered in enzymes (prs, serA, and pgi) that impact the efflux of intermediates from the autocatalytic CO2 fixation cycle to biomass or in critical regulators (crp and ppsR) of carbon metabolism (Herz et al. 2017).
More studies have also demonstrated an admirable example of carbon metabolism plasticity in carbon-fixing bacteria. In Synechococcus elongatus PCC 7942, the CBB cycle and Embden–Meyerhof pathway were engineered to enhance carbon flow in favor of CO2 fixation. The hexose monophosphate shunt (HMP Shunt) pathway was reconfigured to increase ribulose-5-phosphate (Ru5P) as a precursor to CO2 fixation, which improved glucose metabolism (Kanno et al. 2017). To increase ribulose-5-phosphate to ribose-1,5-bisphosphate conversion and regulate cyanobacteria’s carbon metabolism, part of the operator gene (cp12) in the CBB cycle was removed. In the absence of light, this resulted in increased synthesis of 2,3-butanediol (Kanno et al. 2017). In another case, a biosystem coupling Acetobacterium woodii (an acetogen) with Acinetobacter baylyi ADP1 (a non-native alkane producer) designed for alkane production was proven by Lehtinen et al. (Lehtinen et al. 2018). In their study, nine synthetic two-step alkane biosynthesis pathways were designed and produced in A. baylyi using a combination of aldehyde- and alkane-producing enzymes. Although the generation of drop-in liquid fuels from CO2 was shown, the modular system's alkane productivity remained low, posing a huge research challenge in the future (Lehtinen et al. 2018). Moving forward, these innovations would result to lower emissions than those produced using traditional techniques, and they could even remove the CO2 from the atmosphere. Moreover, recent advances are now concentrating on the biotechnological enhancement of cyanobacteria and microalgae cultivation through biofilm Photobioreactors (PBRs) as a sustainable alternative to cut the cost of production at the industrial scale. PBRs have the benefits of requiring less water and having a comparatively simple harvesting process (Cheng et al. 2019; Guo et al. 2019). Table 1 summarizes some of the recent advancements in CO2-fixing engineered bacteria.
Future prospects
A few of the problems facing researchers trying to address the problem of excessive CO2 emissions associated with a rise in greenhouse gases is the capacity to enhance CO2 conversion using the same natural mechanisms that have been used for ages to fix inorganic carbon sources. The sole economic drawback is currently related to cultivation techniques, which might be mitigated by excellent production rates due to genetic engineering methods (Senatore et al. 2020). Fortunately, metabolic engineering steps to enhance CO2 fixation via rewiring of central metabolisms, like i) splitting RuBisCO 's catalysis among many enzymes; ii) substituting the CBB cycle with other pathways; and; iii) replacing Rubisco with alternative carboxylation reaction, have the potential to transform CO2 fixation in the long run (Antonovsky et al. 2016; Bar-Even 2018; Claassens 2017; Herz et al. 2017; Hing et al. 2019). Albeit the CBB cycle is the most well-known pathway for photosynthetic CO2 fixation cycle by microorganism (Andorfer and Drennan 2021; Antonovsky et al. 2017), a potential synthetic pathway known as Malonyl-coA-Oxaloacetate-Glyoxylate (MOG) was reported as a better alternate to autotrophs' inherently poor CO2 fixation pathways (Salehizadeh et al. 2020). In comparison to the CBB cycle, the MOG pathway uses quicker carboxylases (e.g., phosphoenolpyruvate carboxylase or pyruvate carboxylase). These enzymes are highly oxygen-tolerant and have reduced ATP costs (Salehizadeh et al. 2020). Furthermore, the rapid advances in metabolic engineering techniques involving genome-scale modeling and sequencing of bacteria and yeast (E. coli and Saccharomyces cerevisiae), might present a whole new field in CO2 fixation by heterotrophic bacteria. Interestingly, with the aid of Maximum Driving Force (MDF), it is very possible now to identify thermodynamically viable metabolic pathways and even assess the CO2 fixation capability of heterotrophs such as E. coli via the innovative OptMDFpathway, particularly in the case of cell factories' metabolic design (Hädicke et al. 2018; Kanno et al. 2017; Savakis et al. 2015; Tabita 2005; Tracy et al. 2012). Now it is left for biotechnological engineering to tackle the exciting issue of increasing the efficiency of producing fuel, commodities, and food from CO2. Not surprisingly, the absence of infrastructure for manufacturing and storing hydrogen from water, as well as safety concerns and capital intensity, limits the utilization of CO2 into fuels. New manufacturing strategies including the usage of a succession of biological stages employed in contemporary biorefinery projects are still needed. This might be an intriguing method for implementing and developing new CO2 capture and conversion systems by bacteria.
Conclusion
CO2 capture by bacteria is an appealing option for climate change mitigation and immediately creating bio-based commodities with added value from CO2. However, to extend the production of these valuable commodities from CO2, revolutionary innovations encompassing major biotechnological methods (synthetic biology, and metabolic and genetic engineering) will need to be used and further developed. In this review, various novel genetic engineering and synthetic approaches employed in the engineering of bacteria for improved CO2 capture and utilization were discussed. With a growing emphasis on climate remediation, the use of bacteria targeted at a severe lowering in the addition of value to the carbon dioxide extracted, the use of waste raw materials, and footprint will be the ones to watch in the future. The emerging picture from this review highlights the need for future studies should focus on the selection of efficient bacteria, genetically engineering alterations as well as designing and building synthetic metabolic pathways. Doing so will help to reduce the cost of production of value-added bio-based products via CO2 capture and conversion by bacteria. Finally, integrating bacteria CO2 fixation in addition to other industrial operations such as treatment of exhaust gas and wastewater, refining biogas, and direct manufacture of commodities from CO2 might be more productive. This might help address the primary ecological issues of global warming while also reducing the usual cost and performance constraints in microbiological CO2 capture and conversion on a large scale as well as technological advances.
Availability of data and materials
Not applicable.
References
Andorfer MC, Drennan CL (2021) Fixing Nature’s Carbon Inefficiencies Joule 5(4):765–767
Antoniewicz MR (2019) Synthetic methylotrophy: Strategies to assimilate methanol for growth and chemicals production. Curr Opin Biotechnol 59:165–174
Antonovsky N, Gleizer S, Milo R (2017) Engineering carbon fixation in E. coli: from heterologous RuBisCO expression to the Calvin–Benson–Bassham cycle. Curr Opin Biotechnol 47:83–91
Antonovsky N, Gleizer S, Noor E et al (2016) Sugar synthesis from CO2 in Escherichia coli. Cell 166(1):115–125
Aresta M, Dibenedetto A (2007) Utilisation of CO2 as a chemical feedstock: opportunities and challenges. Dalton Trans 28:2975–2992
Aresta M, Dibenedetto A, Angelini A (2014) Catalysis for the valorization of exhaust carbon: from CO2 to chemicals, materials, and fuels Technological use of CO2. Chem Rev 114(3):1709–1742
Ashley AE, Thompson AL, O’Hare D (2009) Non-metal-mediated homogeneous hydrogenation of CO2 to CH3OH. Angew Chem Int Ed 48(52):9839–9843
Bains P, Psarras P, Wilcox J (2017) CO2 capture from the industry sector. Prog Energy Combust Sci 63:146–172
Bar-Even A (2018) Daring metabolic designs for enhanced plant carbon fixation. Plant Sci 273:71–83
Barati B, Zeng K, Baeyens J et al (2021) Recent progress in genetically modified microalgae for enhanced carbon dioxide sequestration. Biomass Bioenerg 145:105927
Bharti RK, Srivastava S, Thakur IS (2014) Production and characterization of biodiesel from carbon dioxide concentrating chemolithotrophic bacteria, Serratia sp. ISTD04. Biores Technol 153:189–197
Budinis S, Krevor S, Mac Dowell N, Brandon N, Hawkes A (2018) An assessment of CCS costs, barriers and potential. Energ Strat Rev 22:61
Callaway E (2019) E. coli bacteria engineered to eat carbon dioxide. Nature 576(7785):19–20
Carvalho AP, Meireles LA, Malcata FX (2006) Microalgal reactors: a review of enclosed system designs and performances. Biotechnol Prog 22(6):1490–1506
Chang K, Wang T, Chen JG (2017) Hydrogenation of CO2 to methanol over CuCeTiOx catalysts. Appl Catal B 206:704–711
Chen FY-H, Jung H-W, Tsuei C-Y, Liao JC (2020) Converting Escherichia coli to a synthetic methylotroph growing solely on methanol. Cell 182(4):933–946
Cheng J, Zhu Y, Zhang Z, Yang W (2019) Modification and improvement of microalgae strains for strengthening CO2 fixation from coal-fired flue gas in power plants. Biores Technol 291:121850
Chiranjeevi P, Bulut M, Breugelmans T, Patil SA, Pant D (2019) Current trends in enzymatic electrosynthesis for CO2 reduction. Current Opinion in Green and Sustain Chem 16:65–70
Choi KR, Shin JH, Cho JS, Yang D, Lee SY (2016) Systems metabolic engineering of Escherichia coli. EcoSal plus. https://doi.org/10.1128/ecosalplus.ESP-0010-2015
Chu S, Cui Y, Liu N (2017) The path towards sustainable energy. Nat Mater 16(1):16–22
Claassens NJ (2017) A warm welcome for alternative CO2 fixation pathways in microbial biotechnology. Microb Biotechnol 10(1):31
Claassens NJ, Sánchez-Andrea I, Sousa DZ, Bar-Even A (2018) Towards sustainable feedstocks: A guide to electron donors for microbial carbon fixation. Curr Opin Biotechnol 50:195–205
Costa JAV, de Morais MG, Dalcanton F, da Cruz RC, Durante AJ (2006) Simultaneous cultivation of Spirulina platensis and the toxigenic cyanobacteria Microcystis aeruginosa. Zeitschrift Für Naturforschung C 61(1–2):105–110
Cuéllar-Franca RM, Azapagic A (2015) Carbon capture, storage and utilisation technologies: A critical analysis and comparison of their life cycle environmental impacts. Journal of CO2 Utilization 9:82–102
Dasgupta CN, Gilbert JJ, Lindblad P et al (2010) Recent trends on the development of photobiological processes and photobioreactors for the improvement of hydrogen production. Int J Hydrogen Energy 35(19):10218–10238
Data E (2019) 800,000-year Ice-Core Records of Atmospheric Carbon Dioxide (CO2). In. https://cdiac.ess-dive.lbl.gov/trends/co2/ice_core_co2.html Accessed 26 June 2021
de Blas I, Mediavilla M, Capellán-Pérez I, Duce C (2020) The limits of transport decarbonization under the current growth paradigm. Energ Strat Rev 32:100543
De Vietro N, Tursi A, Beneduci A et al (2019) Photocatalytic inactivation of Escherichia coli bacteria in water using low pressure plasma deposited TiO 2 cellulose fabric. Photochem Photobiol Sci 18(9):2248–2258
Ding Y, Bertram JR, Eckert C et al (2019) Nanorg microbial factories: light-driven renewable biochemical synthesis using quantum dot-bacteria nanobiohybrids. J Am Chem Soc 141(26):10272–10282
du Pont YR, Jeffery ML, Gütschow J, Christoff P, Meinshausen M (2016) National contributions for decarbonizing the world economy in line with the G7 agreement. Environ Res Lett 11(5):054005
Ekwebelem OC, Ofielu ES, Nnorom-Dike OV, Aleke JC, Nwachukwu GA (2020) Towards Sustainable Energy: The Requisite Role of Microorganisms in the Production of Biogas and Bioethanol. Journal of Energy Research and Reviews:20–32
Fixen KR, Zheng Y, Harris DF et al (2016) Light-driven carbon dioxide reduction to methane by nitrogenase in a photosynthetic bacterium. Proc Natl Acad Sci 113(36):10163–10167
Fraccascia L, Giannoccaro I (2019) Analyzing CO2 emissions flows in the world economy using global emission chains and global emission trees. J Clean Prod 234:1399–1420
Fry BA, Peel J (2016) Autotrophic micro-organisms. Cambridge University Press
Gebicki J, Modigell M, Schumacher M, Van Der Burg J, Roebroeck E (2009) Development of photobioreactors for anoxygenic production of hydrogen by purple bacteria. Chem Eng Trans 18:363–366
Ghosh A, Kiran B (2017) Carbon concentration in algae: reducing CO2 from exhaust gas. Trends Biotechnol 35(9):806–808
Gleizer S, Bar-On YM, Ben-Nissan R, Milo R (2020) Engineering microbes to produce fuel, commodities, and food from CO2. Cell Reports Physical Science:100223
Gleizer S, Ben-Nissan R, Bar-On YM, et al. (2019) Conversion of Escherichia coli to generate all biomass carbon from CO2. Cell 179(6):1255–1263. e12
Global Carbon Project (2020) Carbon dioxide emissions (CO2), thousand metric tons of CO2. In. https://www.globalcarbonproject.org/carbonneutral/index.htm Accessed 17 June 2021
Goli A, Shamiri A, Talaiekhozani A, Eshtiaghi N, Aghamohammadi N, Aroua MK (2016) An overview of biological processes and their potential for CO2 capture. J Environ Manage 183:41–58
Gong F, Cai Z, Li Y (2016) Synthetic biology for CO2 fixation. Science China Life Sciences 59(11):1106–1114
Gong F, Liu G, Zhai X, Zhou J, Cai Z, Li Y (2015) Quantitative analysis of an engineered CO2-fixing Escherichia coli reveals great potential of heterotrophic CO2 fixation. Biotechnol Biofuels 8(1):1–10
Gong F, Zhu H, Zhang Y, Li Y (2018) Biological carbon fixation: from natural to synthetic. Journal of CO2 Utilization 28:221–227
Greer K, Zeller D, Woroniak J et al (2019a) Global trends in carbon dioxide (CO2) emissions from fuel combustion in marine fisheries from 1950 to 2016. Mar Policy 107:103382
Greer K, Zeller D, Woroniak J, et al. (2019b) Reply to Ziegler et al.“Adding perspectives to: Global trends in carbon dioxide (CO2) emissions from fuel combustion in marine fisheries from 1950–2016” and addressing concerns of using fishing effort to predict carbon dioxide emissions. Marine Policy 107:103491
Guo C, Duan D, Sun Y, Han Y, Zhao S (2019) Enhancing Scenedesmus obliquus biofilm growth and CO2 fixation in a gas-permeable membrane photobioreactor integrated with additional rough surface. Algal Res 43:101620
Hädicke O, von Kamp A, Aydogan T, Klamt S (2018) OptMDFpathway: Identification of metabolic pathways with maximal thermodynamic driving force and its application for analyzing the endogenous CO2 fixation potential of Escherichia coli. PLoS Comput Biol 14(9):e1006492
Hart A, Onyeaka H (2020) Eggshell and Seashells Biomaterials Sorbent for Carbon Dioxide Capture. Carbon Capture
Hashimoto K (2019) Global temperature and atmospheric carbon dioxide concentration Global Carbon Dioxide Recycling. Springer, p 5–17
HASSOUN SES, Hicham A, (2020) Renewable Energy and Sustainable Development: Evidence from 17 OECD Countries/Renewable Energy and Sustainable Development: Evidence from 17 OECD Countries. Uluslararası Ekonomi İşletme Ve Politika Dergisi 4(1):41–60
Herz E, Antonovsky N, Bar-On Y et al (2017) The genetic basis for the adaptation of E coli to sugar synthesis from CO2. Nature Commun 8(1):1–10
Hing NYK, Liang F, Lindblad P, Morgan JA (2019) Combining isotopically non-stationary metabolic flux analysis with proteomics to unravel the regulation of the calvin-benson-bassham cycle in synechocystis sp. Pcc 6803. Metab Eng 56:77–84
Holz C, Kartha S, Athanasiou T (2018) Fairly sharing 1.5: national fair shares of a 1.5 C-compliant global mitigation effort. International Environmental Agreements: Politics, Law and Economics 18(1):117–134
Hu B, Guild C, Suib SL (2013) Thermal, electrochemical, and photochemical conversion of CO2 to fuels and value-added products. J of CO2 Utilization 1: 18–27
Hu G, Li Y, Ye C, Liu L, Chen X (2019) Engineering microorganisms for enhanced CO2 sequestration. Trends Biotechnol 37(5):532–547
Jaganmoha M (2021) Global large-scale carbon sequestration projects 2020, by status. In. https://www.statista.com/statistics/726624/large-scale-carbon-capture-and-storage-projects-worldwide-by-status/ Accessed 25 June 2021
Jajesniak P, Ali H, Wong TS (2014) Carbon dioxide capture and utilization using biological systems: opportunities and challenges. J Bioprocess Biotech 4(155):2
Jiang W, Villamor DH, Peng H et al (2021) Metabolic engineering strategies to enable microbial utilization of C1 feedstocks. Nat Chem Biol 17(8):845–855
Jiang Y, May HD, Lu L, Liang P, Huang X, Ren ZJ (2019) Carbon dioxide and organic waste valorization by microbial electrosynthesis and electro-fermentation. Water Res 149:42–55
Kanno M, Carroll AL, Atsumi S (2017) Global metabolic rewiring for improved CO2 fixation and chemical production in cyanobacteria. Nat Commun 8(1):1–11
Ketheesan B, Nirmalakhandan N (2012) Feasibility of microalgal cultivation in a pilot-scale airlift-driven raceway reactor. Biores Technol 108:196–202
Kim S, Lindner SN, Aslan S et al (2020) Growth of E coli on formate and methanol via the reductive glycine pathway. Nature Chem Biol 16(5):538–545
Kumar M, Sahoo PC, Srikanth S, Bagai R, Puri S, Ramakumar S (2019a) Photosensitization of electro-active microbes for solar assisted carbon dioxide transformation. Biores Technol 272:300–307
Kumar M, Saxena R, Rai PK, et al. (2019b) Genetic diversity of methylotrophic yeast and their impact on environments Recent advancement in white biotechnology through fungi. Springer, pp. 53–71
Kumar M, Sundaram S, Gnansounou E, Larroche C, Thakur IS (2018) Carbon dioxide capture, storage and production of biofuel and biomaterials by bacteria: a review. Biores Technol 247:1059–1068
Kumar M, Tomar RS, Lade H, Paul D (2016) Methylotrophic bacteria in sustainable agriculture. World J Microbiol Biotechnol 32(7):1–9
Lee JW, Na D, Park JM, Lee J, Choi S, Lee SY (2012) Systems metabolic engineering of microorganisms for natural and non-natural chemicals. Nat Chem Biol 8(6):536–546
Lehtinen T, Virtanen H, Santala S, Santala V (2018) Production of alkanes from CO2 by engineered bacteria. Biotechnol Biofuels 11(1):1–11
Li H, Opgenorth PH, Wernick DG et al (2012) Integrated electromicrobial conversion of CO2 to higher alcohols. Science 335(6076):1596–1596
Li Y, Lin Z, Huang C et al (2015) Metabolic engineering of Escherichia coli using CRISPR–Cas9 meditated genome editing. Metab Eng 31:13–21
Liew F, Martin ME, Tappel RC, Heijstra BD, Mihalcea C, Köpke M (2016) Gas fermentation—a flexible platform for commercial scale production of low-carbon-fuels and chemicals from waste and renewable feedstocks. Front Microbiol 7:694
Liu C, Colón BC, Ziesack M, Silver PA, Nocera DG (2016) Water splitting–biosynthetic system with CO2 reduction efficiencies exceeding photosynthesis. Science 352(6290):1210–1213
Liu R, Liang L, Wu M et al (2013) CO2 fixation for succinic acid production by engineered Escherichia coli co-expressing pyruvate carboxylase and nicotinic acid phosphoribosyltransferase. Biochem Eng J 79:77–83
Lüthi D, Le Floch M, Bereiter B et al (2008) High-resolution carbon dioxide concentration record 650,000–800,000 years before present. Nature 453(7193):379–382. https://doi.org/10.1038/nature06949
Ma XY, Ai JQ, Shan JX, Li YH Aerodynamic shape deformation of underwing nacelle-pylon configuration based on B-spline surface. In: Applied Mechanics and Materials, 2014. vol 444. Trans Tech Publ, p 191–195
Majidian P, Tabatabaei M, Zeinolabedini M, Naghshbandi MP, Chisti Y (2018) Metabolic engineering of microorganisms for biofuel production. Renew Sustain Energy Rev 82:3863–3885
Markewitz P, Kuckshinrichs W, Leitner W et al (2012) Worldwide innovations in the development of carbon capture technologies and the utilization of CO2. Energy Environ Sci 5(6):7281–7305
Marlin DS, Sarron E, Sigurbjörnsson Ó (2018) Process advantages of direct CO2 to methanol synthesis. Front Chem 6:446
Mikhno I, Koval V, Shvets G, Garmatiuk O, Tamošiūnienė R (2021) Green economy in sustainable development and improvement of resource efficiency. Central Eur Bus Rev (CEBR) 10(1):99–113
Mohan SV, Modestra JA, Amulya K, Butti SK, Velvizhi G (2016) A circular bioeconomy with biobased products from CO2 sequestration. Trends Biotechnol 34(6):506–519
Molitor HR, Moore EJ, Schnoor JL (2019) Maximum CO2 utilization by nutritious microalgae. ACS Sustain Chem Eng 7(10):9474–9479
Mustafa A, Lougou BG, Shuai Y, Wang Z, Tan H (2020) Current technology development for CO2 utilization into solar fuels and chemicals: a review. J Energy Chem 49:96–123
NASA (2021) 2020 Tied for Warmest Year on Record, NASA Analysis Shows. In. https://www.nasa.gov/press-release/2020-tied-for-warmest-year-on-record-nasa-analysis-shows Accessed 26 June 2021
Nathan VK, Ammini P (2019) Carbon dioxide sequestering ability of bacterial carbonic anhydrase in a mangrove soil microcosm and its bio-mineralization properties. Water Air Soil Pollut 230(8):1–12
NOAA (2021) In. https://www.co2.earth/ Accessed 26 June 2021
Norsker N-H, Barbosa MJ, Vermuë MH, Wijffels RH (2011) Microalgal production—a close look at the economics. Biotechnol Adv 29(1):24–27
Obileke K, Nwokolo N, Makaka G, Mukumba P, Onyeaka H (2021) Anaerobic digestion: technology for biogas production as a source of renewable energy—A review. Energy & Environ 32(2):191–225
Olivier J, Peters, J (2020) Trends in global CO2 and total greenhouse gas emissions. In. https://www.pbl.nl/en/trends-in-global-co2-emissions Accessed 26 June 2021
Omoregbe O, Mustapha AN, Steinberger-Wilckens R, El-Kharouf A, Onyeaka H (2020) Carbon capture technologies for climate change mitigation: a bibliometric analysis of the scientific discourse during 1998–2018. Energy Rep 6:1200–1212
Park J-Y, Kim B-N, Kim Y-H, Min J (2018a) Whole-genome sequence of purple non-sulfur bacteria, Rhodobacter sphaeroides strain MBTLJ-8 with improved CO2 reduction capacity. J Biotechnol 288:9–14
Park SY, Yang D, Ha SH, Lee SY (2018b) Metabolic engineering of microorganisms for the production of natural compounds. Adv Biosyst 2(1):1700190
Pattharaprachayakul N, Choi J-i, Incharoensakdi A, Woo HM (2020) Metabolic Engineering and Synthetic Biology of Cyanobacteria for Carbon Capture and Utilization. Biotechnology and Bioprocess Engineering pp. 1–19
Pisaric M, Smol JP (2021) Arctic Ecology–A Paleoenvironmental Perspective. Arctic Ecology:23–55
Pontrelli S, Chiu T-Y, Lan EI, Chen FY-H, Chang P, Liao JC (2018) Escherichia coli as a host for metabolic engineering. Metab Eng 50:16–46
Rodemerck U, Holeňa M, Wagner E, Smejkal Q, Barkschat A, Baerns M (2013) Catalyst development for CO2 hydrogenation to fuels. ChemCatChem 5(7):1948–1955
Saeidi S, Amin NAS, Rahimpour MR (2014) Hydrogenation of CO2 to value-added products—A review and potential future developments. Journal of CO2 Utilization 5: 66–81
Sahoo PC, Pant D, Kumar M, Puri S, Ramakumar S (2020) Material–microbe interfaces for solar-driven CO2 bioelectrosynthesis. Trends Biotechnol 38(11):1245–1261
Saini R, Kapoor R, Kumar R, Siddiqi T, Kumar A (2011) CO2 utilizing microbes—a comprehensive review. Biotechnol Adv 29(6):949–960
Sakimoto KK, Wong AB, Yang P (2016) Self-photosensitization of nonphotosynthetic bacteria for solar-to-chemical production. Science 351(6268):74–77
Salehizadeh H, Yan N, Farnood R (2020) Recent advances in microbial CO2 fixation and conversion to value-added products. Chem Eng J 390:124584
Savakis P, Tan X, Du W, dos Santos FB, Lu X, Hellingwerf KJ (2015) Photosynthetic production of glycerol by a recombinant cyanobacterium. J Biotechnol 195:46–51
Schweitzer H, Aalto NJ, Busch W et al (2021) Innovating carbon-capture biotechnologies through ecosystem-inspired solutions. One Earth 4(1):49–59
Senatore A, Lania I, Corrente GA, Basile A (2020) CO2 capture by bacteria and their enzymes Advances in Carbon Capture. Elsevier, pp. 407–429
Senftle TP, Carter EA (2017) The holy grail: chemistry enabling an economically viable CO2 capture, utilization, and storage strategy. Acc Chem Res 50(3):472–475
Sierra E, Acién F, Fernández J, García J, González C, Molina E (2008) Characterization of a flat plate photobioreactor for the production of microalgae. Chem Eng J 138(1–3):136–147
Singh B, Strømman AH, Hertwich EG (2011) Comparative life cycle environmental assessment of CCS technologies. Int J Greenhouse Gas Control 5(4):911–921
Su Y, Cestellos-Blanco S, Kim JM et al (2020) Close-packed nanowire-bacteria hybrids for efficient solar-driven CO2 fixation. Joule 4(4):800–811
Sultana S, Sahoo PC, Martha S, Parida K (2016) A review of harvesting clean fuels from enzymatic CO2 reduction. RSC Adv 6(50):44170–44194
Tabita FR (2005) Research on carbon dioxide fixation in photosynthetic microorganisms (1971-present). Discover Photosynthesis pp. 771–788
Taheri Najafabadi A (2013) CO2 chemical conversion to useful products: an engineering insight to the latest advances toward sustainability. Int J Energy Res 37(6):485–499
Tracy BP, Jones SW, Fast AG, Indurthi DC, Papoutsakis ET (2012) Clostridia: the importance of their exceptional substrate and metabolite diversity for biofuel and biorefinery applications. Curr Opin Biotechnol 23(3):364–381
Tu W, Zhou Y, Zou Z (2014) Photocatalytic conversion of CO2 into renewable hydrocarbon fuels: state-of-the-art accomplishment, challenges, and prospects. Adv Mater 26(27):4607–4626
Tursi A (2019) A review on biomass: importance, chemistry, classification, and conversion. Biofuel Res J 6(2):962
Tursi A, De Vietro N, Beneduci A et al (2019) Low pressure plasma functionalized cellulose fiber for the remediation of petroleum hydrocarbons polluted water. J Hazard Mater 373:773–782
United Nations (2015) Adoption of the Paris Agreement. 21932, 32. In. http://unfccc.int/resource/docs/2015/cop21/eng/l09r01.pdf
Vidales AG, Omanovic S, Tartakovsky B (2019) Combined energy storage and methane bioelectrosynthesis from carbon dioxide in a microbial electrosynthesis system. Bioresource Technol Rep 8:100302
von der Assen N, Bardow A (2014) Life cycle assessment of polyols for polyurethane production using CO2 as feedstock: insights from an industrial case study. Green Chem 16(6):3272–3280
von der Assen N, Voll P, Peters M, Bardow A (2014) Life cycle assessment of CO2 capture and utilization: a tutorial review. Chem Soc Rev 43(23):7982–7994
Wang B, Jiang Z, Jimmy CY, Wang J, Wong PK (2019) Enhanced CO2 reduction and valuable C 2+ chemical production by a CdS-photosynthetic hybrid system. Nanoscale 11(19):9296–9301
Wang L, Wang L, Zhang J et al (2018) Selective hydrogenation of CO2 to ethanol over cobalt catalysts. Angew Chem Int Ed 57(21):6104–6108
Wang S, Zhao S, Uzoejinwa BB et al (2020) A state-of-the-art review on dual purpose seaweeds utilization for wastewater treatment and crude bio-oil production. Energy Convers Manage 222:113253
Weisser D (2007) A guide to life-cycle greenhouse gas (GHG) emissions from electric supply technologies. Energy 32(9):1543–1559
Yadav AN (2021) Microbial biotechnology for bio-prospecting of microbial bioactive compounds and secondary metabolites. J Appl Biol Biotechnol 9(2):1–6
Yang D, Park SY, Park YS, Eun H, Lee SY (2020) Metabolic engineering of Escherichia coli for natural product biosynthesis. Trends Biotechnol 38(7):745–765
Ye J, Yu J, Zhang Y et al (2019) Light-driven carbon dioxide reduction to methane by Methanosarcina barkeri-CdS biohybrid. Appl Catal B 257:117916
Yishai O, Lindner SN, de la Cruz JG, Tenenboim H, Bar-Even A (2016) The formate bio-economy. Curr Opin Chem Biol 35:1–9
Yoro KO, Daramola MO (2020) CO2 emission sources, greenhouse gases, and the global warming effect Advances in Carbon Capture. Elsevier, p 3–28
Zahed MA, Movahed E, Khodayari A, Zanganeh S, Badamaki M (2021) Biotechnology for carbon capture and fixation: Critical review and future directions. J Environ Manage 293:112830
Zaimes GG, Khanna V (2013) Microalgal biomass production pathways: evaluation of life cycle environmental impacts. Biotechnol Biofuels 6(1):1–11
Zapp P, Schreiber A, Marx J, Haines M, Hake J-F, Gale J (2012) Overall environmental impacts of CCS technologies—A life cycle approach. Int J Greenhouse Gas Control 8:12–21
Zhang H, Liu H, Tian Z et al (2018) Bacteria photosensitized by intracellular gold nanoclusters for solar fuel production. Nat Nanotechnol 13(10):900–905
Zhang L, Liu B, Du J, Liu C, Wang S (2019) CO2 emission linkage analysis in global construction sectors: Alarming trends from 1995 to 2009 and possible repercussions. J Clean Prod 221:863–877
Zhou J, Zhu T, Cai Z, Li Y (2016) From cyanochemicals to cyanofactories: a review and perspective. Microb Cell Fact 15(1):1–9
Acknowledgements
We are thankful to the invited reviewers for their insightful comments.
Funding
None.
Author information
Authors and Affiliations
Contributions
Both authors contributed equally.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Editorial responsibility: Q. Aguilar-Virgen.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Onyeaka, H., Ekwebelem, O.C. A review of recent advances in engineering bacteria for enhanced CO2 capture and utilization. Int. J. Environ. Sci. Technol. 20, 4635–4648 (2023). https://doi.org/10.1007/s13762-022-04303-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-022-04303-8