Abstract
The low incidence of pediatric severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection and the associated multisystem inflammatory syndrome (MIS-C) lack a unifying pathophysiological explanation, impeding effective prevention and therapy. Activation of the NACHT, LRR, and PYD domains-containing protein (NLRP) 3 inflammasome in SARS-CoV-2 with perturbed regulation in MIS-C, has been reported. We posit that, early age physiological states and genetic determinants, such as certain polymorphisms of renin-angiotensin aldosterone system (RAAS) molecules, promote a controlled RAAS hyperactive state, and form an evolutionary landscape involving an age-dependent erythropoietin (EPO) elevation, mediating ancestral innate immune defenses that, through appropriate NLRP3 regulation, mitigate tissue injury and pathogen invasion. SARS-CoV-2-induced downregulation of angiotensin-converting enzyme (ACE)2 expression in endothelial cells (EC), impairment of endothelial nitric oxide (NO) synthase (eNOS) activity and downstream NO bioavailability, may promote a hyperactive RAAS with elevated angiotensin II and aldosterone that, can trigger, and accelerate NLRP3 inflammasome activation, while EPO-eNOS/NO abrogate it. Young age and a protective EPO evolutionary landscape may successfully inhibit SARS-CoV-2 and contain NLRP3 inflammasome activation. By contrast, increasing age and falling EPO levels, in genetically susceptible children with adverse genetic variants and co-morbidities, may lead to unopposed RAAS hyperactivity, NLRP3 inflammasome dysregulation, severe endotheliitis with pyroptotic cytokine storm, and development of autoantibodies, as already described in MIS-C. Our haplotype estimates, predicted from allele frequencies in population databases, are in concordance with MIS-C incidence reports in Europeans but indicate lower risks for Asians and African Americans. Targeted Mendelian approaches dissecting the influence of relevant genetic variants are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection, the cause of the coronavirus disease 2019 (COVID-19) pandemic, remains a continuing global threat. Despite the usually asymptomatic or mild SARS-CoV-2 in children, the latent multisystem inflammatory syndrome secondary to SARS-CoV-2 observed in this particular population (MIS-C) is worrisome [1, 2]. MIS-C remains a diagnosis of exclusion with elusive pathophysiological mechanisms [3]. Its rare occurrence (annual incidence of two per 100,000 individuals under 21) and ethnic disparities suggest a genetic predisposition [1,2,3]. Kawasaki disease (KD), KD Shock Syndrome, and MIS-C appear to be on the same immunopathological continuum, in which host immune responses precipitate vascular endothelial inflammation and a cytokine storm that result in cardiomyopathy and enteropathy [4]. Indeed, endothelial hyperinflammation-endotheliitis is the pathophysiological hallmark of acute, severe COVID-19 and MIS-C [5,6,7]. MIS-C, however, displays an even greater degree of inflammation, increased levels of circulating SARS-CoV-2 spike (S) protein, dysregulated inflammasome activation with pyroptosis, and presence of autoantibodies [8,9,10].
Viral infections, including SARS-CoV-2, summon innate immune system intracellular pattern recognition receptors (PRRs) that detect pathogen-associated molecular patterns (PAMPs), such as viral RNA, proteins, and cell wall fragments, and danger-associated molecular patterns (DAMPs) released from damaged host cells [11,12,13,14,15,16,17]. One of the most studied PRRs, the NACHT, LRR, and PYD domains-containing protein 3 (NLRP3), senses PAMPs or DAMPs and triggers signaling cascades to assemble cytosolic, oligomeric, protein platforms called inflammasomes [11, 17]. Following a two-step process of priming and activation, canonical NLRP3 inflammasome activation leads, through caspase-1 activation, to cytokine (interleukin (IL)-1β and IL-18) release, and gasdermin D (GSDMD)-induced pyroptosis, a form of lytic, inflammatory programmed cell death [11, 17]. Controlled, canonical inflammasome activation and moderate pyroptosis is necessary to effectively contain an infection [18, 19]. Dysregulated NLRP3 inflammasome activation in response to acute SARS-CoV-2 infection associates with COVID-19 severity symptoms, indicating a crucial role in the pathophysiology underlying the massive inflammation observed in severe and fatal cases [13, 20,21,22]. Additionally, secretory phospholipase A2 increase in acute pediatric COVID-19 and MIS-C implies an active role for inflammasome activation in their pathogeneses [23]. Furthermore, non-canonical (involving caspase 4/5) NLRP3 inflammasome activation, also leading to canonical NLRP3 inflammasome activation, appears unique for MIS-C [8]. If uninhibited, aberrant activation of pyroptosis leads to massive DAMPs release, able to amplify and perpetuate uncontrolled inflammatory immune responses, potentially leading to autoantibody formation and autoimmunity [24]. Stringent host regulation of NLRP3 inflammasome activation is necessary, to avoid detrimental inflammatory reactions, as seen in numerous autoinflammatory and autoimmune diseases [18, 19, 24]. Thus, loss of control of inflammasome regulation in genetically predisposed children, could potentially pave the way towards MIS-C [22, 25].
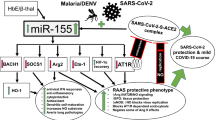
SARS-CoV-2 spike protein (S) interaction with angiotensin-converting enzyme (ACE) 2 has been shown to downregulate ACE2 expression in endothelial cells (EC) and impair endothelial nitric oxide (NO) synthase (eNOS) activity and downstream NO bioavailability [6, 7]. ACE2 has an important role in counterbalancing renin-angiotensin aldosterone system (RAAS) activation of ACE in the vascular endothelium of the lungs and kidneys by cleaving circulating angiotensin II (Ang II) to Ang 1–7 and promoting eNOS activation (Fig. 1) [26]. Therefore, putative ACE2 activity and signaling is essential to maintain homeostatic endothelial biology. Constitutive NO production by ECs is also involved in maintaining normal endothelial function and defense against insults, injuries, and inflammation [27, 28]. Bioavailable NO potently inhibits leukocyte adhesion and displays significant antithrombotic, antiproliferative, antioxidative, immunoregulatory and microbicidal properties [27, 28]. Thus, through the latent suppression of endothelial expression of ACE2 and eNOS/NO, SARS-CoV-2 may promote a state of RAAS hyperactivity with elevated Ang II levels and impaired NO bioavailability that trigger and accelerate NLRP3 inflammasome activation, contributing to the endotheliitis, and resultant organ injuries observed in MIS-C (Fig. 1) [6, 7, 15, 29,30,31,32,33,34].
Schematic representation of the renin-angiotensin aldosterone system (RAAS) cascade, RAAS regulation of erythropoietin (EPO), and interactions with the fibroblast growth factor (FGF)23/α-Klotho system. Single nucleotide polymorphisms (SNPs) leading to RAAS hyperactivity and FGF23 elevation are presented. Upon binding of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) to angiotensin-converting enzyme (ACE)2, ACE/ACE2 imbalance arises, earlier in children due to lower ACE2 expression, and amplified by the ACE2 T-allele, leading to RAAS hyperactivity with angiotensin II (Ang II) elevation, further augmented by the ACE D-allele. The angiotensinogen (AGT) G-allele increases AGT, RAAS’ primary substrate, while the Ang II type 1 receptor gene (AGTR1) C-allele amplifies Ang II action. Elevated FGF23, further potentiated by its C-allele, will lower 1alpha,25-dihydroxyvitamin D3 (1,25(OH)2D3), in turn increasing renin and sustaining a RAAS hyperactive state. FGF23 elevation lowers α-Klotho, both negatively impacting on eNOS and NO generation. Interactions between Ang II and FGF23 promote adverse cardiac pathology. Elevated Ang II will raise EPO levels that through the EPO- endothelial nitric oxide (NO) synthase (eNOS) cascade will attempt to restore NO impairments and inhibit SARS-CoV-2 replication and cell entry. Ang II effects through the Ang II type 2 (AT2R) and Mas receptors (MasR) lead to delayed and sustained NO increases offering additional cardiovascular protection
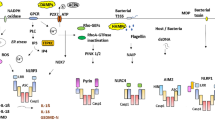
Despite the resulting imbalance in ACE and ACE2 activities during latent SARS-CoV-2-related disease, amplified in children by lower ACE2 expression, prior work suggests that the host leverages the higher circulating levels of Ang II to evoke erythropoietin (EPO) secretion in an effort to restore eNOS activity and homeostatic NO signaling (Fig. 1) [35,36,37,38,39,40,41,42,43]. Elevated EPO with enhanced eNOS/NO pathway activity, and subsequently increased NO generation and bioavailability, is known to suppress the NLRP3 inflammasome, potentially effectively inhibiting early SARS-CoV-2 replication and cell entry, and the development of endotheliitis [32, 44,45,46,47,48,49,50]. Such an ancestral, evolutionary landscape involving an age-dependent EPO elevation, is already known to occur at an early age and provides the host with a fitness advantage against malaria while forming constraints against pathogen adaptation and invasion [39]. All molecules in the above evolutionary landscape involving RAAS-EPO-eNOS interactions are under significant genetic control aiming to support, augment, and extend this early age EPO elevation and eNOS activity upon insult, as witnessed by protective single nucleotide polymorphisms (SNPs) in malaria [39, 51,52,53]. Increasing age and SNPs for members of the RAAS hormonal axis and NOS3, the gene responsible for eNOS expression, may differentially and significantly impact EPO secretion, NO production and bioavailability, and substantially influence regulation of inflammasome activation in genetically susceptible children [27, 28, 54]. Synergism with other genetic variants like the fibroblast growth factor (FGF) 23, reportedly associated with KD, may aggravate endothelial dysfunction, and adversely impact the heart [55]. The purpose of this narrative review is to provide key insights into the evolutionary landscape of EPO-mediated eNOS regulation and the genetic jigsaw of SNPs involving the above molecules that may disturb this signaling axis and affect NLRP3 regulation, potentially contributing to MIS-C pathobiology (Fig. 2).
Schematic interactions between endothelial nitric oxide (NO) synthase (eNOS) activity modulations by erythropoietin (EPO), age, and genetic determinants for members of the renin-angiotensin aldosterone system (RAAS) hormonal axis, nitric oxide synthase (NOS)3, β-common receptor (βcR), and fibroblast growth factor (FGF)23, may result in opposing effects in NO generation and bioavailability, differentially impacting nucleotide-binding oligomerisation domain (NOD)-like receptor protein 3 (NLRP3) inflammasome activation. Genetically augmented EPO levels through relevant SNPs for members of the RAAS hormonal axis along with eNOS activating SNPs will synergistically enhance NO generation and bioavailability, subsequently resulting in an extended age span for SARS-CoV-2 protection, successful NLRP3 inflammasome regulation, and asymptomatic/mild infections. Genetic determinants for members of the RAAS hormonal axis and NOS3, that are unable to counteract a declining EPO due to increasing age, may result in an unopposed RAAS and FGF23 proinflammatory state, possibly potentiated by angiotensin II type 1 receptor autoantibodies (AT1-AA), that through a cytokine storm and gravely reduced NO bioavailability, induce NLRP3 inflammasome dysregulation, and predispose for multisystem inflammatory syndrome in children (MIS-C) with cardiac and/or enteric affliction
EPO-eNOS interactions suppress NLRP3 inflammasome activation and promote protection against SARS-CoV-2 in children
NLRP3 inflammasome activation in SARS-CoV-2 may occur through, at least, five different pathways: (1) directly through PAMPs recognition of virus-IgG complexes, viral RNAs and proteins, S-protein-ACE2 interaction [14, 15, 22]; (2) SARS-CoV-2-induced RAAS hyperactivity increases Ang II, a well-known trigger of NLRP3 inflammasome activation through the Ang II type 1 receptor (AT1R), in the heart, lung, kidney and bowel [15, 21, 56,57,58,59,60,61,62]; (3) increased aldosterone due to SARS-CoV-2-induced RAAS hyperactivity increases the expression of the NLRP3 inflammasome, known to play a crucial role in aldosterone-induced vascular damage [33, 34]; (4) elevated Ang II also stimulates the upregulation of stimulator of interferon genes (STING), a powerful meditator of innate immunity, known to meditate heart inflammation and fibrosis by activating NLRP3 inflammasome and GSDMD-induced pyroptosis [63,64,65]; and finally, (5) eNOS-NO pathway impairments have been reported to accelerate NLRP3 inflammasome activation while the NLRP3 inflammasome perpetuates its activation through downregulation and proteolysis of eNOS [32, 61, 66, 67].
It is well-known that an initial innate immune response, involving activation of the NLRP3 inflammasome (pathway 1: PAMPs-induced), is a necessary antiviral frontline defense and limits pathogen dissemination until the adaptive immunity arm commences its antibody-driven warfare [11]. By contrast, dysregulated inflammasome control with pathological inflammasome hyperactivation, as might occur through pathways 2–5, is associated with massive inflammation, oxidative stress, fibrosis, and cytokine storm with bystander tissue damage [21, 68]. In this disconcerted milieu, EPO has the potential to alleviate inflammation and elicit tissue protection though activation of survival pathways (eNOS/NO) and inhibition of pro-inflammatory cascades (IL-1β) [32, 45, 47,48,49,50]. EPO has been repeatedly reported to alleviate ischemic sequalae and inflammation in the heart, lung, kidney, and central nervous system through abrogation of NLRP3 inflammasome activation [48, 50, 69, 70].
A highly significant, age-dependent, anemia-independent EPO elevation, highest in the youngest but declining during a child’s development, has been reported during the first 13 years of life [39, 51]. Maximum EPO response occurs very early, prior to the age of 5, at a time when cerebral malaria and MIS-C are uncommon [39, 71]. This origin of this age-dependent EPO elevation is unknown but could be attributed to the significantly higher, age- and genotype-related ACE activities in serum, physiologically found in newborns, healthy children, and teenagers but not in adults [35,36,37, 72, 73]. A lower nasal ACE2 expression in newborns and children, would appear to further amplify ACE activities [40,41,42]. These early age, physiological states promoting a controlled RAAS hyperactive state with elevated EPO levels, can consequently enhance EPO-eNOS/NO pathway responsiveness, and potentially mediate protection against SARS-CoV-2; indeed, children below the age of 5 generally experience asymptomatic or mild SARS-CoV-2 infections [1, 38, 44, 74]. Furthermore, working in tandem, EPO-augmenting SNPs of the RAAS hormonal axis and eNOS activity amplifying NOS3 SNPs will ensure abundant catalysts and substrates to sustain EPO generation and NO bioavailability to exert protective effects over a wider age span, potentially beyond age 13, as seen in young Indian adults with malaria (Fig. 1) [44,45,46,47, 52, 53, 75]. We posit that, in children, this age-dependent EPO elevation within the EPO evolutionary landscape, can effectively contain an acute SARS-CoV-2 infection and appropriately regulate the initial SARS-CoV-2-PAMP-induced NLRP3 inflammasome activation, while the ensuing SARS-CoV-2-induced RAAS hyperactivity with Ang II and aldosterone elevations could be leveraged to further enhance EPO secretion [35,36,37], rather than aggravating an ongoing NLRP3 inflammasome activation through pathways 2–5 [12,13,14,15,16, 34, 44, 63]. Under the protection of this evolutionary landscape, consequent inflammatory and ischemic sequalae in the lung, heart, kidney, and central nervous system, can be prevented by EPO-mediated abrogation of further NLRP3 inflammasome activation involving pathways 2–5 [48,49,50, 69, 70]. It is, however, obvious that children can still get infected by SARS-CoV-2, and that some may progress to MIS-C [1, 2]. Attenuation of the EPO-eNOS/NO protection provided by this EPO evolutionary landscape, through increasing age and adverse genotypes, may allow augmented, perpetual NLRP3 inflammasome activation, ultimately resulting in a cataclysmic COVID-19 cytokine storm and MIS-C in genetically susceptible individuals (Fig. 2) [12, 15, 22, 39, 72, 73].
While available information on EPO levels in SARS-CoV-2 patients is sparse and limited to adults, it is also supportive of a protective effect. Nasopharyngeal swab samples in asymptomatic or mild COVID-19 patients, demonstrate 2.6 times elevated EPO mRNA levels, correlating well with whole blood [76]. Profound EPO elevation in moderate cases, significant decline with disease advancement, and profoundly low EPO levels in severe disease, have all been recently reported [77, 78]. EPO’s strong negative correlation with thromboembolism and tissue injury markers imply that its induction may counter the adverse effects of a COVID-19 cytokine storm [77]. These findings collectively lend support to the protective link between elevated EPO and asymptomatic or mild/moderate COVID-19 [76,77,78,79,80].
Lastly, a child’s immune system and EPO physiology may contribute to initial protection from severe SARS-CoV-2 infection by modulating host systemic immunological response towards increased tolerance [81, 82]. EPO-regulated increases in circulating CD71+ erythroid cells (abundant in children while absent in adults) contribute to an attenuated inflammatory response to pathogens, lowering the burden of infection in this age group [81, 82]. Furthermore, EPO mediates reduction of auto- and alloantibody formation [83], while its binding to T cell-expressed EPO receptor (EPOR) inhibits Th17 cell induction preventing collateral damage and autoimmune pathology [84]. Loss of EPO protection may allow immune autoreactivity and development of agonist AT1R autoantibodies (AT1-AA) perpetuating Ang II pro-inflammatory actions, even in the absence of ACE D-allele, as reported in long COVID-19 patients [85]. AT1-AA correlate with blood pressure dysregulation and COVID-19 disease severity and could account for the 6–8-week lag observed in MIS-C (Fig. 2) [85, 86]. Finally, while endothelial progenitor cell (EPC) mobilization through the hematopoietic actions of EPO may contribute to additional tissue protection [45], EPO loss may allow pyroptotic NLRP3 damage of host stem cell reservoir, jeopardizing the proliferative potential of the vascular endothelium [15].
The NO genetic pathway to MIS-C
Suppression of eNOS appears to be a critical determinant of SARS-CoV-2 infection severity as suggested by improved COVID-19 outcomes with the use of a wide range of repurposed drugs with known capacity for eNOS activation (e.g., fluvoxamine, dehydroepiandrosterone (DHEA)/DHEA-sulfate (DHEAS), raloxifene, and metformin) (Fig. 2) [87,88,89,90]. Furthermore, as lower soluble eNOS levels were associated with worse acute respiratory distress syndrome (ARDS) severity in adults with COVID-19, these data suggest that eNOS may be an important therapeutic target during SARS-CoV-2 infection to mitigate serious lung complications [91]. Interestingly, inhibitory effects of NO on NLRP3 have been reported through NO-mediated inhibition of caspase-1, IL-1β, and IL-18 release, suggesting an obligatory role of eNOS in mediating anti-inflammatory effects and endothelial protection, with grave cardiovascular consequences when NO bioavailability is deranged [32, 45, 47, 61, 67, 92]. Furthermore, demonstrating a critical link between EPO and eNOS effects, NO can induce expression of the EPOR and EPO, while cardio-, reno- and vasculoprotective effects of EPO are eNOS-dependent as eNOS antagonism or ablation abrogate them [45,46,47, 50]. Evidently, eNOS activity-reducing SNPs impairing NO generation and bioavailability, could accelerate NLRP3 inflammasome dysregulation, allowing the development of systemic hyperinflammation/cytokine storm, subsequently leading to MIS-C in genetically predisposed children [32, 54, 93].
NOS3 genetic polymorphisms
Impairments of the eNOS/NO pathway through uncoupling, inhibition, and/or genetic polymorphisms, and the resulting reduced availability of NO, accelerate NLRP3 inflammasome activation and progression of endothelial dysfunction through infiltration of proinflammatory macrophages [32]. Commonly researched NOS3 polymorphisms and their functional effects are summarized in Table 1 [54, 74, 93]. Numerous studies have demonstrated important clinical implications of eNOS activity-reducing polymorphisms in hypertension and anti-hypertensive treatment, pre-eclampsia, coronary artery disease (CAD) and KD, thrombosis, metabolic syndrome, obesity, and diabetes [54, 94, 95]. Malaria, Dengue, and Puumala Hanta virus infections have all been associated with NOS3 polymorphisms [52, 53, 96]. NO production may vary up to 30.5%, as reported from genotype-based simulations of combined NOS3 polymorphisms, with obvious clinical implications for endothelial dysfunction in several diseases [54, 93, 97]. Haplotype differences between ethnicities may underlie disparities in susceptibility to a variety of diseases involving alterations in NO formation and could thus also explain ethnic differences in MIS-C incidence [93]. While the C-b-Asp haplotype may enhance eNOS expression and NO production, the C–4b–Glu haplotype is associated with lower NO formation in healthy Caucasian and African Americans, but also in CAD in patients and their first-degree relatives [98,99,100]. NOS3 haplotype-related variability in vasculoprotective NO bioavailability may consequently affect NLRP3 regulation and be the tipping point for MIS-C development (Fig. 2) [32, 66].
Genetic polymorphisms in members of the RAAS hormonal axis
As RAAS activity is an important regulator of eNOS activity, genetic polymorphisms for molecules in the RAAS signaling axis (Table 2) are likely to have significant impact on NO generation, NLRP3 regulation, and endothelial inflammation during MIS-C (Fig. 2) [15, 21, 56,57,58,59,60,61,62, 103, 104]. Polymorphisms of the ACE gene explain 20–50% of the variability in ACE levels and up to 15% of hypertensive cases [105]. Given the fundamental role of RAAS in cardiovascular homeostasis and SARS-CoV-2, RAAS molecule polymorphisms could significantly modulate Ang II activity and increase the risk of a RAAS-induced hyperinflammation with excessive NLRP3 inflammasome activation, when increasing age and co-inherited eNOS activity-reducing NOS3 haplotypes, attenuate EPO effects (Fig. 1, 2) [15, 21, 22, 30, 56,57,58,59,60,61,62, 104, 106,107,108,109,110].
By contrast, combination of the ACE D-/ACE2 T-alleles with the NO enhancing C-b-Asp haplotype, as opposed to the NO reducing C-b-Glu haplotype, associate with protection against malaria through increased Ang II and NO bioavailability [52, 53]. In females, specifically, the ACE2 T-allele by further reducing ACE2 expression, may be instrumental in Ang II elevation, enhancing EPO levels, and potentially conferring protection against SARS-CoV-2 [52, 111]. SNPs involving all the above-mentioned RAAS molecules, (ACE/ACE2/AGT/AGTR1), while protective in malaria, have been implicated in severe adult COVID-19 outcomes (Table 2) [104, 106, 115]. Their net haplotype effect could significantly impact Ang II and EPO levels, eNOS activity, NO generation and bioavailability, perturbing NLRP3 inflammasome regulation, endothelial function, peripheral vascular resistance, and blood pressure (Table 2) [52, 57, 58, 108,109,110, 116].
FGF23 genetic polymorphisms
Elevated FGF23 (a phosphaturic hormone), reduced α-Klotho (α-kl: an anti-ageing hormone, vasculoprotective factor, and FGF23 co-receptor), and RAAS hyperactivity are critically linked to reduced eNOS activity and NO bioavailability, NLRP3 inflammasome activation, endothelial dysfunction, and adverse cardiovascular pathology [117,118,119,120,121]. FGF23 directly suppresses ACE2 and 1,25-dihydroxyvitamin D3 thereby increasing Ang II and renin expression, respectively (Fig. 1) [119]. Both these actions promote further RAAS upregulation and are obviously potentiated by the ACE D- and ACE2 T-alleles [117, 118]. Moreover, elevated FGF23 adversely regulates innate immune responses towards a pro-inflammatory state, blocking myocardial macrophage transition to M2 and resolution of inflammation [117]. Furthermore, elevated FGF23 decreases endothelial α-kl expression, severely impacting eNOS activation and NO synthesis (additionally reduced by relevant NOS3 SNPs), while EPO mitigates reductions in renal α-Klotho expression (Fig. 1) [118, 122, 123]. Increased EPO and α-kl, both effectively abrogate NLRP3 inflammasome activation, preventing the maturation of proinflammatory cytokines IL-1β and IL-18 and pyroptotic cell death [48, 70, 120, 124, 125]. As FGF23 expression is under the control of IL-1β, the product of an activated NLRP3 inflammasome, alleviating NLRP3 activation would lower IL-1β and not only reduce FGF-23 production, but also minimize local and systemic inflammatory responses [121]. Elevated Ang II stimulates systemic release of FGF-23 and its ectopic expression in the heart, subsequently augmenting the adverse cardiac effects of Ang II (Fig. 2) [117]. Significantly higher FGF23 levels reported in KD patients associate positively with impaired endothelial vasodilation, coronary artery aneurysms, and adverse cardiovascular and renal events and death [55, 117, 126, 127]. The FGF23 rs3832879 (c.212-37insC) polymorphism, is significantly associated with both elevated serum FGF23 levels and coronary artery dilatations and aneurysms in KD [55, 127, 128]. AGT rs5050 G-, ACE D- and AGTR1 C-alleles, all are synergistically associated with adverse cardiovascular pathology and coronary artery lesions in KD (Fig. 1) [55, 103, 114, 126]. KD aneurysmal endothelium demonstrated histological signs of vascular senescence with lack of eNOS immunostaining compared to controls, confirming that decreases in vasodilative factors, such as eNOS/NO, play operative roles in KD aneurysm development [129]. To our knowledge, FGF23 levels and genetic polymorphisms in MIS-C have not been investigated to date.
Finally, pertaining to MIS-C gastrointestinal (GI) predilection, recent studies pinpoint the GI tract as a potential theater for MIS-C initiating events [130, 131]. Increased zonulin, lipopolysaccharide (LPS), and LPS binding protein (LBP) levels, indicating gut mucosal barrier breakdown, are specific for MIS-C [130, 131]. LBP alone [132], or LBP-transported LPS from exposed dysbiotic gut microbial flora [130, 131], can initiate a non-canonical NLRP3 inflammasome activation, as reported in inflammatory bower disease (IBD) and observed uniquely in MIS-C [8, 133]. Increased levels of circulating SARS-CoV-2 S protein, reported in MIS-C, bind LPS, and through Toll-like receptor 4 (TLR4) recognition, lead to an overactive immune response and hyperinflammation [134]. Apart from abrogating NLRP3 inflammasome activation [70], EPO reduces TLR4 expression levels, thereby improving necrotizing enterocolitis [135]. Moreover, all components of the RAAS are present in the GI tract and Ang II is produced locally [136]. Ang II exerts potent pro-inflammatory effects in the colonic microcirculation [137] that, along with reduced NO bioavailability and P-glycoprotein (Pgp) inhibition, may be the reasons for the enteropathy in MIS-C [138, 139]. Pgp downregulation is under the control of Ang II and AT1R, both of which are involved in the pathogenesis and treatment of IBD [139]. By contrast, Pgp induction/activation exerts potent anti-NLRP3 inflammasome effects that may provide potential therapeutic anti-inflammatory effects for IBD patients [140]. An overactive NLRP3/IL-1β axis further aggravates genetically reduced eNOS activity through eNOS downregulation and proteolysis, gravely impairing the ability of the endothelium to produce NO, leading to unopposed Ang II-AT1R-dependent leukocyte-endothelial cell interactions, potentially resulting in the vascular lesions that occur in hypertension, atherosclerosis, and myocardial ischemia–reperfusion injury [66, 141, 142]. Consequently, increasing age and detrimental combinatory haplotypes of SNPs in NOS3 and members of the RAAS, that significantly compromise eNOS and NO generation and bioavailability, along with FGF23 genetic variants that elevate FGF23 and potentiate Ang II effects on the endothelium of various organs (heart, GI tract, kidney) through NLRP3 inflammasome dysregulation, suggest a pathway to MIS-C with different organ phenotypes (Fig. 2).
Gene clusters predisposing for MIS-C
Allele co-expression analysis of key target genes in hypertension and across several ethnicities provide important insights in the search for MIS-C-prone haplotypes [143, 144]. A gene cluster encompassing the ACE, AGT, AGTR1, and NOS3 genes has been described linked to hypertension and the components of the metabolic syndrome [143]. The implicated risk haplotypes were overwhelmingly composed of variant alleles coding for RAAS hyperactivity and eNOS inhibition, confirmed by higher ACE activity and lower NO levels in plasma [144]. The C–4b–Glu haplotype associated with lower NO formation, together with relevant alleles of FGF23 and members of the RAAS, could be a causative haplotype in children with MIS-C (Table 3) [98, 99]. Similar genetic cluster findings have been reported in SARS-CoV-2, thus haplotypes with the above effects, together with an age-dependent loss of EPO protection, might induce higher levels of NLRP3 and result in MIS-C in genetically susceptible children [107].
As the three NOS3 SNPs in the C-4b-Glu haplotype are in linkage disequilibrium (LD), we used the population allele frequencies of the involved genes to calculate haplotype prevalence estimates (Table 3) [99, 145,146,147]. Epidemiological reports of C-4b-Glu haplotype point to its scarcity in the population at 2.4%, consistent with the rarity of MIS-C [1, 2, 148]. The estimated haplotype prevalence of all detrimental alleles per 100,000 in the general population was 9.8851, 1.01376, 4.5421 for Europeans, African Americans, and Asians, respectively. Based on the percentage of the United States and 27-state European Union population under 18 at 20.3%, our MIS-C prevalence estimates were 2.0, 0.2057, and 0.922 per 100,000 for European, African American and Asian populations under 18 years, respectively [149, 150]. Our European ancestry risk estimates are in concordance with the reported MIS-C incidence [1, 2]. Our Asian MIS-C risk estimate was less than half of that for Europeans, while African Americans appear to enjoy a 10 times lower risk compared to Europeans. The observed overrepresentation of Blacks and Hispanics in epidemiological studies may also be due to socioeconomic factors [1, 71]. However, recent reports are not in support of any differences between racial or ethnic groups [151]. Limitations of our estimates include the presumption that included alleles retain their purported singular effect when occurring in a haplotype with the other alleles, unknown LD between the implicated genes, unknown gene co-expressions, and gene–gene or gene-environment interactions.
EPOR and β-common receptor (βcR) genetic polymorphisms
EPO-induced eNOS activation requires the βcR in the formation of a βcR-EPOR-eNOS complex (Fig. 2) [45, 152]. While the EPOR mediates hematopoietic EPO effects, the βcR mediates EPO’s anti-inflammatory, antiapoptotic, and antioxidative tissue protective functions by forming an EPOR/βcR heterodimer (Fig. 2) [45, 152]. Both EPOR and βcR have thus the potential to limit the EPO-eNOS activation cascade [45, 152]. A truncating mutation (p.Gln82Ter; rs370865377) resulting in a hypo-responsive EPOR has been detected in 1 in 550 Icelanders associated with a three-fold EPO increase, normal hemoglobin, and no adverse cardiovascular associations [153]. It is intriguing to speculate whether carriers of this EPOR mutation might also enjoy SARS-CoV-2 protection, but this remains unknown. Apart from being an integral part of the βcR-EPOR-eNOS complex [152], βcR is also a shared receptor subunit of IL-3, IL-5, and granulocyte–macrophage colony stimulation factor (GM-CSF) receptors. Polymorphisms in the CSF2RB (βcR-coding gene) would attenuate EPO-eNOS effects [45]. The effect of co-inherited CSF2RB and NOS3 polymorphisms is unknown. CSF2RB polymorphisms will also affect various functions of IL-3, IL-5, and GM-CSF, as reported in schizophrenia, where NO is implicated in its pathogenesis and symptomatology, and could account for the excess COVID-19 mortality reported [154,155,156]. GM-CSF is a key regulator of the NLRP3 inflammasome and IL-1β production, thus impaired βc-cytokine function, already described in KD, may have widespread immunological implications in SARS-CoV-2 [156,157,158,159,160,161,162,163].
Conclusion
We posit that an evolutionary landscape involving an age-dependent EPO elevation, supported by genetic polymorphisms of members of the RAAS, promotes innate defenses that actively suppress viral replication or transmission and tolerance mechanisms, including appropriate NLRP3 inflammasome regulation, that, at an early age, can lower the burden of infection [38, 39]. SARS-CoV-2-ACE2 binding appears as a well-rehearsed host act since low ACE2 expression in children [40] will swiftly and early mediate a RAAS hyperactive state [33, 62] resulting in Ang II/aldosterone-mediated, heightened EPO secretion [35,36,37], that through EPO-eNOS-mediated increases in NO generation aims to contain NLRP3 activation, and inhibit the imminent endotheliitis, SARS-CoV-2 replication and cell entry [7, 32, 44, 48]. All steps in the above cascade are under significant genetic control aiming to enhance EPO levels and amplify eNOS activity at an early age [39, 51,52,53]. Regulation of EPO secretion is under substantial control by the ACE I/D polymorphism [164] while EPO-eNOS signaling is modifiable by βcR [152] and NOS3 SNPs [165]. Polymorphisms in several RAAS molecules, e.g., ACE, ACE2, AGT, AGTR1 may additionally amplify a RAAS hyperactive state, elevate EPO and protect against SARS-CoV-2 in the face of eNOS-augmenting SNPs [52, 53], but appear detrimental with increasing age and co-morbidities, when EPO secretion, eNOS activity and NO generation and bioavailability wane, allowing NLRP3 dysregulation [112, 142, 166]. Finally, genetically amplified FGF23 and Ang II levels may synergistically elicit detrimental cardiac and GI phenotypes through excessive NLRP3 activation [117, 121, 136, 137, 139]. The variability and duration of EPO’s protective age span will depend on host haplotypes. The probability of a child presenting with a “perfect storm haplotype”, where all detrimental NOS3-RAAS molecule-FGF23 candidate polymorphisms are present is currently unknown, but presumably very low, given the rarity of MIS-C [2]. Our haplotype estimates, predicted from allele frequencies in population databases, are in concordance with MIS-C incidence reports in Europeans but indicate lower risks for Asians and African Americans [1, 2]. Early age (0–5 years) and EPO-augmenting RAAS genetic determinants might remedy eNOS activity-reducing genetic polymorphisms and sustain adequate NO generation and bioavailability, allowing appropriate NLRP3 regulation, apposite innate immune response, and successful resolution of the infection. Increasing age (6–18 years) with declining EPO levels, in the presence of relevant genetic variants and co-morbidities, could substantially attenuate EPO secretion and override its protection. The resulting unopposed RAAS proinflammatory state with lower EPO and vasculoprotective NO levels, plausibly leads to protracted and dysregulated NLRP3 inflammasome activation, allowing transition to MIS-C in genetically susceptible children. Targeted Mendelian approaches dissecting the influence of relevant genetic variants are needed.
References
Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, et al. Multisystem inflammatory syndrome in children in New York state. N Engl J Med. 2020;383(4):347–58. https://doi.org/10.1056/NEJMoa2021756.
Holm M, Hartling UB, Schmidt LS, Glenthøj JP, Kruse A, Rytter MH, et al. Multisystem inflammatory syndrome in children occurred in one of four thousand children with severe acute respiratory syndrome coronavirus 2. Acta Paediatr. 2021;110(9):2581–3. https://doi.org/10.1111/apa.15985.
Organization WH. WHO Coronavirus Disease (COVID-19) Dashboard. World Health Organization., Geneva, Switzerland. 2020. https://www.who.int/publications/i/item/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19. Accessed July 1 2022.
Ghosh P, Katkar GD, Shimizu C, Kim J, Khandelwal S, Tremoulet AH, et al. An artificial intelligence-guided signature reveals the shared host immune response in MIS-C and Kawasaki disease. Nat Commun. 2022;13(1):2687. https://doi.org/10.1038/s41467-022-30357-w.
Gelzo M, Giannattasio A, Maglione M, Muzzica S, D’Anna C, Scialò F, et al. Biomarkers of endothelial damage in distinct phases of multisystem inflammatory syndrome in children. Metabolites. 2022. https://doi.org/10.3390/metabo12080680.
Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–8. https://doi.org/10.1016/s0140-6736(20)30937-5.
Lei Y, Zhang J, Schiavon CR, He M, Chen L, Shen H, et al. SARS-CoV-2 spike protein impairs endothelial function via downregulation of ACE 2. Circ Res. 2021;128(9):1323–6. https://doi.org/10.1161/CIRCRESAHA.121.318902.
Wang W-T, He M, Shimizu C, Croker BA, Hoffman HM, Tremoulet AH, et al. Inflammasome activation in children with Kawasaki disease and multisystem inflammatory syndrome. Arterioscler Thromb Vasc Biol. 2021;41(9):2509–11. https://doi.org/10.1161/ATVBAHA.121.316210.
Sacco K, Castagnoli R, Vakkilainen S, Liu C, Delmonte OM, Oguz C, et al. Immunopathological signatures in multisystem inflammatory syndrome in children and pediatric COVID-19. Nat Med. 2022;28(5):1050–62. https://doi.org/10.1038/s41591-022-01724-3.
Spracklen TF, Mendelsohn SC, Butters C, Facey-Thomas H, Stander R, Abrahams D, et al. IL27 gene expression distinguishes multisystem inflammatory syndrome in children from febrile illness in a South African cohort. Front Immunol. 2022;13:992022. https://doi.org/10.3389/fimmu.2022.992022.
Guo H, Callaway JB, Ting JPY. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21(7):677–87. https://doi.org/10.1038/nm.3893.
Ratajczak MZ, Kucia M. SARS-CoV-2 infection and overactivation of Nlrp3 inflammasome as a trigger of cytokine “storm” and risk factor for damage of hematopoietic stem cells. Leukemia. 2020;34(7):1726–9. https://doi.org/10.1038/s41375-020-0887-9.
Rodrigues TS, de Sá KSG, Ishimoto AY, Becerra A, Oliveira S, Almeida L, et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J Exp Med. 2021. https://doi.org/10.1084/jem.20201707.
Kucia M, Ratajczak J, Bujko K, Adamiak M, Ciechanowicz A, Chumak V, et al. An evidence that SARS-CoV-2/COVID-19 spike protein (SP) damages hematopoietic stem/progenitor cells in the mechanism of pyroptosis in Nlrp3 inflammasome-dependent manner. Leukemia. 2021;35(10):3026–9. https://doi.org/10.1038/s41375-021-01332-z.
Ratajczak MZ, Bujko K, Ciechanowicz A, Sielatycka K, Cymer M, Marlicz W, et al. SARS-CoV-2 entry receptor ACE2 is expressed on very small CD45(-) precursors of hematopoietic and endothelial cells and in response to virus spike protein activates the Nlrp3 inflammasome. Stem Cell Rev Rep. 2021;17(1):266–77. https://doi.org/10.1007/s12015-020-10010-z.
Harris J, Borg NA. The multifaceted roles of NLRP3-modulating proteins in virus infection. Front Immunol. 2022;13:987453. https://doi.org/10.3389/fimmu.2022.987453.
Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19(8):477–89. https://doi.org/10.1038/s41577-019-0165-0.
Dutta D, Liu J, Xiong H. NLRP3 inflammasome activation and SARS-CoV-2-mediated hyperinflammation, cytokine storm and neurological syndromes. Int J Physiol Pathophysiol Pharmacol. 2022;14(3):138–60.
van den Berg DF, Te Velde AA. Severe COVID-19: NLRP3 inflammasome dysregulated. Front Immunol. 2020;11:1580. https://doi.org/10.3389/fimmu.2020.01580.
Toldo S, Bussani R, Nuzzi V, Bonaventura A, Mauro AG, Cannatà A, et al. Inflammasome formation in the lungs of patients with fatal COVID-19. Inflamm Res. 2021;70(1):7–10. https://doi.org/10.1007/s00011-020-01413-2.
Ciechanowicz AK, Lay WX, Prado Paulino J, Suchocki E, Leszczak S, Leszczak C, et al. Angiotensin 1–7 stimulates proliferation of lung bronchoalveolar progenitors-implications for SARS-CoV-2 infection. Cells. 2022. https://doi.org/10.3390/cells11132102.
Yang CA, Chiang BL. Inflammasomes and childhood autoimmune diseases: a review of current knowledge. Clin Rev Allergy Immunol. 2021;61(2):156–70. https://doi.org/10.1007/s12016-020-08825-2.
Kuypers FA, Rostad CA, Anderson EJ, Chahroudi A, Jaggi P, Wrammert J, et al. Secretory phospholipase A2 in SARS-CoV-2 infection and multisystem inflammatory syndrome in children (MIS-C). Exp Biol Med (Maywood). 2021;246(23):2543–52. https://doi.org/10.1177/15353702211028560.
Zhang D, Li Y, Du C, Sang L, Liu L, Li Y, et al. Evidence of pyroptosis and ferroptosis extensively involved in autoimmune diseases at the single-cell transcriptome level. J Transl Med. 2022;20(1):363. https://doi.org/10.1186/s12967-022-03566-6.
Yang CA, Huang YL, Chiang BL. Innate immune response analysis in COVID-19 and Kawasaki disease reveals MIS-C predictors. J Formos Med Assoc. 2022;121(3):623–32. https://doi.org/10.1016/j.jfma.2021.06.009.
Sampaio WO, Souza dos Santos RA, Faria-Silva R, da Mata Machado LT, Schiffrin EL, Touyz RM. Angiotensin-(1–7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension. 2007;49(1):185–92. https://doi.org/10.1161/01.HYP.0000251865.35728.2f.
Zhao Y, Vanhoutte PM, Leung SWS. Vascular nitric oxide: beyond eNOS. J Pharmacol Sci. 2015;129(2):83–94. https://doi.org/10.1016/j.jphs.2015.09.002.
Förstermann U, Münzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113(13):1708–14. https://doi.org/10.1161/circulationaha.105.602532.
Teuwen LA, Geldhof V, Pasut A, Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20(7):389–91. https://doi.org/10.1038/s41577-020-0343-0.
Camargo RL, Bombassaro B, Monfort-Pires M, Mansour E, Palma AC, Ribeiro LC, et al. Plasma angiotensin II is increased in critical coronavirus disease 2019. Front Cardiovasc Med. 2022;9:847809. https://doi.org/10.3389/fcvm.2022.847809.
Carpenter RM, Young MK, Petri WAO, Lyons GR, Gilchrist C, Carey RM et al. Repressed Ang 1–7 in COVID-19 is inversely associated with inflammation and coagulation. mSphere. 2022:0022022. https://doi.org/10.1128/msphere.00220-22.
Sogawa Y, Nagasu H, Itano S, Kidokoro K, Taniguchi S, Takahashi M, et al. The eNOS-NO pathway attenuates kidney dysfunction via suppression of inflammasome activation in aldosterone-induced renal injury model mice. PLoS ONE. 2018;13(10):e0203823. https://doi.org/10.1371/journal.pone.0203823.
Villard O, Morquin D, Molinari N, Raingeard I, Nagot N, Cristol JP, et al. The plasmatic aldosterone and C-reactive protein levels, and the severity of COVID-19: the Dyhor-19 study. J Clin Med. 2020. https://doi.org/10.3390/jcm9072315.
Bruder-Nascimento T, Ferreira NS, Zanotto CZ, Ramalho F, Pequeno IO, Olivon VC, et al. NLRP3 inflammasome mediates aldosterone-induced vascular damage. Circulation. 2016;134(23):1866–80. https://doi.org/10.1161/circulationaha.116.024369.
Yasuoka Y, Izumi Y, Nagai T, Fukuyama T, Nakayama Y, Inoue H, et al. Fludrocortisone stimulates erythropoietin production in the intercalated cells of the collecting ducts. Biochem Biophys Res Commun. 2018;503(4):3121–7. https://doi.org/10.1016/j.bbrc.2018.08.102.
Yasuoka Y, Izumi Y, Fukuyama T, Inoue H, Oshima T, Yamazaki T, et al. Effects of angiotensin II on erythropoietin production in the kidney and liver. Molecules. 2021;26(17):5399.
Kim YC, Mungunsukh O, Day RM. Erythropoietin regulation by angiotensin II. Vitam Horm. 2017;105:57–77. https://doi.org/10.1016/bs.vh.2017.02.001.
Papadopoulos KI, Sutheesophon W, Manipalviratn S, Aw TC. Age and genotype dependent erythropoietin protection in COVID-19. World J Stem Cells. 2021;13(10):1513–29. https://doi.org/10.4252/wjsc.v13.i10.1513.
O’Donnell A, Premawardhena A, Arambepola M, Allen SJ, Peto TE, Fisher CA, et al. Age-related changes in adaptation to severe anemia in childhood in developing countries. Proc Natl Acad Sci USA. 2007;104(22):9440–4. https://doi.org/10.1073/pnas.0703424104.
Bunyavanich S, Do A, Vicencio A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA. 2020;323(23):2427–9. https://doi.org/10.1001/jama.2020.8707.
Hasan MR, Ahmad MN, Dargham SR, Zayed H, Al Hashemi A, Ngwabi N, et al. Nasopharyngeal expression of angiotensin-converting enzyme 2 and transmembrane serine protease 2 in children within SARS-CoV-2-infected family clusters. Microbiol Spectr. 2021;9(3):e0078321. https://doi.org/10.1128/Spectrum.00783-21.
Heinonen S, Helve O, Andersson S, Janér C, Süvari L, Kaskinen A. Nasal expression of SARS-CoV-2 entry receptors in newborns. Arch Dis Child Fetal Neonatal Ed. 2022;107(1):95–7. https://doi.org/10.1136/archdischild-2020-321334.
Osman IO, Melenotte C, Brouqui P, Million M, Lagier JC, Parola P, et al. Expression of ACE2, soluble ACE2, angiotensin I, angiotensin II and angiotensin-(1–7) is modulated in COVID-19 patients. Front Immunol. 2021;12:625732. https://doi.org/10.3389/fimmu.2021.625732.
Akaberi D, Krambrich J, Ling J, Luni C, Hedenstierna G, Jarhult JD, et al. Mitigation of the replication of SARS-CoV-2 by nitric oxide in vitro. Redox Biol. 2020;37:101734. https://doi.org/10.1016/j.redox.2020.101734.
Suresh S, Rajvanshi PK, Noguchi CT. The many facets of erythropoietin physiologic and metabolic response. Front Physiol. 2019;10:1534. https://doi.org/10.3389/fphys.2019.01534.
Keswani SC, Bosch-Marcé M, Reed N, Fischer A, Semenza GL, Höke A. Nitric oxide prevents axonal degeneration by inducing HIF-1-dependent expression of erythropoietin. Proc Natl Acad Sci USA. 2011;108(12):4986–90. https://doi.org/10.1073/pnas.1019591108.
Teng R, Calvert JW, Sibmooh N, Piknova B, Suzuki N, Sun J, et al. Acute erythropoietin cardioprotection is mediated by endothelial response. Basic Res Cardiol. 2011;106(3):343–54. https://doi.org/10.1007/s00395-011-0158-z.
Cao F, Tian X, Li Z, Lv Y, Han J, Zhuang R, et al. Suppression of NLRP3 inflammasome by erythropoietin via the EPOR/JAK2/STAT3 pathway contributes to attenuation of acute lung injury in mice. Front Pharmacol. 2020;11:306. https://doi.org/10.3389/fphar.2020.00306.
Liu F, Wen Y, Kang J, Wei C, Wang M, Zheng Z, et al. Regulation of TLR4 expression mediates the attenuating effect of erythropoietin on inflammation and myocardial fibrosis in rat heart. Int J Mol Med. 2018;42(3):1436–44. https://doi.org/10.3892/ijmm.2018.3707.
Khan AI, Coldewey SM, Patel NS, Rogazzo M, Collino M, Yaqoob MM, et al. Erythropoietin attenuates cardiac dysfunction in experimental sepsis in mice via activation of the β-common receptor. Dis Model Mech. 2013;6(4):1021–30. https://doi.org/10.1242/dmm.011908.
Abugri J, Tetteh JK, Oseni LA, Mensah-Brown HE, Delimini RK, Obuobi DO, et al. Age-related pattern and monocyte-acquired haemozoin associated production of erythropoietin in children with severe malarial anaemia in Ghana. BMC Res Notes. 2014;7:551. https://doi.org/10.1186/1756-0500-7-551.
Dhangadamajhi G, Mohapatra BN, Kar SK, Ranjit M. Gene polymorphisms in angiotensin I converting enzyme (ACE I/D) and angiotensin II converting enzyme (ACE2 C–>T) protect against cerebral malaria in Indian adults. Infect Genet Evol. 2010;10(2):337–41. https://doi.org/10.1016/j.meegid.2010.01.009.
Dhangadamajhi G, Mohapatra BN, Kar SK, Ranjit M. Endothelial nitric oxide synthase gene polymorphisms and Plasmodium falciparum infection in Indian adults. Infect Immun. 2009;77(7):2943–7. https://doi.org/10.1128/IAI.00083-09.
Cotta Filho CK, Oliveira-Paula GH, Rondon Pereira VC, Lacchini R. Clinically relevant endothelial nitric oxide synthase polymorphisms and their impact on drug response. Expert Opin Drug Metab Toxicol. 2020;16(10):927–51. https://doi.org/10.1080/17425255.2020.1804857.
Falcini F, Rigante D, Masi L, Covino M, Franceschelli F, Leoncini G, et al. Fibroblast growth factor 23 (FGF23) gene polymorphism in children with Kawasaki syndrome (KS) and susceptibility to cardiac abnormalities. Ital J Pediatr. 2013;39:69. https://doi.org/10.1186/1824-7288-39-69.
Espitia-Corredor JA, Boza P, Espinoza-Pérez C, Lillo JM, Rimassa-Taré C, Machuca V, et al. Angiotensin II triggers NLRP3 inflammasome activation by a Ca(2+) signaling-dependent pathway in rat cardiac fibroblast Ang-II by a Ca(2+)-dependent mechanism triggers NLRP3 inflammasome in CF. Inflammation. 2022. https://doi.org/10.1007/s10753-022-01707-z.
Cau SB, Bruder-Nascimento A, Silva MB, Ramalho FNZ, Mestriner F, Alves-Lopes R, et al. Angiotensin-II activates vascular inflammasome and induces vascular damage. Vascul Pharmacol. 2021;139:106881. https://doi.org/10.1016/j.vph.2021.106881.
Fu H, Shen QR, Zhao Y, Ni M, Zhou CC, Chen JK, et al. Activating α7nAChR ameliorates abdominal aortic aneurysm through inhibiting pyroptosis mediated by NLRP3 inflammasome. Acta Pharmacol Sin. 2022. https://doi.org/10.1038/s41401-022-00876-9.
Ren XS, Tong Y, Ling L, Chen D, Sun HJ, Zhou H, et al. NLRP3 gene deletion attenuates angiotensin II-induced phenotypic transformation of vascular smooth muscle cells and vascular remodeling. Cell Physiol Biochem. 2017;44(6):2269–80. https://doi.org/10.1159/000486061.
Ren P, Wu D, Appel R, Zhang L, Zhang C, Luo W, et al. Targeting the NLRP3 inflammasome with inhibitor MCC950 prevents aortic aneurysms and dissections in mice. J Am Heart Assoc. 2020;9(7):e014044. https://doi.org/10.1161/jaha.119.014044.
Li X, Zhang Z, Luo M, Cheng Z, Wang R, Liu Q, et al. NLRP3 inflammasome contributes to endothelial dysfunction in angiotensin II-induced hypertension in mice. Microvasc Res. 2022;143:104384. https://doi.org/10.1016/j.mvr.2022.104384.
Wu Z, Hu R, Zhang C, Ren W, Yu A, Zhou X. Elevation of plasma angiotensin II level is a potential pathogenesis for the critically ill COVID-19 patients. Crit Care. 2020;24(1):290. https://doi.org/10.1186/s13054-020-03015-0.
Zhang Y, Chen W, Wang Y. STING is an essential regulator of heart inflammation and fibrosis in mice with pathological cardiac hypertrophy via endoplasmic reticulum (ER) stress. Biomed Pharmacother. 2020;125:110022. https://doi.org/10.1016/j.biopha.2020.110022.
Li N, Zhou H, Wu H, Wu Q, Duan M, Deng W, et al. STING-IRF3 contributes to lipopolysaccharide-induced cardiac dysfunction, inflammation, apoptosis and pyroptosis by activating NLRP3. Redox Biol. 2019;24:101215. https://doi.org/10.1016/j.redox.2019.101215.
Han J, Dai S, Zhong L, Shi X, Fan X, Zhong X, et al. GSDMD (Gasdermin D) mediates pathological cardiac hypertrophy and generates a feed-forward amplification cascade via mitochondria-STING (stimulator of interferon genes) axis. Hypertension. 2022. https://doi.org/10.1161/hypertensionaha.122.20004.
Hu S, Pi Q, Luo M, Cheng Z, Liang X, Luo S, et al. Contribution of the NLRP3/IL-1β axis to impaired vasodilation in sepsis through facilitation of eNOS proteolysis and the protective role of melatonin. Int Immunopharmacol. 2021;93:107388. https://doi.org/10.1016/j.intimp.2021.107388.
Valle Raleigh J, Mauro AG, Devarakonda T, Marchetti C, He J, Kim E, et al. Reperfusion therapy with recombinant human relaxin-2 (Serelaxin) attenuates myocardial infarct size and NLRP3 inflammasome following ischemia/reperfusion injury via eNOS-dependent mechanism. Cardiovasc Res. 2017;113(6):609–19. https://doi.org/10.1093/cvr/cvw246.
Benigni A, Cassis P, Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med. 2010;2(7):247–57. https://doi.org/10.1002/emmm.201000080.
Kwak J, Kim JH, Jang HN, Jung MH, Cho HS, Chang SH, et al. Erythropoietin ameliorates ischemia/reperfusion-induced acute kidney injury via inflammasome suppression in mice. Int J Mol Sci. 2020. https://doi.org/10.3390/ijms21103453.
Heinisch O, Zeyen T, Goldmann T, Prinz M, Huber M, Jung J, et al. Erythropoietin abrogates post-ischemic activation of the NLRP3, NLRC4, and AIM2 inflammasomes in microglia/macrophages in a TAK1-dependent manner. Transl Stroke Res. 2022;13(3):462–82. https://doi.org/10.1007/s12975-021-00948-8.
Payne AB, Gilani Z, Godfred-Cato S, Belay ED, Feldstein LR, Patel MM, et al. Incidence of multisystem inflammatory syndrome in children among us persons infected with SARS-CoV-2. JAMA Netw Open. 2021;4(6):e2116420. https://doi.org/10.1001/jamanetworkopen.2021.16420.
Lopez-Sublet M, Caratti di Lanzacco L, Danser AHJ, Lambert M, Elourimi G, Persu A. Focus on increased serum angiotensin-converting enzyme level: From granulomatous diseases to genetic mutations. Clin Biochem. 2018;59:1–8. https://doi.org/10.1016/j.clinbiochem.2018.06.010.
Cambien F, Alhenc-Gelas F, Herbeth B, Andre JL, Rakotovao R, Gonzales MF, et al. Familial resemblance of plasma angiotensin-converting enzyme level: the Nancy Study. Am J Hum Genet. 1988;43(5):774–80.
Guan SP, Seet RCS, Kennedy BK. Does eNOS derived nitric oxide protect the young from severe COVID-19 complications? Ageing Res Rev. 2020;64:101201. https://doi.org/10.1016/j.arr.2020.101201.
Dhangadamajhi G, Mohapatra BN, Kar SK, Ranjit MR. A new allele (eNOS4e) in the intron 4 (VNTR) of eNOS gene in malaria infected individuals of the population of Orissa (an eastern Indian state). Nitric Oxide. 2010;22(1):58–9. https://doi.org/10.1016/j.niox.2009.11.010.
Mpekoulis G, Frakolaki E, Taka S, Ioannidis A, Vassiliou AG, Kalliampakou KI, et al. Alteration of l-Dopa decarboxylase expression in SARS-CoV-2 infection and its association with the interferon-inducible ACE2 isoform. PLoS ONE. 2021;16(6):e0253458. https://doi.org/10.1371/journal.pone.0253458.
Gupta A, Jayakumar MN, Saleh MA, Kannan M, Halwani R, Qaisar R, et al. SARS-CoV-2 infection- induced growth factors play differential roles in COVID-19 pathogenesis. Life Sci. 2022;304:120703. https://doi.org/10.1016/j.lfs.2022.120703.
Revin VV, Balykova LA, Radaeva OA, Shchapov VV, Revina ES, Pinyaev SI, et al. Morphofunctional characteristics of erythrocytes and blood erythropoietin level in patients as predictors of severe course of COVID-19. Bull Exp Biol Med. 2022;173(1):46–50. https://doi.org/10.1007/s10517-022-05490-7.
Arias-Reyes C, Zubieta-DeUrioste N, Poma-Machicao L, Aliaga-Raduan F, Carvajal-Rodriguez F, Dutschmann M, et al. Does the pathogenesis of SARS-CoV-2 virus decrease at high-altitude? Respir Physiol Neurobiol. 2020;277:103443. https://doi.org/10.1016/j.resp.2020.103443.
Viruez-Soto A, Lopez-Davalos MM, Rada-Barrera G, Merino-Luna A, Molano-Franco D, Tinoco-Solorozano A, et al. Low serum erythropoietin levels are associated with fatal COVID-19 cases at 4,150 meters above sea level. Respir Physiol Neurobiol. 2021;292:103709. https://doi.org/10.1016/j.resp.2021.103709.
Elahi S. Neonatal and children’s immune system and COVID-19: biased immune tolerance versus resistance strategy. J Immunol. 2020;205(8):1990–7. https://doi.org/10.4049/jimmunol.2000710.
Grzywa TM, Nowis D, Golab J. The role of CD71(+) erythroid cells in the regulation of the immune response. Pharmacol Ther. 2021;228:107927. https://doi.org/10.1016/j.pharmthera.2021.107927.
Guglielmo C, Bin S, Cantarelli C, Hartzell S, Angeletti A, Donadei C, et al. Erythropoietin reduces auto- and alloantibodies by inhibiting T follicular helper cell differentiation. J Am Soc Nephrol. 2021;32(10):2542–60. https://doi.org/10.1681/asn.2021010098.
Donadei C, Angeletti A, Cantarelli C, D’Agati VD, La Manna G, Fiaccadori E, et al. Erythropoietin inhibits SGK1-dependent TH17 induction and TH17-dependent kidney disease. JCI Insight. 2019. https://doi.org/10.1172/jci.insight.127428.
Wallukat G, Hohberger B, Wenzel K, Furst J, Schulze-Rothe S, Wallukat A, et al. Functional autoantibodies against G-protein coupled receptors in patients with persistent long-COVID-19 symptoms. J Transl Autoimmun. 2021;4:100100. https://doi.org/10.1016/j.jtauto.2021.100100.
Briquez PS, Rouhani SJ, Yu J, Pyzer AR, Trujillo J, Dugan HL, et al. Severe COVID-19 induces autoantibodies against angiotensin II that correlate with blood pressure dysregulation and disease severity. Sci Adv. 2022;8(40):eabn3777. https://doi.org/10.1126/sciadv.abn3777.
Papadopoulos KI, Sutheesophon W, Aw T-C. Anti-SARS-CoV-2 action of fluvoxamine may be mediated by endothelial nitric oxide synthase. Pharmacopsychiatry. 2022;55(01):57.
Papadopoulos KI, Papadopoulou A, Sutheesophon W, Aw TC. Anti-SARS-CoV-2 action of 5α-reductase inhibitors may be mediated by dehydroepiandrosterone. Letter J Urol. 2022;207(5):1163–4. https://doi.org/10.1097/ju.0000000000002469.
Ma Z, Patel N, Vemparala P, Krishnamurthy M. Metformin is associated with favorable outcomes in patients with COVID-19 and type 2 diabetes mellitus. Sci Rep. 2022;12(1):5553. https://doi.org/10.1038/s41598-022-09639-2.
Nicastri E, Marinangeli F, Pivetta E, Torri E, Reggiani F, Fiorentino G, et al. A phase 2 randomized, double-blinded, placebo-controlled, multicenter trial evaluating the efficacy and safety of raloxifene for patients with mild to moderate COVID-19. eClinicalMedicine. 2022. https://doi.org/10.1016/j.eclinm.2022.101450.
Vassiliou AG, Zacharis A, Keskinidou C, Jahaj E, Pratikaki M, Gallos P, et al. Soluble angiotensin converting enzyme 2 (ACE2) is upregulated and soluble endothelial nitric oxide synthase (eNOS) is downregulated in COVID-19-induced acute respiratory distress syndrome (ARDS). Pharmaceuticals (Basel). 2021;14(7):695. https://doi.org/10.3390/ph14070695.
Kim YM, Talanian RV, Li J, Billiar TR. Nitric oxide prevents IL-1beta and IFN-gamma-inducing factor (IL-18) release from macrophages by inhibiting caspase-1 (IL-1beta-converting enzyme). J Immunol. 1998;161(8):4122–8.
Oliveira-Paula GH, Lacchini R, Tanus-Santos JE. Endothelial nitric oxide synthase: from biochemistry and gene structure to clinical implications of NOS3 polymorphisms. Gene. 2016;575(2 Pt 3):584–99. https://doi.org/10.1016/j.gene.2015.09.061.
Matsa LS, Rangaraju A, Vengaldas V, Latifi M, Jahromi HM, Ananthapur V, et al. Haplotypes of NOS3 gene polymorphisms in dilated cardiomyopathy. PLoS ONE. 2013;8(7):e70523. https://doi.org/10.1371/journal.pone.0070523.
Luo Z, Jia A, Lu Z, Muhammad I, Adenrele A, Song Y. Associations of the NOS3 rs1799983 polymorphism with circulating nitric oxide and lipid levels: a systematic review and meta-analysis. Postgrad Med J. 2019;95(1125):361–71. https://doi.org/10.1136/postgradmedj-2019-136396.
Koskela S, Laine O, Makela S, Pessi T, Tuomisto S, Huhtala H, et al. Endothelial nitric oxide synthase G894T polymorphism associates with disease severity in puumala hantavirus infection. PLoS ONE. 2015;10(11):e0142872. https://doi.org/10.1371/journal.pone.0142872.
Katkam SK, Indumathi B, Tasneem FSD, Rajasekhar L, Kutala VK. Impact of eNOS 27-bp VNTR (4b/a) gene polymorphism with the risk of systemic lupus erythematosus in south Indian subjects. Gene. 2018;658:105–12. https://doi.org/10.1016/j.gene.2018.03.021.
Kumar GR, Spurthi KM, Kumar GK, Aiyengar TM, Chiranjeevi P, Nivas S, et al. Genetic polymorphisms of eNOS (− 786T/C, Intron 4b/4a & 894G/T) and its association with asymptomatic first degree relatives of coronary heart disease patients. Nitric Oxide. 2016;60:40–9. https://doi.org/10.1016/j.niox.2016.09.001.
Metzger IF, Ishizawa MH, Rios-Santos F, Carvalho WA, Tanus-Santos JE. Endothelial nitric oxide synthase gene haplotypes affect nitrite levels in black subjects. Pharmacogenomics J. 2011;11(6):393–9. https://doi.org/10.1038/tpj.2010.52.
Maurer P, Barbisan F, Azzolin VF, Berro LF, Montagner R, Duarte M, et al. Polymorphism eNOS Glu298Asp modulates the inflammatory response of human peripheral blood mononuclear cells. Cytokine. 2020;125:154812. https://doi.org/10.1016/j.cyto.2019.154812.
Wang XL, Sim AS, Wang MX, Murrell GA, Trudinger B, Wang J. Genotype dependent and cigarette specific effects on endothelial nitric oxide synthase gene expression and enzyme activity. FEBS Lett. 2000;471(1):45–50. https://doi.org/10.1016/s0014-5793(00)01356-9.
Wang J, Dudley D, Wang XL. Haplotype-specific effects on endothelial NO synthase promoter efficiency: modifiable by cigarette smoking. Arterioscler Thromb Vasc Biol. 2002;22(5):e1-4. https://doi.org/10.1161/01.atv.0000016248.51577.1f.
Liu Y, Fu L, Pi L, Che D, Xu Y, Zheng H, et al. An angiotensinogen gene polymorphism (rs5050) is associated with the risk of coronary artery aneurysm in southern Chinese children with Kawasaki disease. Dis Markers. 2019;2019:2849695. https://doi.org/10.1155/2019/2849695.
Izmailova O, Shlykova O, Vatsenko A, Ivashchenko D, Dudchenko M, Koval T, et al. Allele C (rs5186) of at1r is associated with the severity of COVID-19 in the Ukrainian population. Infect Genet Evol. 2022;98:105227. https://doi.org/10.1016/j.meegid.2022.105227.
Bautista LE, Vargas CI, Oróstegui M, Gamarra G. Population-based case-control study of renin-angiotensin system genes polymorphisms and hypertension among Hispanics. Hypertens Res. 2008;31(3):401–8. https://doi.org/10.1291/hypres.31.401.
Yamamoto N, Ariumi Y, Nishida N, Yamamoto R, Bauer G, Gojobori T, et al. SARS-CoV-2 infections and COVID-19 mortalities strongly correlate with ACE1 I/D genotype. Gene. 2020;758:144944. https://doi.org/10.1016/j.gene.2020.144944.
Cafiero C, Rosapepe F, Palmirotta R, Re A, Ottaiano MP, Benincasa G, et al. Angiotensin system polymorphisms’ in SARS-CoV-2 positive patients: assessment between symptomatic and asymptomatic patients: a pilot study. Pharmgenomics Pers Med. 2021;14:621–9. https://doi.org/10.2147/pgpm.S303666.
Mokretar K, Velinov H, Postadzhiyan A, Apostolova M. Association of polymorphisms in endothelial nitric oxide synthesis and renin-angiotensin-aldosterone system with developing of coronary artery disease in bulgarian patients. Genet Test Mol Biomarkers. 2016;20(2):67–73. https://doi.org/10.1089/gtmb.2015.0195.
Riad M, Adhikari P, Bhattarai S, Gupta A, Ali E, Ali M, et al. Risk assessment using the association between renin-angiotensin genes polymorphisms and coronary artery disease. Cureus. 2021;13(3):e14083. https://doi.org/10.7759/cureus.14083.
Pan Y, Lu H. Angiotensin-converting enzyme insertion/deletion polymorphism and susceptibility to Kawasaki disease: a meta-analysis. Afr Health Sci. 2017;17(4):991–9. https://doi.org/10.4314/ahs.v17i4.6.
De A, Tiwari A, Dash M, Sinha A. ACE2 mutation might explain lower COVID-19 burden in malaria endemic areas. Hum Cell. 2021;34(2):702–5. https://doi.org/10.1007/s13577-021-00489-0.
Dhangadamajhi G, Singh S. Malaria link of hypertension: a hidden syndicate of angiotensin II, bradykinin and sphingosine 1-phosphate. Hum Cell. 2021;34(3):734–44. https://doi.org/10.1007/s13577-021-00513-3.
van Geel PP, Pinto YM, Voors AA, Buikema H, Oosterga M, Crijns HJ, et al. Angiotensin II type 1 receptor A1166C gene polymorphism is associated with an increased response to angiotensin II in human arteries. Hypertension. 2000;35(3):717–21. https://doi.org/10.1161/01.hyp.35.3.717.
Fukazawa R, Sonobe T, Hamamoto K, Hamaoka K, Sakata K, Asano T, et al. Possible synergic effect of angiotensin-I converting enzyme gene insertion/deletion polymorphism and angiotensin-II type-1 receptor 1166A/C gene polymorphism on ischemic heart disease in patients with Kawasaki disease. Pediatr Res. 2004;56(4):597–601. https://doi.org/10.1203/01.Pdr.0000139426.16381.C8.
Feng S, Song F, Guo W, Tan J, Zhang X, Qiao F, et al. Potential genes associated with COVID-19 and comorbidity. Int J Med Sci. 2022;19(2):402–15. https://doi.org/10.7150/ijms.67815.
Silva RFD, Lacchini R, Pinheiro LC, Ferezin LP, Tanus-Santos JE, Luizon MR, et al. Association between endothelial nitric oxide synthase and the renin-angiotensin-aldosterone system polymorphisms, blood pressure and training status in normotensive/pre-hypertension and hypertensive older adults: a pilot study. Clin Exp Hypertens. 2021;43(7):661–70. https://doi.org/10.1080/10641963.2021.1937202.
Pi M, Ye R, Han X, Armstrong B, Liu X, Chen Y, et al. Cardiovascular interactions between fibroblast growth factor-23 and angiotensin II. Sci Rep. 2018;8(1):12398. https://doi.org/10.1038/s41598-018-30098-1.
Dai B, David V, Martin A, Huang J, Li H, Jiao Y, et al. A comparative transcriptome analysis identifying FGF23 regulated genes in the kidney of a mouse CKD model. PLoS ONE. 2012;7(9):e44161. https://doi.org/10.1371/journal.pone.0044161.
Freundlich M, Gamba G, Rodriguez-Iturbe B. Fibroblast growth factor 23—Klotho and hypertension: experimental and clinical mechanisms. Pediatr Nephrol. 2021;36(10):3007–22. https://doi.org/10.1007/s00467-020-04843-6.
Li X, Li Z, Li B, Zhu X, Lai X. Klotho improves diabetic cardiomyopathy by suppressing the NLRP3 inflammasome pathway. Life Sci. 2019;234:116773. https://doi.org/10.1016/j.lfs.2019.116773.
McKnight Q, Jenkins S, Li X, Nelson T, Marlier A, Cantley LG, et al. IL-1β drives production of FGF-23 at the onset of chronic kidney disease in mice. J Bone Miner Res. 2020;35(7):1352–62. https://doi.org/10.1002/jbmr.4003.
Sugiura H, Yoshida T, Mitobe M, Shiohira S, Nitta K, Tsuchiya K. Recombinant human erythropoietin mitigates reductions in renal klotho expression. Am J Nephrol. 2010;32(2):137–44. https://doi.org/10.1159/000315864.
Chung CP, Chang YC, Ding Y, Lim K, Liu Q, Zhu L, et al. α-Klotho expression determines nitric oxide synthesis in response to FGF-23 in human aortic endothelial cells. PLoS ONE. 2017;12(5):e0176817. https://doi.org/10.1371/journal.pone.0176817.
Typiak M, Piwkowska A. Antiinflammatory actions of klotho: implications for therapy of diabetic nephropathy. Int J Mol Sci. 2021;22(2):956.
Junho CVC, González-Lafuente L, Neres-Santos RS, Navarro-García JA, Rodríguez-Sánchez E, Ruiz-Hurtado G, et al. Klotho relieves inflammation and exerts a cardioprotective effect during renal ischemia/reperfusion-induced cardiorenal syndrome. Biomed Pharmacother. 2022;153:113515. https://doi.org/10.1016/j.biopha.2022.113515.
Geng YN, Zhang HY. Association of FGF23 gene polymorphism with Kawasaki disease and coronary artery lesions. Zhongguo Dang Dai Er Ke Za Zhi. 2015;17(10):1107–11.
Masi L, Franceschelli F, Leoncini G, Gozzini A, Rigante D, La Torre F, et al. Can fibroblast growth factor (FGF)-23 circulating levels suggest coronary artery abnormalities in children with Kawasaki disease? Clin Exp Rheumatol. 2013;31(1):149–53.
Masi LFF, Leoncini G, Gozzini A, Rigante D, La Torre F, Matucci-Cerinic M, Brandi ML, Falcini F. Can fibroblast growth factor (FGF)-23 circulating levels suggest coronary artery abnormalities in children with Kawasaki disease? Clin Exp Rheumatol. 2013;31(1):149–53.
Fukazawa R, Ikegam E, Watanabe M, Hajikano M, Kamisago M, Katsube Y, et al. Coronary artery aneurysm induced by Kawasaki disease in children show features typical senescence. Circ J. 2007;71(5):709–15. https://doi.org/10.1253/circj.71.709.
Yonker LM, Gilboa T, Ogata AF, Senussi Y, Lazarovits R, Boribong BP, et al. Multisystem inflammatory syndrome in children is driven by zonulin-dependent loss of gut mucosal barrier. J Clin Invest. 2021. https://doi.org/10.1172/jci149633.
Kumar NP, Venkataraman A, Hanna LE, Putlibai S, Karthick M, Rajamanikam A, et al. Systemic inflammation and microbial translocation are characteristic features of SARS-CoV-2-related multisystem inflammatory syndrome in children. Open Forum Infect Dis. 2021;8(7):ofab279. https://doi.org/10.1093/ofid/ofab279.
Kopp F, Kupsch S, Schromm AB. Lipopolysaccharide-binding protein is bound and internalized by host cells and colocalizes with LPS in the cytoplasm: implications for a role of LBP in intracellular LPS-signaling. Biochimica et Biophysica Acta (BBA)—Mol Cell Res. 2016;1863(4):660–72. https://doi.org/10.1016/j.bbamcr.2016.01.015.
Yi Y-S. Dual roles of the caspase-11 non-canonical inflammasome in inflammatory bowel disease. Int Immunopharmacol. 2022;108:108739. https://doi.org/10.1016/j.intimp.2022.108739.
Samsudin F, Raghuvamsi P, Petruk G, Puthia M, Petrlova J, MacAry P, et al. SARS-CoV-2 spike protein as a bacterial lipopolysaccharide delivery system in an overzealous inflammatory cascade. J Mol Cell Biol. 2022. https://doi.org/10.1093/jmcb/mjac058.
Qi W, Shen Q, Zhang L, Han LP, Wang S. Study on the inflammatory intervention of erythropoietin on NEC. Exp Ther Med. 2016;11(6):2221–4. https://doi.org/10.3892/etm.2016.3199.
Fändriks L. The renin–angiotensin system and the gastrointestinal mucosa. Acta Physiol. 2011;201(1):157–67. https://doi.org/10.1111/j.1748-1716.2010.02165.x.
Riaz AA, Wang Y, Schramm R, Sato T, Menger MD, Jeppsson B, et al. Role of angiotensin II in ischemia/reperfusion-induced leukocyte-endothelium interactions in the colon. FASEB J. 2004;18(7):881–3. https://doi.org/10.1096/fj.03-0502fje.
Belay ED, Abrams J, Oster ME, Giovanni J, Pierce T, Meng L, et al. Trends in geographic and temporal distribution of US children with multisystem inflammatory syndrome during the COVID-19 pandemic. JAMA Pediatr. 2021;175(8):837–45. https://doi.org/10.1001/jamapediatrics.2021.0630.
Kumar A, Priyamvada S, Soni V, Anbazhagan AN, Gujral T, Gill RK, et al. Angiotensin II inhibits P-glycoprotein in intestinal epithelial cells. Acta Physiol (Oxf). 2020;228(1):e13332. https://doi.org/10.1111/apha.13332.
Mahmoud N, Hegazy MEF, Wadie W, Elbadawi M, Fleischer E, Klinger A, et al. Naphthoquinone derivatives as P-glycoprotein inducers in inflammatory bowel disease: 2D monolayers, 3D spheroids, and in vivo models. Pharmacol Res. 2022;179:106233. https://doi.org/10.1016/j.phrs.2022.106233.
Alvarez A, Piqueras L, Bello R, Canet A, Moreno L, Kubes P, et al. Angiotensin II is involved in nitric oxide synthase and cyclo-oxygenase inhibition-induced leukocyte-endothelial cell interactions in vivo. Br J Pharmacol. 2001;132(3):677–84. https://doi.org/10.1038/sj.bjp.0703867.
Luo M, Meng J, Yan J, Shang F, Zhang T, Lv D, et al. Role of the nucleotide-binding domain-like receptor protein 3 inflammasome in the endothelial dysfunction of early sepsis. Inflammation. 2020;43(4):1561–71. https://doi.org/10.1007/s10753-020-01232-x.
Mabhida SE, Mashatola L, Kaur M, Sharma JR, Apalata T, Muhamed B, et al. Hypertension in African populations: review and computational insights. Genes (Basel). 2021;12(4):532. https://doi.org/10.3390/genes12040532.
Kumar R, Nejatizadeh A, Arif E, Akhtar S, Gupta M, Tyagi S, et al. Multi-locus interactions of vascular homeostasis genes in essential hypertension: a gender-based study. Clin Chim Acta. 2009;405(1–2):87–93. https://doi.org/10.1016/j.cca.2009.04.010.
dbSNP. National Centre for Biotechnology Information, National Library of Medicine. 2021. https://www.ncbi.nlm.nih.gov/snp/. Accessed Oct 26, 2021.
Gamil S, Erdmann J, Abdalrahman IB, Mohamed AO. Association of NOS3 gene polymorphisms with essential hypertension in Sudanese patients: a case control study. BMC Med Genet. 2017;18(1):128. https://doi.org/10.1186/s12881-017-0491-7.
Tanus-Santos JE, Desai M, Flockhart DA. Effects of ethnicity on the distribution of clinically relevant endothelial nitric oxide variants. Pharmacogenetics. 2001;11(8):719–25. https://doi.org/10.1097/00008571-200111000-00011.
Devendran A, Nampoothiri S, Shewade DG, Chatterjee S, Jayaraman B, Chandrasekharan A. Allele, genotype and haplotype structures of functional polymorphic variants in endothelial nitric oxide synthase (eNOS), angiotensinogen (ACE) and aldosterone synthase (CYP11B2) genes in healthy pregnant women of indian ethnicity. J Reprod Infertil. 2015;16(4):180–92.
U.S. Adult Population Grew Faster Than Nation’s Total Population From 2010 to 2020. United States Census Bureau. 2020. https://www.census.gov/library/stories/2021/08/united-states-adult-population-grew-faster-than-nations-total-population-from-2010-to-2020.html. Accessed Oct 25, 2021.
Being young in Europe today - demographic trends. Eurostat. 2020. https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Being_young_in_Europe_today_-_demographic_trends. Accessed Oct 25, 2021.
Funk AL, Florin TA, Kuppermann N, Tancredi DJ, Xie J, Kim K, et al. Outcomes of SARS-CoV-2–positive youths tested in emergency departments: the global PERN–COVID-19 study. JAMA Netw Open. 2022;5(1):e2142322. https://doi.org/10.1001/jamanetworkopen.2021.42322.
Su KH, Shyue SK, Kou YR, Ching LC, Chiang AN, Yu YB, et al. beta Common receptor integrates the erythropoietin signaling in activation of endothelial nitric oxide synthase. J Cell Physiol. 2011;226(12):3330–9. https://doi.org/10.1002/jcp.22678.
Oskarsson GR, Kristjansson RP, Lee AL, Sveinbjornsson G, Magnusson MK, Ivarsdottir EV, et al. A truncating mutation in EPOR leads to hypo-responsiveness to erythropoietin with normal haemoglobin. Commun Biol. 2018;1:49. https://doi.org/10.1038/s42003-018-0053-3.
Papadopoulos KI, Sutheesophon W, Aw T-C. Genetic polymorphisms affecting nitric oxide and β-cytokine pathways may contribute to increased COVID-19 mortality in schizophrenia. Asian J Psychiatr. 2022;69:102981. https://doi.org/10.1016/j.ajp.2021.102981.
Chen Q, Wang X, O’Neill FA, Walsh D, Fanous A, Kendler KS, et al. Association study of CSF2RB with schizophrenia in Irish family and case–control samples. Mol Psychiatry. 2008;13(10):930–8. https://doi.org/10.1038/sj.mp.4002051.
Borriello F, Galdiero MR, Varricchi G, Loffredo S, Spadaro G, Marone G. Innate immune modulation by GM-CSF and IL-3 in health and disease. Int J Mol Sci. 2019;20(4):834. https://doi.org/10.3390/ijms20040834.
Lotfi N, Thome R, Rezaei N, Zhang GX, Rezaei A, Rostami A, et al. Roles of GM-CSF in the pathogenesis of autoimmune diseases: an update. Front Immunol. 2019;10:1265. https://doi.org/10.3389/fimmu.2019.01265.
Benard A, Jacobsen A, Brunner M, Krautz C, Klosch B, Swierzy I, et al. Interleukin-3 is a predictive marker for severity and outcome during SARS-CoV-2 infections. Nat Commun. 2021;12(1):1112. https://doi.org/10.1038/s41467-021-21310-4.
Kuo HC, Wang CL, Liang CD, Yu HR, Huang CF, Wang L, et al. Association of lower eosinophil-related T helper 2 (Th2) cytokines with coronary artery lesions in Kawasaki disease. Pediatr Allergy Immunol. 2009;20(3):266–72. https://doi.org/10.1111/j.1399-3038.2008.00779.x.
Mu X, Liu K, Li H, Wang FS, Xu R. Granulocyte-macrophage colony-stimulating factor: an immunotarget for sepsis and COVID-19. Cell Mol Immunol. 2021;18(8):2057–8. https://doi.org/10.1038/s41423-021-00719-3.
Stock AT, Hansen JA, Sleeman MA, McKenzie BS, Wicks IP. GM-CSF primes cardiac inflammation in a mouse model of Kawasaki disease. J Exp Med. 2016;213(10):1983–98. https://doi.org/10.1084/jem.20151853.
Hercus TR, Kan WLT, Broughton SE, Tvorogov D, Ramshaw HS, Sandow JJ, et al. Role of the beta common (betac) family of cytokines in health and disease. Cold Spring Harb Perspect Biol. 2018;10(6):a028514. https://doi.org/10.1101/cshperspect.a028514.
Furuya MY, Asano T, Sumichika Y, Sato S, Kobayashi H, Watanabe H, et al. Tofacitinib inhibits granulocyte-macrophage colony-stimulating factor-induced NLRP3 inflammasome activation in human neutrophils. Arthritis Res Ther. 2018;20(1):196. https://doi.org/10.1186/s13075-018-1685-x.
Al-Radeef MY, Fawzi HA, Allawi AA. ACE gene polymorphism and its association with serum erythropoietin and hemoglobin in Iraqi hemodialysis patients. Appl Clin Genet. 2019;12:107–12. https://doi.org/10.2147/TACG.S198992.
Oliveira-Paula GH, Lacchini R, Pinheiro LC, Ferreira GC, Luizon MR, Garcia WNP, et al. Endothelial nitric oxide synthase polymorphisms affect the changes in blood pressure and nitric oxide bioavailability induced by propofol. Nitric Oxide. 2018;75:77–84. https://doi.org/10.1016/j.niox.2018.02.007.
De A, Tiwari A, Pande V, Sinha A. Evolutionary trilogy of malaria, angiotensin II and hypertension: deeper insights and the way forward. J Hum Hypertens. 2022;36(4):344–51. https://doi.org/10.1038/s41371-021-00599-0.
Acknowledgements
We are thankful to James T. A. Marshall for his invaluable editorial assistance.
Funding
The authors have no relevant financial or non-financial interests to disclose.
Author information
Authors and Affiliations
Contributions
KIP conceived and conceptualized the pathophysiology and designed the review, drafted the initial manuscript, and reviewed and revised the manuscript. AP performed the literature search, extracted vital information, contributed to the synthesis of the review, and reviewed and revised the manuscript. TCA coordinated and supervised literature search, made substantial and direct intellectual contributions and critically reviewed the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest or competing interests are reported.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Papadopoulos, K.I., Papadopoulou, A. & Aw, TC. A protective erythropoietin evolutionary landscape, NLRP3 inflammasome regulation, and multisystem inflammatory syndrome in children. Human Cell 36, 26–40 (2023). https://doi.org/10.1007/s13577-022-00819-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-022-00819-w