Abstract
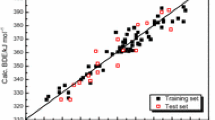
Benzenoid hydrocarbons are a group of the most important π-electron systems having the attention of both experimental and theoretical chemists for the last 100 years. In the present study, based on the general interaction properties function (GIPF) family descriptors, significant one- or two-parametric quantitative structure–property (activity) relationship models were developed for the prediction of properties/activities of benzenoids hydrocarbons. All descriptors were computed in density functional theory (DFT) at the B3LYP/STO-3G level of theory in Gaussian98 software. A large number of physico-chemical properties and two biological activities (e.g. bio-concentration factor and photo-induced toxicity) of these compounds were investigated by using multiple linear regressions. All created models were interpreted in term of selected descriptors. R 2 and R 2cv values of all models are respectively between 0.665–0.994 and 0.609–0.990 for the whole dataset of each property/activity. Maximum R 2 for Y-randomization (R 2max ) test and its cross-validation (R 2cv ,max) are between 0.098–0.485 and 0.002–0.357, respectively.




Similar content being viewed by others
References
H.J. Lee, J. Villaume, D.C. Cullen, B.C. Kim, M.B. Gu, Biosens Bioelectron 18, 571 (2003)
J.C. Drosos, M. Viola-Rhenals, R. Vivas-Reyes, J Chromatogr A 1217, 4411 (2010)
Q. Jun, S. Chang-Hong, W. Jia, Procedia. Environ Sci 2, 1429 (2010)
S. Tao, X.C. Jiao, S.H. Chen, F.L. Xu, Y.J. Li, F.Z. Liu, Environ Pollut 140, 13 (2006)
J. Beyer, G. Jonsson, C. Porte, M.M. Krahn, F. Ariese, Toxicol Phar 30, 224 (2010)
I. Martorell, G. Perelló, R. Martí-Cid, V. Castell, J.M. Llobet, J.L. Domingo, Environ Int 36, 242 (2010)
R. Ghavami, B. Sepehri, J Chromatogr A 1233, 116 (2012)
F. Liu, Y. Liang, C. Cao, N. Zhou, Anal Chim Acta 594, 279 (2007)
R.J. Hu, H.X. Liu, R.S. Zhang, C.X. Xue, X.J. Yao, M.C. Liu, Z.D. Hu, B.T. Fan, Talanta 68, 31 (2005)
X.J. Yao, A. Panaye, J.P. Doucet, R.S. Zhang, H.F. Chen, M.C. Liu, Z.D. Hu, B.T. Fan, J Chem Inf Comput Sci 44, 1257 (2004)
M. Karelson, Molecular Descriptors in QSAR/QSPR (Wiley, New York, 2000)
R. Todeschini, V. Consonni, Molecular Descriptors for Chemoinformatics, Volumes I & II (Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2009)
J. Devillers, A.T. Balaban, Topological Indices and Related Descriptors in QSAR and QSPR (Eds. Gordon and Breach, Amestrdam, 1999)
M.M.C. Ferreira, Chemosphere 44, 125 (2001)
G.N. Lu, Z. Dang, X.O. Tao, C. Yang, X.Y. Yi, Sci Total Environ 373, 289 (2007)
S. Nikolić, A. Miličević, N. Trinajstić, Croat Chem Acta 79, 155 (2006)
P. Politzer, J.S. Murray, Fluid Phase Equilibr 185, 129 (2001)
P. Politzer, J.S. Murray, P. Flodmark, J Phys Chem 100, 5538 (1996)
P. Politzer, J.S. Murray, F.A. Bulat, J Mol. Model 16, 1731 (2010)
P. Jin, T. Brinck, J.S. Murray, P. Politzer, Int J Quantum Chem 95, 632 (2003)
W. Karacher, Spectral Atlas of Polycyclic Aromatic Compounds, vol. 2 (Kluwer academic publishers, Dordrecht, 1988), p. 16
L.C. Sander, S.A. Wise, Adv Chromatogr 25, 139 (1986)
D. Mackay, W.-Y. Shiu, K.C. Ma, Illustrated Handbook of Physical-Chemical Properties and Environmental Fate of Organic Compounds, vol. 2 (Lewis/CRC, Boca Raton, 1992)
D. Mackay, D. Calloct, Partitioning and physical properties of PAHs, in The Handbook of Environmental Chemistry, vol. 3, Part J. PAHs and related compounds, ed. by A.H. Neilson (Springer, Berlin, 1998), pp. 325–346
A.T. Balaban, M. Pompe, J Phys Chem A 111, 2448 (2007)
M. Randić, Chem Rev 103, 3449 (2003)
ISIS Draw 2.3 (MDL Information Systems, Inc., 1990–2000)
HyperChem Release 7.1 for Windows Molecular Modeling System Program Package, (HyperCube, 2002)
M.J. Frisch, G.W. Trucks, H.B. Schlegel, G.E. Scuseria, M.A. Robb, J.R. Cheeseman, V.G. Zakrzewski, J.A. Montgomery, R.E. Stratmann, J.C. Burant, S. Dappich, J.M. Millam, A.D. Daniels, K.N. Kudin, M.C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G. Petersson, P.Y. Aayala, Q. Cui, K. Morokuma, D.K. Malick, A.D. Rubuck, K. Raghavachari, J.B. Foresman, J. Cioslowski, J.V. Ortiz, B.B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R.L. Martin, D.J. Fox, T. Keith, M.A. Al-Laham, C.Y. Peng, A. Nanayakkara, C. Gonzalez, M. Challacombe, P.M.W. Gill, B.G. Johnson, W. Chen, M.W. Wong, J.L. Andres, M. Head-Gordon, E.S. Replogle, J.A. Pople, Gaussian98, Revision A.5 (Gaussian Inc, Pittsburgh, 1998)
H.Y. Xu, J.W. Zou, Q.S. Yu, Y.H. Wang, J.Y. Zhang, H.X. Jin, Chemosphere 66, 1998 (2007)
F.A. Bulat, A. Toro-Labbé, T. Brinck, J.S. Murray, P. Politzer, J Mol Model 16, 1679 (2010)
J.S. Murray, F. Abu-Awwad, P. Politzer, J Phys Chem A 103, 1853 (1999)
Y. Ma, K.C. Gross, C.A. Hollingsworth, P.G. Seybold, J.S. Murray, J Mol Model 10, 235 (2004)
O.G. Gonzalez, J.S. Murray, Z. Peralta-Inga, P. Politzer, Int J Quantum Chem 83, 115 (2001)
P. Kulshrestha, N. Sukumar, J.S. Murray, R.F. Giese, T.D. Wood, J Phys Chem A 113, 756 (2009)
M.N. Hasan, P.C. Jurs, Anal Chem 60, 978 (1988)
J. Olivero, T. Garcia, P. Payares, R. Viva, D. Diaz, E. Daza, P. Geerliger, J Pharm Sci 86, 625 (1997)
R. Ghavami, S. Faham, Chromatographia 72, 893 (2010)
M. Dumarey, A.M.V. Nederkassel, E. Deconinck, Y.V. Heyden, J Chromatogr A 1192, 81 (2008)
J.G. Topliss, R.P. Edwards, J Med Chem 22, 1238 (1979)
K. Varmuza, P. Filzmoser, Introduction to Multivariate Statistical Analysis in Chemometrics (Taylor & Francis Group, LLC, 2009)
H. Kubinyi, QSAR: Hansch analysis and related approaches (VCH VerlagsgesellschaftmbH, D-69451 Weinheirn, Federal Republic of Germany, 1993)
H. Kubinyi, F.A. Hamprecht, T. Mietzner, J Med Chem 41, 2553 (1998)
D.M. Hawkins, J. Kraker, J Chemometr 24, 188 (2010)
R. Ghavami, A. Najafi, M. Sajadi, F. Djannaty, J Mol Graph Modell 27, 105 (2008)
D.M. Hawkins, S.C. Basak, D. Mills, J Chem Inf Comput Sci 43, 579 (2003)
A. Tropsha, P. Gramatica, V. Gombar, Quant Struct Act Relat Comb Sci 22, 69 (2003)
R. Ghavami, F. Sadeghi, Chromatographia 70, 851 (2009)
C. Hansch, R.P.A. Verma, Eur J Med Chem 44, 274 (2009)
R. Christoph, R. Gerta, M. Markus, J Chem Inf Model 47, 2345 (2007)
J.S. Murray, T. Brinck, P. Politzer, J Phys Chem 97, 13807 (1993)
C. Hansch, J.E. Quinlan, G.L. Lawerence, J. Org. Chem. 33, 347 (1968)
T. Harner, M. Shoeib, J Chem Eng Data 47, 228 (2002)
M. Shoeib, T. Harner, Environ Toxicol Chem 21, 984 (2002)
P. Sang, J.W. Zou, P. Zhou, L. Xu, Chemosphere 83, 1045 (2011)
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ghavami, R., Sepehri, B. QSPR/QSAR solely based on molecular surface electrostatic potentials for benzenoid hydrocarbons. J IRAN CHEM SOC 13, 519–529 (2016). https://doi.org/10.1007/s13738-015-0761-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13738-015-0761-2