Abstract
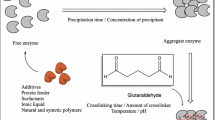
Urease which catalyzes biodegradation of urea to ammonia and carbon dioxide has attracted a great deal of attention as a catalyst in food industry, agriculture and artificial kidneys. Immobilization is a unique approach for enhancement of enzyme stability before enzyme industrialization. In the present work, crude sword bean urease was immobilized in the form of cross-linked enzyme aggregate prior to entrapment in polyacrylamide gel or chitosan beads. No significant change was observed in the kinetic constant value (k m ) of urease upon formation of cross-linked enzyme aggregate. Entrapment of urease cross-linked aggregates in chitosan beads resulted in complete loss of activity while polyacrylamide-embedded cross-linked urease aggregates were enzymatically active. The optimum temperature for urease was 60 °C (soluble and cross-linked aggregate form) and 70 °C (polyacrylamide-embedded cross-linked aggregate). The optimal pH for urease was 7.0 (soluble and cross-linked aggregate form) and 5.0 (polyacrylamide-embedded cross-linked aggregate). In the other words, immobilization in the form of cross-linked aggregate and entrapment in polyacrylamide matrix could be resulted in the stabilization of urease over a wider pH range. Urease cross-linked aggregate showed improved stability at 60 °C, and its half-life increased about two times. Operational stability of cross-linked urease aggregate was also noticeably improved upon entrapment in polyacrylamide gel. In conclusion, entrapment of carrier-free cross-linked urease aggregate in a polyacrylamide matrix could result in a great improvement in both the storage and operational stabilities of urease.






Similar content being viewed by others
References
Akkaya A, Uslan AH (2010) Sequential immobilization of urease to glycidyl methacrylate grafted sodium alginate. J Mol Catal B Enzym 67:195–201
Andrews RK, Blakeley RL, Zerner B (1984) Urea and urease. Adv Inorg Biochem 6:245–283
Andrich L, Esti M, Moresi M (2010) Urea degradation kinetics in model wine solutions by acid urease immobilized onto chitosan-derivative beads of different sizes. Enzyme Microb Technol 46:397–405
Baran AT, Hamarat SB (2007) Immobilization of urease using glycidyl methacrylate grafted nylon-6-membranes. Process Biochem 42:439–443
Blakeley RL, Zerner B (1984) Jack bean urease: the first nickel enzyme. J Mol Catal 23:263–292
Gabrovska K, Godjevargova T (2009) Optimum immobilization of urease on modified acrylonitrile copolymer membranes: inactivation by heavy metal ions. J Mol Catal B Enzym 60:69–75
George S, Chellapandian M, Sivasankar B, Jayaraman K (1997) A new process for the treatment of fertilizer effluent using immobilized urease. Bioprocess Eng 16:83–85
Janssen MH, van Langen LM, Pereira SR, van Rantwijk F, Sheldon RA (2002) Evaluation of the performance of immobilized penicillin G acylase using active-site titration. Biotechnol Bioeng 78:425–432
Katoa K, Nishidaa M, Ito K, Tomita M (2012) Characterization of silica particles prepared via urease-catalyzed urea hydrolysis and activity of urease in sol–gel silica matrix. Appl Surf Sci 262:69–75
Krajewska B (2004) Application of chitin- and chitosan-based materials for enzyme immobilizations: a review. Enzyme Microb Technol 35:126–139
Krajewska B (2009) Urease II. Properties and their customizing by enzyme immobilization: a review. J Mol Catal B Enzym 59:22–40
Krishna BL, Singh AN, Patra S, Dubey VK (2011) Purification, characterization and immobilization of urease from Momordica charantia seeds. Process Biochem 46:1486–1491
Kumar S, Dwevedi A, Kayastha AM (2009) Immobilization of soybean (Glycine max) urease on alginate and chitosan beads showing improved stability: analytical applications. J Mol Catal B Enzym 58:138–145
Lemmen W, Van Baal H (1999) The environmental impact of a Stamicarbon 2000-mtpd urea plant. In: Proceedings of an international workshop on current environmental issues of fertilizer production, Prague, Czech Republic. pp 207–2012
Lloyd AB, Sheaffe MJ (1973) Urease activity in soils. Plant Soil 39:71–80
Lopez-Serrano P, Cao L, Van Rantwijk F, Sheldon R (2002) Cross-linked enzyme aggregates with enhanced activity: application to lipases. Biotechnol Lett 24:1379–1383
Majeau J, Brar SK, Tyagi RD (2010) Laccases for removal of recalcitrant and emerging pollutants. Bioresour Technol 101:2331–2350
Mobley HLT, Cortesia MJ, Rosenthal LE, Jones BD (1988) Characterization of urease from Campylobacter pylori. J Clin Microbiol 26:831–836
Nabatia FM, Habibi-Rezaei B, Amanloua M, Moosavi-Movahedi AA (2011) Dioxane enhanced immobilization of urease on alkyl modified nano-porous silica using reversible denaturation approach. J Mol Catal B Enzym 70:17–22
Ough CS, Crowell EA, Mooney LA (1988) Formation of ethyl carbamate precursors during grape juice (Chardonnay) fermentation. I. Addition of amino acids, urea, and ammonia: effects of fortification on intracellular and extracellular precursors. Am J Enol Viticult 39:243–249
Rahimpour MR, Mottaghi HR (2009) Simultaneous removal of urea, ammonia, and carbon dioxide from industrial wastewater using a thermal hydrolyzer-separator loop. Ind Eng Chem Res 48:10037–10046
Reddy KRC, Srivastava PK, Dey PM, Kayastha AM (2004) Immobilization of pigeonpea (Cajanus cajan) urease on DEAE-cellulose paper strips for urea estimation. Biotechnol Appl Biochem 39:323–327
Schlatter J, Lutz WK (1990) The carcinogenic potential of ethyl carbamate (urethane): risk assessment at human dietary exposure levels. Food Chem Toxicol 28:205–211
Schoevaart R, Wolbers MW, Golubovic M, Ottens M, Kieboom AP, van Rantwijk F, van der Wielen LA, Sheldon RA (2004) Preparation, optimization, and structures of cross-linked enzyme aggregates (CLEAs). Biotechnol Bioeng 87:754–762
Sheldon RA (2007) Enzyme immobilization: the quest for optimum performance. Adv Synth Catal 349:1289–1307
Sun WQ, Davidson P, Chan HS (1998) Protein stability in the amorphous carbohydrate matrix: relevance to anhydrobiosis. BBA Gen Subj 1425:245–254
Theil F (2000) Enhancement of selectivity and reactivity of lipases by additives. Tetrahedron 56:2905–2919
van Roon JL, Joerink M, Rijkers MP, Tramper J, Schroën CG, Beeftink HH (2003) Enzyme distribution derived from macroscopic particle behavior of an industrial immobilized penicillin-G acylase. Biotechnol Prog 19:1510–1518
Wang M, Qi W, Jia C, Ren Y, Su R, He Z (2011) Enhancement of activity of cross-linked enzyme aggregates by a sugar-assisted precipitation strategy: technical development and molecular mechanism. J Biotechnol 156:30–38
Acknowledgements
This work was supported by the Research Institute of Petroleum Industry (RIPI), Tehran, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Additional information
Editorial responsibility: M. Abbaspour.
Rights and permissions
About this article
Cite this article
Zeinali, M., Lenjannezhadian, H. Degradation of urea by entrapped cross-linked urease aggregates: a combinatorial approach to urease stabilization for environmental and industrial applications. Int. J. Environ. Sci. Technol. 15, 49–56 (2018). https://doi.org/10.1007/s13762-017-1337-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-017-1337-8