Abstract
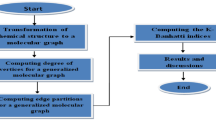
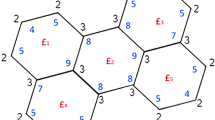
The study of benzenoid systems has been steadily gaining momentum due to their extensive applications in many emerging fields including nanosciences. Topological descriptors provide a mathematical expression of the molecular structure of chemical compounds and their properties. They serve as efficient and cost-effective tools to theoretically predict the properties of compounds using quantitative structure–activity (QSAR) and structure–property relationship (QSPR) studies. This paper demonstrates the computation of degree-based and irregularity-based topological descriptors using edge-partition techniques for two benzenoid structures. This analysis of degree-based descriptors for these structures can lay the basis for further exploration into benzenoids and their properties.





Similar content being viewed by others
References
N. Trinajstic, Chemical Graph Theory (Routledge, Abingdon, 2018)
J. Devillers, A.T. Balaban (eds.), Topological Indices and Related Descriptors in QSAR and QSPAR (CRC Press, Boca Raton, 2000)
F. Emmert-Streib, Statistical Modelling of Molecular Descriptors in QSAR/QSPR (Wiley, Hoboken, 2012)
M.I. Skvortsova, I.I. Baskin, O.L. Slovokhotova, V.A. Palyulin, N.S. Zefirov, Inverse problem in QSAR/QSPR studies for the case of topological indexes characterizing molecular shape (Kier indices). J. Chem. Inf. Comput. Sci. 33(4), 630–634 (1993)
I. Gutman, Degree-based topological indices. Croat. Chem. Acta 86(4), 351–361 (2013)
G. Rücker, C. Rücker, On topological indices, boiling points, and cycloalkanes. J. Chem. Inf. Comput. Sci. 39(5), 788–802 (1999)
M. Črepnjak, N. Tratnik, P.Ž. Pleteršek, Predicting melting points of hydrocarbons by the Graovac-Pisanski index. Fuller. Nanotub. Carbon Nanostruct. 26(5), 239–245 (2018)
C. Guan, M. Lu, W. Zeng, D. Yang, D. Han, Prediction of standard enthalpies of formation based on hydrocarbon molecular descriptors and active subspace methodology. Ind. Eng. Chem. Res. 59(10), 4785–4791 (2020)
L.L. Zhou, J.C. Jiang, Y. Pan, Z.R. Wang, A mathematical method for predicting heat of reaction of organic peroxides. J. Loss Prev. Process Ind. 38, 254–259 (2015)
D.H. Rouvray, W. Tatong, Novel applications of topological indices. 3. Prediction of the vapor pressure in polychlorinated biphenyls. Int. J. Environ. Stud. 33(4), 247–257 (1989)
M. Pompe, M. Novic, Prediction of gas-chromatographic retention indices using topological descriptors. J. Chem. Inf. Comput. Sci. 39(1), 59–67 (1999)
F. Liu, Y. Liang, C. Cao, N. Zhou, Theoretical prediction of the Kovat’s retention index for oxygen-containing organic compounds using novel topological indices. Anal. Chim. Acta 594(2), 279–289 (2007)
R. Garcia-Domenech, P. Alarcon-Elbal, G. Bolas, R. Bueno-Marí, F.A. Chordá-Olmos, S.A. Delacour, M.C. Mouriño, A. Vidal, J. Gálvez, Prediction of acute toxicity of organophosphorus pesticides using topological indices. SAR QSAR Environ. Res. 18(7–8), 745–755 (2007)
S.C. Basak, D. Mills, B.D. Gute, G.D. Grunwald, A.T. Balaban, Applications of topological indices in the property/bioactivity/toxicity prediction of chemicals, in Topology in Chemistry, ed. by D. Rouvray, R. Bruce King (Woodhead Publishing, 2002), pp 113–184
P.F. Sheridan, D.B. Adolf, A.V. Lyulin, I. Neelov, G.R. Davies, Computer simulations of hyperbranched polymers: the influence of the Wiener index on the intrinsic viscosity and radius of gyration. J. Chem. Phys. 117(16), 7802–7812 (2002)
P.V. Khadikar, J. Singh, M. Ingle, Topological estimation of aromatic stabilities of polyacenes and helicenes: modeling of resonance energy and benzene character. J. Math. Chem. 42(3), 433–446 (2007)
J.I. Aihara, Bond resonance energies of polycyclic benzenoid and non-benzenoid hydrocarbons. J. Chem. Soc. Perkin Trans. 2(10), 2185–2195 (1996)
J. Cioslowski, Additive nodal increments for approximate calculation of the total π-electron energy of benzenoid hydrocarbons. Theor. Chim. Acta 68(4), 315–319 (1985)
I. Gutman, S.J. Cyvin, Introduction to the Theory of Benzenoid Hydrocarbons (Springer, Berlin, 2012)
J.R. Dias, Handbook of Polycyclic Hydrocarbons. Part A: Benzenoid Hydrocarbons. United States: N. p., 1987. Web
J.N. Murrell, The Theory of the Electronic Spectra of Organic Molecules (Springer, New York, US, 1963). eBook ISBN 978-1-5041-1152-2
D. Lloyd, The Chemistry of Conjugated Cyclic Compounds: To be or Not to be Like Benzene? (Wiley, Hoboken, 1989)
R. Taylor, Electrophilic Aromatic Substitution (Wiley, Chichester, 1990)
L.J. Allamandola, A.G.G.M. Tielens, J.R. Barker, Interstellar polycyclic aromatic hydrocarbons—the infrared emission bands, the excitation/emission mechanism, and the astrophysical implications. Astrophys. J. Suppl. Ser. 71, 733–775 (1989)
H.I. Abdel-Shafy, M.S. Mansour, A review on polycyclic aromatic hydrocarbons: source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 25(1), 107–123 (2016)
G. Mastrangelo, E. Fadda, V. Marzia, Polycyclic aromatic hydrocarbons and cancer in man. Environ. Health Perspect. 104(11), 1166–1170 (1996)
R. Canton-Vitoria, Y. Sayed-Ahmad-Baraza, M. Pelaez-Fernandez, R. Arenal, C. Bittencourt, C.P. Ewels, N. Tagmatarchis, Functionalization of MoS 2 with 1, 2-dithiolanes: toward donor-acceptor nanohybrids for energy conversion. NPJ 2D Mater. Appl. 1(1), 1–9 (2017)
B.K. Shah, D.C. Neckers, J. Shi, E.W. Forsythe, D. Morton, Anthanthrene derivatives as blue emitting materials for organic light-emitting diode applications. Chem. Mater. 18(3), 603–608 (2006)
J.E. Anthony, Functionalized acenes and heteroacenes for organic electronics. Chem. Rev. 106(12), 5028–5048 (2006)
S.E. Stein, R.L. Brown, pi-Electron properties of large condensed polyaromatic hydrocarbons. J. Am. Chem. Soc. 109(12), 3721–3729 (1987)
M.J.S. Dewar, The Molecular Orbital Theory of Organic Chemistry (McGraw-Hill, New York, 1969)
B.D. Gute, S.C. Basak, Predicting acute toxicity (LC50) of benzene derivatives using theoretical molecular descriptors: a hierarchical QSAR approach. SAR QSAR Environ. Res. 7(1–4), 117–131 (1997)
C. Bertinetto, C. Duce, R. Solaro, K. Héberger, Modeling of the acute toxicity of benzene derivatives by complementary QSAR methods. Match Commun. Math. Comput. Chem. 70(3), 1005–1021 (2013)
J. Wu, W. Pisula, K. Müllen, Graphenes as potential material for electronics. Chem. Rev. 107(3), 718–747 (2007)
Z. Zeng, X. Huang, Z. Yin, H. Li, Y. Chen, H. Li, Q. Zhang, J. Ma, F. Boey, H. Zhang, Fabrication of graphene nanomesh by using an anodic aluminum oxide membrane as a template. Adv. Mater. 24(30), 4138–4142 (2012)
G. Korinth, T. Wellner, K.H. Schaller, H. Drexler, Potential of the octanol-water partition coefficient (log P) to predict the dermal penetration behaviour of amphiphilic compounds in aqueous solutions. Toxicol. Lett. 215(1), 49–53 (2012)
R.S. Braga, P.M.V.B. Barone, D.S. Galvao, Identifying carcinogenic activity of methylated and non-methylated polycyclic aromatic hydrocarbons (PAHs) through electronic and topological indices. Braz. J. Phys. 30(3), 560–568 (2000)
D.B. West, Introduction to Graph Theory, vol. 2 (Prentice Hall, Upper Saddle River, 1996)
B. Lučić, N. Trinajstić, B. Zhou, Comparison between the sum-connectivity index and product-connectivity index for benzenoid hydrocarbons. Chem. Phys. Lett. 475(1–3), 146–148 (2009)
K.C. Das, M. Dehmer, Comparison between the zeroth-order Randić index and the sum-connectivity index. Appl. Math. Comput. 274, 585–589 (2016)
S.C. Basak, G.J. Niemi, G.D. Veith, Predicting properties of molecules using graph invariants. J. Math. Chem. 7(1), 243–272 (1991)
B. Zhao, J. Gan, H. Wu, Redefined Zagreb indices of some nano structures. Appl. Math. Nonlinear Sci. 1(1), 291–300 (2016)
M. Eliasi, D. Vukičević, Comparing the multiplicative Zagreb indices. MATCH Commun. Math. Comput. Chem. 69, 765–773 (2013)
B. Furtula, A. Graovac, D. Vukičević, Augmented zagreb index. J. Math. Chem. 48(2), 370–380 (2010)
D. Vukičević, M. Gašperov, Bond additive modeling 1. Adriatic indices. Croat. Chem. Acta 83(3), 243–260 (2010)
C.K. Gupta, V. Lokesha, S.B. Shwetha, P.S. Ranjini, On the symmetric division deg index of graph. Southeast Asian Bull. Math. 40(1), 59–80 (2016)
B. Furtula, I. Gutman, A forgotten topological index. J. Math. Chem. 53(4), 1184–1190 (2015)
F.K. Bell, A note on the irregularity of graphs. Linear Algebra Appl. 161, 45–54 (1992)
T. Réti, E. Tóth-Laufer, On the construction and comparison of graph irregularity indices. Kragujev. J. Sci. 39, 53–75 (2017)
T. Réti, R. Sharafdini, A. Dregelyi-Kiss, H. Haghbin, Graph irregularity indices used as molecular descriptors in QSPR studies. MATCH Commun. Math. Comput. Chem. 79, 509–524 (2018)
M. Arockiaraj, J. Clement, K. Balasubramanian, Analytical expressions for topological properties of polycyclic benzenoid networks. J. Chemom. 30(11), 682–697 (2016)
H. Wiener, Structural determination of paraffin boiling points. J. Am. Chem. Soc. 69(1), 17–20 (1947)
A. Miličević, S. Nikolić, N. Trinajstić, On reformulated Zagreb indices. Mol. Divers. 8(4), 393–399 (2004)
V.S. Shegehalli, R. Kanabur, Arithmetic-geometric indices of some class of graph. J. Comput. Math. Sci. 6(4), 194–199 (2015)
A. Usha, P.S. Ranjini, V. Lokesha, Zagreb co-indices, augmented zagreb index, redefined zagreb indices and their polynomials for phenylene and hexagonal squeeze, in Proceedings of International Congress in Honour of Dr. Ravi. P. Agarwal, Uludag University, Bursa, Turkey (2014)
V.S. Shigehalli, R. Kanabur, Computation of new degree-based topological indices of graphene. J. Math. 2016, 4341919 (2016). https://doi.org/10.1155/2016/4341919
B. Zhou, N. Trinajstić, On a novel connectivity index. J. Math. Chem. 46(4), 1252–1270 (2009)
B. Zhou, N. Trinajstić, On general sum-connectivity index. J. Math. Chem. 47(1), 210–218 (2010)
Acknowledgements
The authors wish to thank the management of Sri Sivasubramaniya Nadar College of Engineering, Kalavakkam-603110, for their continuous support and encouragement to carry out this research work.
Funding
Funding was provided by National Natural Science Foundation (Grant Nos. 11971142, 11871202, 61673169, 11701176, 11626101, 11601485).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Rights and permissions
About this article
Cite this article
Chu, YM., Julietraja, K., Venugopal, P. et al. Degree- and irregularity-based molecular descriptors for benzenoid systems. Eur. Phys. J. Plus 136, 78 (2021). https://doi.org/10.1140/epjp/s13360-020-01033-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1140/epjp/s13360-020-01033-z