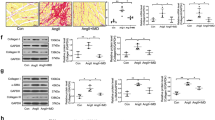
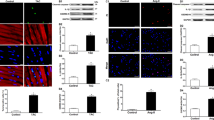
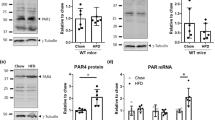
Abstract
Angiotensin II (Ang-II) is a widely studied hypertensive, profibrotic, and pro-inflammatory peptide. In the heart, cardiac fibroblasts (CF) express type 1 angiotensin II receptors (AT1R), Toll-like receptor-4 (TLR4), and the NLRP3 inflammasome complex, which play important roles in pro-inflammatory processes. When activated, the NLRP3 inflammasome triggers proteolytic cleavage of pro-IL-1, resulting in its activation. However, in CF the mechanism by which Ang-II assembles and activates the NLRP3 inflammasome remains not fully known. To elucidate this important point, we stimulated TLR4 receptors in CF and evaluated the signaling pathways by which Ang-II triggers the assembly and activity. In cultured rat CF, pro-IL-1β levels, NLRP3, ASC, and caspase-1 expression levels were determined by Western blot. NLRP3 inflammasome complex assembly was analyzed by immunocytochemistry, whereas by ELISA, we analyzed NLRP3 inflammasome activity and \(IL-1\beta\) release. In CF, Ang-II triggered NLRP3 inflammasome assembly and caspase-1 activity; and in LPS-pretreated CF, Ang-II also triggered \(IL-1\beta\) secretion. These effects were blocked by losartan (AT1R antagonist), U73221 (PLC inhibitor), 2-APB (IP3R antagonist), and BAPTA-AM (Ca2+ chelator) indicating that the AT1R/PLC/IP3R/Ca2+ pathway is involved. Finally, bafilomycin A1 prevented Ang-II-induced \(IL-1\beta\) secretion, indicating that a non-classical protein secretion mechanism is involved. These findings suggest that in CF, Ang-II by a Ca2+-dependent mechanism triggers NLRP3 inflammasome assembly and activation leading to \(IL-1\beta\) secretion through a non-conventional protein secretion mechanism.






Similar content being viewed by others
Availability of Data and Materials
All data generated or analyzed and materials used during this study are included in this published article and its supplementary figure files.
References
Forrester, S.J., G.W. Booz, C.D. Sigmund, T.M. Coffman, T. Kawai, V. Rizzo, R. Scalia, and S. Eguchi. 2018. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiological Reviews 98: 1627–1738. https://doi.org/10.1152/physrev.00038.2017.
Wu, Y., Y. Li, C. Zhang, Y. Wang, W. Cui, H. Li, and J. Du. 2014. S100a8/a9 released by CD11b+Gr1+ neutrophils activates cardiac fibroblasts to initiate angiotensin II–induced cardiac inflammation and injury. Hypertension 63: 1241–1250. https://doi.org/10.1161/HYPERTENSIONAHA.113.02843.
Wang, M., L. Jiang, R.E. Monticone, and E.G. Lakatta. 2014. Proinflammation: the key to arterial aging. Trends in Endocrinology & Metabolism 25: 72–79. https://doi.org/10.1016/j.tem.2013.10.002.
Cheng, Z.J., H. Vapaatalo, and E. Mervaala. 2005. Angiotensin II and vascular inflammation. Med Sci Monit 11: Ra194–205.
Gan, W., J. Ren, T. Li, S. Lv, C. Li, Z. Liu, and M. Yang. 2018. The SGK1 inhibitor EMD638683, prevents angiotensin II–induced cardiac inflammation and fibrosis by blocking NLRP3 inflammasome activation. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1864: 1–10. https://doi.org/10.1016/j.bbadis.2017.10.001.
Kawaguchi, M., M. Takahashi, T. Hata, Y. Kashima, F. Usui, H. Morimoto, A. Izawa, Y. Takahashi, J. Masumoto, J. Koyama, M. Hongo, T. Noda, J. Nakayama, J. Sagara, and Taniguchi, S.i. and Ikeda, U. 2011. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation 123: 594–604. https://doi.org/10.1161/CIRCULATIONAHA.110.982777.
Sandanger, Ø., T. Ranheim, L.E. Vinge, M. Bliksøen, K. Alfsnes, A.V. Finsen, C.P. Dahl, E.T. Askevold, G. Florholmen, G. Christensen, K.A. Fitzgerald, E. Lien, G. Valen, T. Espevik, P. Aukrust, and A. Yndestad. 2013. The NLRP3 inflammasome is up-regulated in cardiac fibroblasts and mediates myocardial ischaemia–reperfusion injury. Cardiovascular Research 99: 164–174. https://doi.org/10.1093/cvr/cvt091.
Palmer, J.N., W.E. Hartogensis, M. Patten, F.D. Fortuin, and C.S. Long. 1995. Interleukin-1 beta induces cardiac myocyte growth but inhibits cardiac fibroblast proliferation in culture. The Journal of Clinical Investigation. https://doi.org/10.1172/JCI117956.
Abbate, A., S. Toldo, C. Marchetti, J. Kron, B.W. Van Tassell, and C.A. Dinarello. 2020. Interleukin-1 and the inflammasome as therapeutic targets in cardiovascular disease. Circulation Research 126: 1260–1280. https://doi.org/10.1161/CIRCRESAHA.120.315937.
Takahashi, M. 2019. Role of NLRP3 inflammasome in cardiac inflammation and remodeling after myocardial infarction. Biological and Pharmaceutical Bulletin 42: 518–523. https://doi.org/10.1248/bpb.b18-00369.
Xiao, H., H. Li, J.-J. Wang, J.-S. Zhang, J. Shen, X.-B. An, C.-C. Zhang, J.-M. Wu, Y. Song, X.-Y. Wang, H.-Y. Yu, X.-N. Deng, Z.-J. Li, M. Xu, Z.-Z. Lu, J. Du, W. Gao, A.-H. Zhang, Y. Feng, and Y.-Y. Zhang. 2018. IL-18 cleavage triggers cardiac inflammation and fibrosis upon β-adrenergic insult. European Heart Journal 39: 60–69. https://doi.org/10.1093/eurheartj/ehx261.
Abderrazak, A., T. Syrovets, D. Couchie, K. El Hadri, B. Friguet, T. Simmet, and M. Rouis. 2015. NLRP3 inflammasome: from a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biology 4: 296–307. https://doi.org/10.1016/j.redox.2015.01.008.
Man, S.M., and T.-D. Kanneganti. 2015. Regulation of inflammasome activation. Immunological Reviews 265: 6–21. https://doi.org/10.1111/imr.12296.
Diaz-Araya, G., R. Vivar, C. Humeres, P. Boza, S. Bolivar, and C. Munoz. 2015. Cardiac fibroblasts as sentinel cells in cardiac tissue: receptors, signaling pathways and cellular functions. Pharmacological Research 101: 30–40. https://doi.org/10.1016/j.phrs.2015.07.001.
Aránguiz-Urroz, P., D. Soto, A. Contreras, R. Troncoso, M. Chiong, J. Montenegro, D. Venegas, C. Smolic, P. Ayala, W.G. Thomas, S. Lavandero, and G. Díaz-Araya. 2009. Differential participation of angiotensin II type 1 and 2 receptors in the regulation of cardiac cell death triggered by angiotensin II. American Journal of Hypertension 22: 569–576. https://doi.org/10.1038/ajh.2009.32.
Vivar, R., C. Soto, M. Copaja, F. Mateluna, P. Aranguiz, J.P. Muñoz, M. Chiong, L. Garcia, A. Letelier, W.G. Thomas, S. Lavandero, and G. Díaz-Araya. 2008. Phospholipase C/protein kinase C pathway mediates angiotensin II-dependent apoptosis in neonatal rat cardiac fibroblasts expressing AT1 receptor. Journal of Cardiovascular Pharmacology. https://doi.org/10.1097/FJC.0b013e318181fadd.
Boza, P., P. Ayala, R. Vivar, C. Humeres, F.T. Cáceres, C. Muñoz, L. García, M.A. Hermoso, and G. Díaz-Araya. 2016. Expression and function of toll-like receptor 4 and inflammasomes in cardiac fibroblasts and myofibroblasts: IL-1β synthesis, secretion, and degradation. Molecular Immunology 74: 96–105. https://doi.org/10.1016/j.molimm.2016.05.001.
Turner, N.A. 2016. Inflammatory and fibrotic responses of cardiac fibroblasts to myocardial damage associated molecular patterns (DAMPs). Journal of Molecular and Cellular Cardiology 94: 189–200. https://doi.org/10.1016/j.yjmcc.2015.11.002.
Bolívar, S., R. Santana, P. Ayala, R. Landaeta, P. Boza, C. Humeres, R. Vivar, C. Muñoz, V. Pardo, S. Fernandez, R. Anfossi, and G. Diaz-Araya. 2017. Lipopolysaccharide activates Toll-like receptor 4 and prevents cardiac fibroblast-to-myofibroblast differentiation. Cardiovascular Toxicology 17: 458–470. https://doi.org/10.1007/s12012-017-9404-4.
Olivares-Silva, F., R. Landaeta, P. Aranguiz, S. Bolivar, C. Humeres, R. Anfossi, R. Vivar, P. Boza, C. Munoz, V. Pardo-Jimenez, C. Peiro, C.F. Sanchez-Ferrer, and G. Diaz-Araya. 2018. Heparan sulfate potentiates leukocyte adhesion on cardiac fibroblast by enhancing Vcam-1 and Icam-1 expression. Biochimica et Biophysica Acta, Molecular Basis of Disease 1864: 831–842. https://doi.org/10.1016/j.bbadis.2017.12.002.
Humeres, C., R. Vivar, P. Boza, C. Munoz, S. Bolivar, R. Anfossi, J.M. Osorio, F. Olivares-Silva, L. Garcia, and G. Diaz-Araya. 2016. Cardiac fibroblast cytokine profiles induced by proinflammatory or profibrotic stimuli promote monocyte recruitment and modulate macrophage M1/M2 balance in vitro. Journal of Molecular and Cellular Cardiology. https://doi.org/10.1016/j.yjmcc.2016.10.014.
Bracey, N.A., B. Gershkovich, J. Chun, A. Vilaysane, H.C. Meijndert, J.R. Wright Jr., P.W. Fedak, P.L. Beck, D.A. Muruve, and H.J. Duff. 2014. Mitochondrial NLRP3 protein induces reactive oxygen species to promote Smad protein signaling and fibrosis independent from the inflammasome *. Journal of Biological Chemistry 289: 19571–19584. https://doi.org/10.1074/jbc.M114.550624.
Gombault, A., L. Baron, and I. Couillin. 2013. ATP release and purinergic signaling in NLRP3 inflammasome activation. Frontiers in Immunology. https://doi.org/10.3389/fimmu.2012.00414.
Li, J., X. Teng, S. Jin, J. Dong, Q. Guo, D. Tian, and Y. Wu. 2019. Hydrogen sulfide improves endothelial dysfunction by inhibiting the vicious cycle of NLRP3 inflammasome and oxidative stress in spontaneously hypertensive rats. Journal of Hypertension 37: 1. https://doi.org/10.1097/HJH.0000000000002101.
Zhou, B., Y. Qiu, N. Wu, A.-D. Chen, H. Zhou, Q. Chen, Y.-M. Kang, Y.-H. Li, and G.-Q. Zhu. 2020. FNDC5 attenuates oxidative stress and NLRP3 inflammasome activation in vascular smooth muscle cells via activating the AMPK-SIRT1 signal pathway. Oxidative Medicine and Cellular Longevity 2020: 6384803. https://doi.org/10.1155/2020/6384803.
Bryan, N.B., A. Dorfleutner, Y. Rojanasakul, and C. Stehlik. 2009. Activation of inflammasomes requires intracellular redistribution of the apoptotic speck-like protein containing a caspase recruitment domain. The Journal of Immunology 182: 3173. https://doi.org/10.4049/jimmunol.0802367.
Jo, E.-K., J.K. Kim, D.-M. Shin, and C. Sasakawa. 2016. Molecular mechanisms regulating NLRP3 inflammasome activation. Cellular & Molecular Immunology 13: 148–159. https://doi.org/10.1038/cmi.2015.95.
Liang, Y.-D., W.-J. Bai, C.-G. Li, L.-H. Xu, H.-X. Wei, H. Pan, X.-H. He, and D.-Y. Ouyang. 2016. Piperine suppresses pyroptosis and interleukin-1β release upon ATP triggering and bacterial infection. Frontiers in Pharmacology. https://doi.org/10.3389/fphar.2016.00390.
Guo, H., J.B. Callaway, and J.P.Y. Ting. 2015. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nature medicine 21: 677–687. https://doi.org/10.1038/nm.3893.
Netea, M.G., A. Simon, F. van de Veerdonk, B.-J. Kullberg, J.W.M. Van der Meer, and L.A.B. Joosten. 2010. IL-1β processing in host defense: beyond the inflammasomes. PLOS Pathogens 6: e1000661. https://doi.org/10.1371/journal.ppat.1000661.
Rossol, M., M. Pierer, N. Raulien, D. Quandt, U. Meusch, K. Rothe, K. Schubert, T. Schöneberg, M. Schaefer, U. Krügel, S. Smajilovic, H. Bräuner-Osborne, C. Baerwald, and U. Wagner. 2012. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nature communications 3: 1329–1329. https://doi.org/10.1038/ncomms2339.
Wang, F., L. Huang, Z.Z. Peng, Y.T. Tang, M.M. Lu, Y. Peng, W.J. Mel, L. Wu, Z.H. Mo, J. Meng, and L.J. Tao. 2014. Losartan inhibits LPS + ATP-induced IL-1beta secretion from mouse primary macrophages by suppressing NALP3 inflammasome. Die Pharmazie 69: 680–684. https://doi.org/10.1691/ph.2014.3926.
Usui, F., K. Shirasuna, H. Kimura, K. Tatsumi, A. Kawashima, T. Karasawa, K. Yoshimura, H. Aoki, H. Tsutsui, T. Noda, J. Sagara, and Taniguchi, S.i. and Takahashi, M. 2015. Inflammasome activation by mitochondrial oxidative stress in macrophages leads to the development of angiotensin II–induced aortic aneurysm. Arteriosclerosis, Thrombosis, and Vascular Biology 35: 127–136. https://doi.org/10.1161/ATVBAHA.114.303763.
Horng, T. 2014. Calcium signaling and mitochondrial destabilization in the triggering of the NLRP3 inflammasome. Trends in Immunology 35: 253–261. https://doi.org/10.1016/j.it.2014.02.007.
Zhang, L.-L., S. Huang, X.-X. Ma, W.-Y. Zhang, D. Wang, S.-Y. Jin, Y.-P. Zhang, Y. Li, and X. Li. 2016. Angiotensin(1–7) attenuated angiotensin II-induced hepatocyte EMT by inhibiting NOX-derived H2O2-activated NLRP3 inflammasome/IL-1β/Smad circuit. Free Radical Biology and Medicine 97: 531–543. https://doi.org/10.1016/j.freeradbiomed.2016.07.014.
Zhao, M., M. Bai, G. Ding, Y. Zhang, S. Huang, Z. Jia, and A. Zhang. 2018. Angiotensin II stimulates the NLRP3 inflammasome to induce podocyte injury and mitochondrial dysfunction. Kidney Diseases 4: 83–94. https://doi.org/10.1159/000488242.
Malhotra, V. 2013. Unconventional protein secretion: an evolving mechanism. The EMBO Journal 32: 1660–1664. https://doi.org/10.1038/emboj.2013.104.
Lopez-Castejon, G., and D. Brough. 2011. Understanding the mechanism of IL-1β secretion. Cytokine & Growth Factor Reviews 22: 189–195. https://doi.org/10.1016/j.cytogfr.2011.10.001.
Eder, C. 2009. Mechanisms of interleukin-1β release. Immunobiology 214: 543–553. https://doi.org/10.1016/j.imbio.2008.11.007.
Qu, Y., L. Franchi, G. Nunez, and G.R. Dubyak. 2007. Nonclassical IL-1β secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. The Journal of Immunology 179: 1913. https://doi.org/10.4049/jimmunol.179.3.1913.
Piccioli, P., and A. Rubartelli. 2013. The secretion of IL-1β and options for release. Seminars in Immunology 25: 425–429. https://doi.org/10.1016/j.smim.2013.10.007.
Katsnelson, M., L. Rucker, H. Russo, and G. Dubyak. (2015). K+ Efflux agonists induce NLRP3 inflammasome activation independently of Ca2+ signaling. Journal of immunology (Baltimore, Md. : 1950) 194. https://doi.org/10.4049/jimmunol.1402658.
Galliher-Beckley, A.J., L.-Q. Lan, S. Aono, L. Wang, and J. Shi. 2013. Caspase-1 activation and mature interleukin-1β release are uncoupled events in monocytes. World journal of biological chemistry 4: 30–34. https://doi.org/10.4331/wjbc.v4.i2.30.
Butts, B., R.A. Gary, S.B. Dunbar, and J. Butler. 2015. The importance of NLRP3 inflammasome in heart failure. Journal of Cardiac Failure 21: 586–593. https://doi.org/10.1016/j.cardfail.2015.04.014.
Acknowledgements
We thank ANID and ACCDiS institutions for funding this work.
Funding
This work was supported by the FONDECYT (grant 1170425 and grant 1210627 to G. Díaz-Araya) and FONDAP ACCDiS grant 15130011.
Author information
Authors and Affiliations
Contributions
Jenaro Espitia-Corredor and Pía Boza: formal analysis, investigation, validation, writing–original draft, and writing–review and editing. Claudio Espinoza-Pérez, José Miguel Lillo, Constanza Rimassa-Taré, Victor Machuca, José Osorio-Sandoval, Raúl Vivar, Samir Bolívar, and Viviana Pardo-Jiménez: Formal analysis and investigation. Concepción Peiró, Carlos F. Sánchez-Ferrer, and Guillermo Díaz-Araya: Conceptualization, writing–review and editing, and supervision.
Corresponding author
Ethics declarations
Ethics Approval
The Bioethics Committee of the Faculty of Chemical and Pharmaceutical Sciences of the University of Chile with the code CBE2012-20 approved the management and care of laboratory animals used for the isolation of cardiac fibroblasts.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Espitia-Corredor, J.A., Boza, P., Espinoza-Pérez, C. et al. Angiotensin II Triggers NLRP3 Inflammasome Activation by a Ca2+ Signaling-Dependent Pathway in Rat Cardiac Fibroblast Ang-II by a Ca2+-Dependent Mechanism Triggers NLRP3 Inflammasome in CF. Inflammation 45, 2498–2512 (2022). https://doi.org/10.1007/s10753-022-01707-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-022-01707-z