Abstract
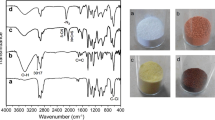
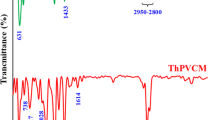
Various chemical modification methods have been tried on PVC polymer materials for recycling and functionalization purpose. These methods employ substitution, elimination (dehydrochlorination) and grafting polymerization processes. PVC is treated with ethylenediamine (EDA) for amine grafting since it is a reactive amine for substitution and efficient removal of chlorides. Nevertheless, optimum conditions for substitution (amination) and significance of its competition with elimination have not been reported in detail so far. In this study, PVC resin has been treated with 99% EDA and 80% EDA (aqueous), and also 9.1% THF solution of PVC has been treated with 99% EDA at varying temperatures (RT to 85 °C or RT to 65 °C). Additionally, PVC membranes have been treated with 99% EDA and 80% EDA (aqueous) at three different temperatures (RT, 50 °C, 75 °C). Effects of temperature and solvent on the degrees of amination and dehydrochlorination have been elaborated. Up to 99% dechlorination and 34% amination is possible with the use of EDA. Direct amination of membranes facilitates production of ready-cast aminated PVC membranes. FTIR Spectroscopy was used to study chemical structures. Morphology was observed via scanning electron microscopy (SEM) and atomic contents were analyzed via X-ray photoelectron spectroscopy (XPS) in order to survey the degree of amination etc. UV-Visible Spectroscopy was used to characterize the length of conjugation.

Similar content being viewed by others
References
P. Jia, L. Hu, G. Feng, C. Bo, M. Zhang, and Y. Zhou, Mater. Chem. Phys., 190, 25 (2017).
C. Lăzăroaie, E. Rusen, B. Mărculescu, T. Zecheru, and G. Hubcă, UPB Sci. Bull. Ser. B, 72, 127 (2010).
Y. Saeki and T. Emura, Prog. Polym. Sci., 27, 2055 (2002).
G. R. Krishnan, K. S. Niveditha, and K. Sreekumar, Indian J. Chem., 52, 428 (2013).
J. Zhu, Y. Su, X. Zhao, Y. Li, J. Zhao, X. Fan, and Z. Jiang, Ind. Eng. Chem. Res., 53, 14046 (2014).
S. M. Hosseini, M. Askari, P. Koranian, S. S. Madaeni, and A. R. Moghadassi, J. Ind. Eng. Chem., 20, 2510 (2014).
Q. Shi, Q. Fan, W. Ye, J. Hou, S. C. Wong, X. Xu, and J. Yin, ACS Appl. Mater. Interfaces, 12, 6 (2014).
J. Simmchen, R. Ventura, and J. Segura, Trans. Med. Rev., 26, 1 (2016).
S. Moulay, Prog. Polym. Sci., 35, 303 (2010).
G. Sneddon, J. C. McGlynn, M. S. Neumann, H. M. Aydin, H. H. Yiu, and A. Y. Ganin, J. Mater. Chem. A, 5, 11864 (2017).
S. Balci, O. Birer, and S. Suzer, Polymer, 45, 7123 (2014).
T. Kameda, M. Ono, G. Grause, T. Mizoguchi, and T. Yoshioka, Polym. Degrad. Stab., 94, 107 (2009).
T. Yoshinaga, M. Yamaye, T. Kito, T. Ichiki, M. Ogata, J. Chen, H. Fujino, T. Tanimura, and T. Yamanobe, Polym. Degrad. Stab., 86, 541 (2004).
B. Balakrishnan, D. S. Kumar, Y. Yoshida, and A. Jayakrishnan, Biomaterials 26, 3495 (2005).
J. T. Allan, L. E. Prest, and E. B. Easton, J. Membr. Sci., 489, 175 (2005).
M. S. Mohy Eldin, T. M. Tamer, M. A. Abu Saied, E. A. Soliman, N. K. Madi, L. Ragab, and I. Fadel, Adv. Polym. Technol., 0, 21640 (2015).
J. Liu, Y. Q. Li, and W. J. Zheng, Monatsh. Chem., 140, 1425 (2009).
B. Balakrishnan, N. R. James, and A. Jayakrishnan, Polym. Int., 54, 1304 (2005).
M. de AMMS, M. Helena, R. M. N. De Assunção, H. M. Soares, A. P. Cangani, D. A. Cerqueira, and C. D. S. Meireles, J. Appl. Polym. Sci., 115, 1474 (2010).
L. Guo, G. Shi, and Y. Liang, Polymer, 42, 5581 (2001).
R. Q. Fu, J. J. Woo, S. J. Seo, J. S. Lee, and S. H. Moon, J. Membr. Sci., 309, 156 (2008).
C. W. Hwang, C. M. Oh, and T. S. Hwang, J. Adhes. Interface, 15, 1 (2014).
J. T. Allan, L. E. Prest, and E. B. Easton, J. Membr. Sci., 489, 175 (2015).
A. M. Saxman, R. Liepins, and M. Aldissi, Prog. Polym. Sci., 11, 57 (1985).
T. Masuda, Encyclopedia of Polymeric Nanomaterials, Springer-Verlag, Heidelberg, 2015.
J. A. Stowell, A. J. Amass, M. S. Beevers, and T. R. Farren, Polymer, 30, 195 (1989).
A. Singh, M. S. M. Rawat, and C. S. Pande, J. Appl. Polym. Sci., 118, 876 (2010).
M. Beltran and A. Marcilla, Eur. Polym. J., 33, 1135 (1997).
L. Guo, G. Shi, and Y. Liang, Synth. Met., 104, 129 (1999).
A. Baruya, D. L. Gerrard, and W. F. Maddams, Macromolecules, 16, 578 (1983).
M. Ghaemy and I. Gharaebi, Eur. Polym. J., 36, 1967 (2000).
F. Sondheimer, D. A. Ben-Efraim, and R. Wolovsky, J. Am. Chem. Soc., 83, 1675 (1961).
D. Braun and D. Sonderhof, Polym. Bull., 14, 39 (1985).
K. Aouachria and N. Belhaneche-Bensemra, Polym. Degrad. Stab., 91, 504 (2006).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Acknowledgments: This work was supported by the Commercializations Promotion Agency for R&D Outcomes Grant funded by the Korean Government (MSIP) (2017, 2017K000215, Joint Research Corporations Support Program). This work was also supported by the Human Resource Training Program for Regional Innovation and Creativity through the Ministry of Education and National Research Foundation of Korea (NRF- 2015H1C1A1035652). This research was supported by the Ministry of Trade, Industry and Energy (MOTIE) and the Korea Institute for the Advancement of Technology (KIAT) (N0002383, 2018).
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Park, E.J., Park, B.C., Kim, Y.J. et al. Elimination and Substitution Compete During Amination of Poly(vinyl chloride) with Ehtylenediamine: XPS Analysis and Approach of Active Site Index. Macromol. Res. 26, 913–923 (2018). https://doi.org/10.1007/s13233-018-6123-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13233-018-6123-z