Abstract
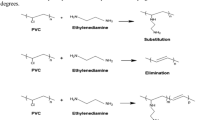
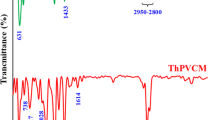
Chemical modification of polyvinyl chloride by nucleophiles is a versatile method for preparation of new functional polymers. Modification of PVC was carried out using different nucleophiles (Nu) in ethylene glycol (EG)/N,N-dimethylformamide (DMF) (1:1 by volume) solution. OH−, N3−, and SCN− were examined as nucleophiles in this study. The FTIR spectrum confirmed substitution of Cl by nucleophiles and also the elimination of HCl in the modification reaction of PVC. The glass transition temperatures (Tgs) of modified-PVC samples were in the order N3− > SCN− > OH− and were equal to 87.6, 86.8 and 78.15 °C, respectively. The T5% (the temperature at which weight loss is 5%) of PVC was 266 °C, while after 1 h modification by OH− it reduced to 197 °C. After alkaline modification, the scanning electron microscopy (SEM) images not only showed an increase in surface roughness and porosity, but also revealed a relatively large drop in average particle size from 160 to 80 µm. Molecular weight and molecular weight distribution were determined by gel permeation chromatography (GPC). The GPC results showed that the number average molecular weight (Mn) and weight average molecular weight (Mw) were decreased from 79,950 and 176,360 g mol−1 in crude PVC to 45,370 and 99,930 g mol−1, respectively, after 1 h modification by OH−. Alkaline treatment also decreased the mechanical strength of PVC.











Similar content being viewed by others
References
Khan F, Kausar A, Siddiq M (2016) Buckypapers of 4, 4′-oxydianiline-modified polyvinylchloride and functional nano-filler obtained by resin infusion method. Iran Polym J 25:213–228
Langer E, Waśkiewicz S, Bortel K, Lenartowicz-Klik M, Jurczyk S (2017) Application of new oligomeric plasticizers based on waste poly (ethylene terephthalate) for poly (vinyl chloride) compositions. Iran Polym J 26:115–123
Pakbaz M, Maghsoud Z (2017) Performance evaluation of polyvinylchloride/polyacrylonitrile ultrafiltration blend membrane. Iran Polym J 26:833–849
Xu N, Cao J, Lu Y (2016) Electrospun polyvinyl chloride/poly (butyl methacrylate-co-butyl acrylate) fibrous mat for absorption of organic matters. Iran Polym J 25:251–262
Yoshioka T, Kameda T, Imai S, Okuwaki A (2008) Dechlorination of poly (vinyl chloride) using NaOH in ethylene glycol under atmospheric pressure. Polym Degrad Stabil 93:1138–1141
Jia P, Lihong H, Xiaohui Y, Meng Z, Qianqian S, Yonghong Z (2017) Internally plasticized PVC materials via covalent attachment of aminated tung oil methyl ester. RSC Adv 48:30101–30108
Guo L, Shi G, Liang Y (2001) Poly (ethylene glycol)s catalyzed homogeneous dehydrochlorination of poly (vinyl chloride) with potassium hydroxide. Polymer 42:5581–5587
Kameda T, Ono M, Grause G, Mizoguchi T, Yoshioka T (2009) Chemical modification of poly (vinyl chloride) by nucleophilic substitution. Polym Degrad Stabil 94:107–112
Jia P, Lihong H, Qianqian S, Rui W, Meng Z, Yonghong Z (2017) Self-plasticization of PVC materials via chemical modification of mannich base of cardanol butyl ether. ACS Sustain Chem Eng 5:6665–6673
Jia P, Lihong H, Meng Z, Guodong F, Yonghong Z (2017) Phosphorus containing castor oil based derivatives: potential non-migratory flame retardant plasticizer. Eur Polym J 87:209–220
Yoshioka T, Kameda T, Ieshige M, Okuwaki A (2008) Dechlorination behaviour of flexible poly (vinyl chloride) in NaOH/EG solution. Polym Degrad Stabil 93:1822–1825
Kameda T, Imai K, Grause G, Mizoguchi T, Yoshioka T (2009) Kinetics of the dehydrochlorination of poly (vinyl chloride) in the presence of NaOH and various diols as solvents. Polym Degrad Stabil 94:1595–1597
Kameda T, Ono M, Grause G, Mizoguchi T, Yoshioka T (2009) Effect of a phase-transfer catalyst on the chemical modification of poly (vinyl chloride) by substitution with thiocyanate as a nucleophile. Mater ChemPhys 118:362–366
Bigot S, Louarn G, Kébir N, Burel F (2013) Click grafting of seaweed polysaccharides onto PVC surfaces using an ionic liquid as solvent and catalyst. Carbohyd Polym 98:1644–1649
Jia P, Hu L, Feng G, Bo C, Zhang M, Zhou Y (2017) PVC materials without migration obtained by chemical modification of azide-functionalized PVC and triethyl citrate plasticizer. Mater Chem Phys 190:25–30
Miyazaki K, Sato H, Kikuchi S, Nakatani H (2014) Dehydrochlorination of poly (vinyl chloride) modified with titanium dioxide/poly (ethylene oxide) based paint photocatalysts. J Appl Polym Sci 131:40760
Mohamed NA, El-Ghany NAA (2017) Evaluation of the stability of rigid poly (vinyl chloride)/biologically active phthalimido phenyl urea composites using thermogravimetric analysis. Polym Degrad Stabil 140:95–103
Szakács T, Iván B (2004) Epoxidation of thermally degraded poly (vinyl chloride). Polym Degrad Stabil 85:1035–1039
Luo Z, Wang H, Chen K, Liu J, Wu C, Wei Z (2015) UV-Visible spectrophotometry for the determination of conjugated polyene structures of poly (vinyl chloride) in 1, 2-dichloroethane. Int J Polym Anal Chem 20:240–249
Zhao P, Li Z, Li T, Yan W, Ge S (2017) The study of nickel effect on the hydrothermal dechlorination of PVC. J Clean Prod 152:38–46
Zhao T, Zhou Q, He X-L, Wei S-D, Wang L, van Kasteren JM, Wang Y-Z (2010) A highly efficient approach for dehydrochlorinating polyvinyl chloride: catalysis by 1-butyl-3-methylimidazolium chloride. Green Chem 12:1062–1065
Ghaemy M, Gharaebi I (2000) Study of dehydrochlorination of poly (vinyl chloride) in solution and the effect of synthesis conditions on graft copolymerization with styrene. Eur Polym J 36:1967–1979
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Najafi, V., Ahmadi, E. & Ziaee, F. Chemical modification of PVC by different nucleophiles in solvent/non-solvent system at high temperature. Iran Polym J 27, 841–850 (2018). https://doi.org/10.1007/s13726-018-0658-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13726-018-0658-x