Abstract
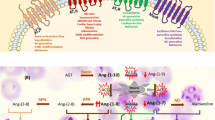
In malaria-endemic countries, the burden of hypertension is on the rise. Although malaria and hypertension seem to have no direct link, several studies in recent years support their possible link. Three bioactive molecules such as angiotensin II (Ang II), bradykinin (BK) and sphingosine 1-phosphate (S1P) are crucial in regulating blood pressure. While the increased level of Ang II and S1P are responsible for inducing hypertension, BK is arthero-protective and anti-hypertensive. Therefore, in the present review, based on available literatures we highlight the present knowledge on the production and bioavailability of these molecules, the mechanism of their regulation of hypertension, and patho-physiological role in malaria. Further, a possible link between malaria and hypertension is hypothesized through various arguments based on experimental evidence. Understanding of their mechanisms of blood pressure regulation during malaria infection may open up avenues for drug therapeutics and management of malaria in co-morbidity with hypertension.


Similar content being viewed by others
References
Dhangadamajhi G, Kar SK, Ranjit M. The survival strategies of malaria parasite in the red blood cell and host cell polymorphisms. Malar Res Treat. 2010;2010.
Sahu PK, Satpathi S, Behera PK, Mishra SK, Mohanty S, Wassmer SC. Pathogenesis of cerebral malaria: new diagnostic tools, biomarkers, and therapeutic approaches. Front Cell Infect Microbiol. 2015;5:75.
Sypniewska P, Duda JF, Locatelli I, Althaus CR, Althaus F, Genton B. Clinical and laboratory predictors of death in African children with features of severe malaria: a systematic review and meta-analysis. BMC Med. 2017;15(1):147.
Danaei G, Finucane MM, Lin JK, Singh GM, Paciorek CJ, Cowan MJ, et al. National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 54 million participants. The Lancet. 2011;377(9765):568–77.
Zhou B, Danaei G, Stevens GA, Bixby H, Taddei C, Carrillo-Larco RM, et al. Long-term and recent trends in hypertension awareness, treatment, and control in 12 high-income countries: an analysis of 123 nationally representative surveys. The Lancet. 2019;394(10199):639–51.
Dhangadamajhi G, Mohapatra BN, Kar SK, Ranjit M. Gene polymorphisms in angiotensin I converting enzyme (ACE I/D) and angiotensin II converting enzyme (ACE2 C→ T) protect against cerebral malaria in Indian adults. Infect Genet Evol. 2010;10(2):337–41.
Etyang AO, Kapesa S, Odipo E, Bauni E, Kyobutungi C, Abdalla M, et al. Effect of previous exposure to malaria on blood pressure in Kilifi, Kenya: a Mendelian randomization study. J Am Heart Assoc. 2019;8(6):e011771.
Etyang AO, Smeeth L, Cruickshank JK, Scott JAG. The malaria-high blood pressure hypothesis. Circ Res. 2016;119(1):36–40.
Eze IC, Bassa FK, Essé C, Koné S, Acka F, Laubhouet-Koffi V, et al. Epidemiological links between malaria parasitaemia and hypertension: findings from a population-based survey in rural Côte d’Ivoire. J Hypertens. 2019;37(7):1384.
Gallego-Delgado J, Walther T, Rodriguez A. The high blood pressure-malaria protection hypothesis. Circ Res. 2016;119(10):1071–5.
Volpe M, Battistoni A. An evolutionary rebus: the complex link between malaria and hypertension. J Hypertens. 2019;37(7):1344–6.
Don-Doncow N, Zhang Y, Matuskova H, Meissner A. The emerging alliance of sphingosine-1-phosphate signalling and immune cells: from basic mechanisms to implications in hypertension. Br J Pharmacol. 2019;176(12):1989–2001.
Nwokocha CR, Bafor EE, Ajayi OI, Ebeigbe AB. The malaria-high blood pressure hypothesis: revisited. Am J Hypertens. 2020;
Koopman JJ, van Bodegom D, Jukema JW, Westendorp RG. Risk of cardiovascular disease in a traditional African population with a high infectious load: a population-based study. PLoS ONE. 2012;7(10):e46855.
Kearney PM, Whelton M, Reynolds K, Whelton PK, He J. Worldwide prevalence of hypertension: a systematic review. J Hypertens. 2004;22(1):11–9.
Adam I, Elhassan EM, Mohmmed AA, Salih MM, Elbashir MI. Malaria and pre-eclampsia in an area with unstable malaria transmission in Central Sudan. Malar J. 2011;10(1):258.
Duley L. Maternal mortality associated with hypertensive disorders of pregnancy in Africa, Asia, Latin America and the Caribbean. BJOG Int J Obstet Gynaecol. 1992;99(7):547–53.
Ndao CT, Dumont A, Fievet N, Doucouré S, Gaye A, Lehesran J-Y. Placental malarial infection as a risk factor for hypertensive disorders during pregnancy in Africa: a case-control study in an urban area of Senegal West Africa. Am J Epidemiol. 2009;170(7):847–53.
Law C, Shiell A, Newsome C, Syddall H, Shinebourne E, Fayers P, et al. Fetal, infant, and childhood growth and adult blood pressure: a longitudinal study from birth to 22 years of age. Circulation. 2002;105(9):1088–92.
Ayoola OO, Omotade OO, Gemmell I, Clayton PE, Cruickshank JK. The impact of malaria in pregnancy on changes in blood pressure in children during their first year of life. Hypertension. 2014;63(1):167–72.
Cruickshank J, Mzayek F, Liu L, Kieltyka L, Sherwin R, Webber L, et al. Origins of the “black/white” difference in blood pressure: roles of birth weight, postnatal growth, early blood pressure, and adolescent body size: the Bogalusa heart study. Circulation. 2005;111(15):1932–7.
Cappuccio FP. Ethnicity and cardiovascular risk: variations in people of African ancestry and South Asian origin. J Hum Hypertens. 1997;11(9):571–6.
Sampson UK, Edwards TL, Jahangir E, Munro H, Wariboko M, Wassef MG, et al. Factors associated with the prevalence of hypertension in the southeastern United States: insights from 69 211 blacks and whites in the southern community cohort study. Circ Cardiovasc Qual Outcomes. 2014;7(1):33–54.
Ferrari R, et al. RAAS inhibition and mortality in hypertension: from pharmacology to clinical evidence. 2013;
Intapad S. Sphingosine-1-phosphate signaling in blood pressure regulation. Am J Physiol-Ren Physiol. 2019;317(3):F638–40.
Katsi V, Katsimichas T, Pittaras A, Grassos C, Katsimichas A, Tousoulis D, et al. Hypertension and bradykinin: a dangerous affair? Cardiovasc Endocrinol Metab. 2012;1(2):24–30.
Foulquier S, Paulis L, Kaschina E, Namsolleck P, Unger T. Hormonal systems. In: Disorders of Blood Pressure Regulation. Springer; 2018. p. 81–106.
te Riet L, van Esch JH, Roks AJ, van den Meiracker AH, Danser AJ. Hypertension: renin–angiotensin–aldosterone system alterations. Circ Res. 2015;116(6):960–75.
van Kats JP, de Lannoy LM, Danser AJ, van Meegen JR, Verdouw PD, Schalekamp MA. Angiotensin II Type 1 (AT1) receptor–mediated accumulation of angiotensin II in tissues and its intracellular half-life in vivo. Hypertension. 1997;30(1):42–9.
Gebre AK, Altaye BM, Atey TM, Tuem KB, Berhe DF. Targeting renin-angiotensin system against Alzheimer’s disease. Front Pharmacol. 2018;9:440.
Royea J, Hamel E. Brain angiotensin II and angiotensin IV receptors as potential Alzheimer’s disease therapeutic targets. GeroScience. 2020;1–20.
Wright JW, Harding JW. Contributions by the brain renin-angiotensin system to memory, cognition, and Alzheimer’s disease. J Alzheimers Dis. 2019;67(2):469–80.
Khalil RA. Hypertension and vascular dysfunction. In: Interdisciplinary concepts in cardiovascular health. Springer; 2013. p. 1–37.
Laurent S, Chatellier G, Azizi M, Calvet D, Choukroun G, Danchin N, et al. Protocol of the SPARTE study: a strategy for preventing cardiovascular and renal events based on arterial stiffness. Artery Res. 2020;
Kaschina E, Unger T. Prehypertension and the renin-angiotensin-aldosterone system. In: Prehypertension and cardiometabolic syndrome. Springer; 2019. p. 307–18.
Liu M, Solomon W, Cespedes JC, Wilson NO, Ford B, Stiles JK. Neuregulin-1 attenuates experimental cerebral malaria (ECM) pathogenesis by regulating ErbB4/AKT/STAT3 signaling. J Neuroinflammation. 2018;15(1):104.
Norlander AE, Saleh MA, Kamat NV, Ko B, Gnecco J, Zhu L, et al. Interleukin-17A regulates renal sodium transporters and renal injury in angiotensin II–induced hypertension. Hypertension. 2016;68(1):167–74.
Silva LS, Peruchetti DB, Silva-Aguiar RP, Abreu TP, Dal-Cheri BK, Takiya CM, et al. The angiotensin II/AT1 receptor pathway mediates malaria-induced acute kidney injury. PLoS ONE. 2018;13(9):e0203836.
Gallego-Delgado J, Basu-Roy U, Ty M, Alique M, Fernandez-Arias C, Movila A, et al. Angiotensin receptors and β-catenin regulate brain endothelial integrity in malaria. J Clin Invest. 2016;126(10):4016–29.
Silva-Filho JL, Caruso-Neves C, Pinheiro AA. Targeting angiotensin II type-1 receptor (AT1R) inhibits the harmful phenotype of Plasmodium-specific CD8+ T cells during blood-stage malaria. Front Cell Infect Microbiol. 2017;7:42.
Silva-Filho JL, Caruso-Neves C, Pinheiro AAS. Angiotensin II type-1 receptor (AT 1 R) regulates expansion, differentiation, and functional capacity of antigen-specific CD8+ T cells. Sci Rep. 2016;6:35997.
Silva-Filho JL, Souza MC, Ferreira-DaSilva CT, Silva LS, Costa MFS, Padua TA, et al. Angiotensin II is a new component involved in splenic T lymphocyte responses during Plasmodium berghei ANKA infection. PLoS ONE. 2013;8(4):e62999.
Maciel C, de Oliveira Junior VX, Fazio MA, Nacif-Pimenta R, Miranda A, Pimenta PF, et al. Anti-plasmodium activity of angiotensin II and related synthetic peptides. PLoS ONE. 2008;3(9):e3296.
Saraiva VB, de Souza SL, Ferreira-DaSilva CT, da Silva-Filho JL, Teixeira-Ferreira A, Perales J, et al. Impairment of the Plasmodium falciparum erythrocytic cycle induced by angiotensin peptides. PLoS ONE. 2011;6(2):e17174.
Gallego-Delgado J, Baravian C, Edagha I, Ty MC, Ruiz-Ortega M, Xu W, et al. Angiotensin II moderately decreases plasmodium infection and experimental cerebral malaria in mice. PLoS ONE. 2015;10(9):e0138191.
Bala R, Abdulazeez A, Kiru A, Abdullahi N, Abubakar B. Effect of an angiotensin converting enzyme inhibitor (Captopril) on liver and kidney function parameters in plasmodium berghei-infected mice. J Adv Med Pharm Sci. 2018;1–8.
Hoffmann BR, Stodola TJ, Wagner JR, Didier DN, Exner EC, Lombard JH, et al. Mechanisms of Mas1 receptor-mediated signaling in the vascular endothelium. Arterioscler Thromb Vasc Biol. 2017;37(3):433–45.
Moreau ME, Garbacki N, Molinaro G, Brown NJ, Marceau F, Adam A. The kallikrein-kinin system: current and future pharmacological targets. J Pharmacol Sci. 2005;99(1):6–38.
Bagnaresi P, Barros NM, Assis DM, Melo PM, Fonseca RG, Juliano MA, et al. Intracellular proteolysis of kininogen by malaria parasites promotes release of active kinins. Malar J. 2012;11(1):156.
Silva LS, Pinheiro AS, Teixeira DE, Silva-Aguiar RP, Peruchetti DB, Scharfstein J, et al. Kinins released by erythrocytic stages of Plasmodium falciparum enhance adhesion of infected erythrocytes to endothelial cells and increase blood brain barrier permeability via activation of bradykinin receptors. Front Med. 2019;6:75.
Ventura PD, Carvalho CP, Barros NM, Martins-Silva L, Dantas EO, Martinez C, et al. Malaria infection promotes a selective expression of kinin receptors in murine liver. Malar J. 2019;18(1):213.
de Souza SL, de Barros PD, Ferreira-Da Silva CT, Ferreira-DaSilva AT, Perales J, Caruso-Neves C, et al. Interaction between bradykinin B2 and Ang-(1–7) Mas receptors regulates erythrocyte invasion by Plasmodium falciparum. Biochim Biophys Acta BBA-Gen Subj. 2016;1860(11):2438–44.
de Moraes LV, Barateiro A, Sousa PM, Penha-Gonçalves C. Bradykinin sequestration by Plasmodium berghei infected erythrocytes conditions B2R signaling and parasite uptake by fetal trophoblasts. Front Microbiol. 2018;9:3106.
Singh S, Chitnis CE. Molecular signaling involved in entry and exit of malaria parasites from host erythrocytes. Cold Spring Harb Perspect Med. 2017;7(10):a026815.
Książek M, Chacińska M, Chabowski A, Baranowski M. Sources, metabolism, and regulation of circulating sphingosine-1-phosphate. J Lipid Res. 2015;56(7):1271–81.
Dhangadamajhi G, Singh S. Sphingosine 1-Phosphate in malaria pathogenesis and its implication in therapeutic opportunities. Front Cell Infect Microbiol. 2020;10.
Bode C, Sensken S-C, Peest U, Beutel G, Thol F, Levkau B, et al. Erythrocytes serve as a reservoir for cellular and extracellular sphingosine 1-phosphate. J Cell Biochem. 2010;109(6):1232–43.
Urtz N, Gaertner F, von Bruehl M-L, Chandraratne S, Rahimi F, Zhang L, et al. Sphingosine 1-phosphate produced by sphingosine kinase 2 intrinsically controls platelet aggregation in vitro and in vivo. Circ Res. 2015;117(4):376–87.
Ancellin N, Colmont C, Su J, Li Q, Mittereder N, Chae S-S, et al. Extracellular export of sphingosine kinase-1 enzyme Sphingosine 1-phosphate generation and the induction of angiogenic vascular maturation. J Biol Chem. 2002;277(8):6667–75.
Venkataraman K, Thangada S, Michaud J, Oo ML, Ai Y, Lee Y-M, et al. Extracellular export of sphingosine kinase-1a contributes to the vascular S1P gradient. Biochem J. 2006;397(3):461–71.
Vu TM, Ishizu A-N, Foo JC, Toh XR, Zhang F, Whee DM, et al. Mfsd2b is essential for the sphingosine-1-phosphate export in erythrocytes and platelets. Nature. 2017;550(7677):524–8.
Hisano Y, Kobayashi N, Yamaguchi A, Nishi T. Mouse SPNS2 functions as a sphingosine-1-phosphate transporter in vascular endothelial cells. PLoS ONE. 2012;7(6):e38941.
Jonnalagadda D, Sunkara M, Morris AJ, Whiteheart SW. Granule-mediated release of sphingosine-1-phosphate by activated platelets. Biochim Biophys Acta BBA-Mol Cell Biol Lipids. 2014;1841(11):1581–9.
Cantalupo A, Zhang Y, Kothiya M, Galvani S, Obinata H, Bucci M, et al. Nogo-B regulates endothelial sphingolipid homeostasis to control vascular function and blood pressure. Nat Med. 2015;21(9):1028–37.
MacRitchie N, Volpert G, Al Washih M, Watson DG, Futerman AH, Kennedy S, et al. Effect of the sphingosine kinase 1 selective inhibitor, PF-543 on arterial and cardiac remodelling in a hypoxic model of pulmonary arterial hypertension. Cell Signal. 2016;28(8):946–55.
Wilson PC, Fitzgibbon WR, Garrett SM, Jaffa AA, Luttrell LM, Brands MW, et al. Inhibition of sphingosine kinase 1 ameliorates angiotensin ii-induced hypertension and inhibits transmembrane calcium entry via store-operated calcium channel. Mol Endocrinol. 2015;29(6):896–908.
Camm J, Hla T, Bakshi R, Brinkmann V. Cardiac and vascular effects of fingolimod: mechanistic basis and clinical implications. Am Heart J. 2014;168(5):632–44.
Cantalupo A, Gargiulo A, Dautaj E, Liu C, Zhang Y, Hla T, et al. S1PR1 (sphingosine-1-phosphate receptor 1) signaling regulates blood flow and pressure. Hypertension. 2017;70(2):426–34.
Kurano M, Yatomi Y. Sphingosine 1-phosphate and atherosclerosis. J Atheroscler Thromb. 2017;RV17010.
Tölle M, Levkau B, Keul P, Brinkmann V, Giebing G, Schönfelder G, et al. Immunomodulator FTY720 Induces eNOS-dependent arterial vasodilatation via the lysophospholipid receptor S1P3. Circ Res. 2005;96(8):913–20.
Salomone S, Soydan G, Ip PC-T, Hopson KMP, Waeber C. Vessel-specific role of sphingosine kinase 1 in the vasoconstriction of isolated basilar arteries. Pharmacol Res. 2010;62(6):465–74.
Park S-J, Im D-S. Sphingosine 1-phosphate receptor modulators and drug discovery. Biomol Ther. 2017;25(1):80.
Galvani S, Sanson M, Blaho VA, Swendeman SL, Obinata H, Conger H, et al. HDL-bound sphingosine 1-phosphate acts as a biased agonist for the endothelial cell receptor S1P1 to limit vascular inflammation. Sci Signal. 2015;8(389):ra79–ra79.
Obinata H, Hla T. Sphingosine 1-phosphate and inflammation. Int Immunol. 2019;31(9):617–25.
Brizuela L, Rábano M, Gangoiti P, Narbona N, Macarulla JM, Trueba M, et al. Sphingosine-1-phosphate stimulates aldosterone secretion through a mechanism involving the PI3K/PKB and MEK/ERK 1/2 pathways. J Lipid Res. 2007;48(10):2264–74.
Chen J, Tang H, Sysol JR, Moreno-Vinasco L, Shioura KM, Chen T, et al. The sphingosine kinase 1/sphingosine-1-phosphate pathway in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2014;190(9):1032–43.
Meissner A, Miro F, Jimenez-Altayo F, Jurado A, Vila E, Planas AM. Sphingosine-1-phosphate signalling—a key player in the pathogenesis of Angiotensin II-induced hypertension. Cardiovasc Res. 2017;113(2):123–33.
Siedlinski M, Nosalski R, Szczepaniak P, Ludwig-Ga\lęzowska AH, Miko\lajczyk T, Filip M, et al. Vascular transcriptome profiling identifies Sphingosine kinase 1 as a modulator of angiotensin II-induced vascular dysfunction. Sci Rep. 2017;7:44131.
Yang K, Jiang K, Xu Z, Song Y, Wang J. Targeting sphingosine kinase 1 for the treatment of pulmonary arterial hypertension. Future Med Chem. 2019;11(22):2939–53.
Pyne NJ, Pyne S. Sphingosine kinase 1: a potential therapeutic target in pulmonary arterial hypertension? Trends Mol Med. 2017;23(9):786–98.
Matloubian M, Lo CG, Cinamon G, Lesneski MJ, Xu Y, Brinkmann V, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 2004;427(6972):355–60.
Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, et al. Role of the T cell in the genesis of angiotensin II–induced hypertension and vascular dysfunction. J Exp Med. 2007;204(10):2449–60.
Itani HA, McMaster WG Jr, Saleh MA, Nazarewicz RR, Mikolajczyk TP, Kaszuba AM, et al. Activation of human T cells in hypertension: studies of humanized mice and hypertensive humans. Hypertension. 2016;68(1):123–32.
Schulze T, Golfier S, Tabeling C, Räbel K, Gräler MH, Witzenrath M, et al. Sphingosine-1-phospate receptor 4 (S1P4) deficiency profoundly affects dendritic cell function and TH17-cell differentiation in a murine model. FASEB J. 2011;25(11):4024–36.
van Hooren KW, Spijkers LJ, van Breevoort D, Fernandez-Borja M, Bierings R, van Buul JD, et al. Sphingosine-1-phosphate receptor 3 mediates sphingosine-1-phosphate induced release of weibel-palade bodies from endothelial cells. PLoS ONE. 2014;9(3):e91346.
Sanchez T, Skoura A, Wu MT, Casserly B, Harrington EO, Hla T. Induction of vascular permeability by the sphingosine-1-phosphate receptor–2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler Thromb Vasc Biol. 2007;27(6):1312–8.
Li N, Zhang F. Implication of sphingosin-1-phosphate in cardiovascular regulation. Front Biosci Landmark Ed. 2016;21:1296.
Sah RK, Saini M, Pati S, Singh S. Plasmodium falciparum growth is regulated by Sphingosine 1 phosphate produced by Host Erythrocyte Membrane Sphingosine kinase 1. bioRxiv. 2019;756502.
Finney CA, Hawkes CA, Kain DC, Dhabangi A, Musoke C, Cserti-Gazdewich C, et al. S1P is associated with protection in human and experimental cerebral malaria. Mol Med. 2011;17(7):717–25.
Nacer A, Movila A, Sohet F, Girgis NM, Gundra UM, Daneman R, et al. Experimental cerebral malaria pathogenesis—hemodynamics at the blood brain barrier. PLoS Pathog. 2014;10(12):e1004528.
Nacer A, Movila A, Baer K, Mikolajczak SA, Kappe SH, Frevert U. Neuroimmunological blood brain barrier opening in experimental cerebral malaria. PLoS Pathog. 2012;8(10):e1002982.
Punsawad C, Viriyavejakul P. Expression of sphingosine kinase 1 and sphingosine 1-phosphate receptor 3 in malaria-associated acute lung injury/acute respiratory distress syndrome in a mouse model. PLoS ONE. 2019;14(9):e0222098.
Birhanu M, Asres Y, Adissu W, Yemane T, Zemene E, Gedefaw L. Hematological parameters and hemozoin-containing leukocytes and their association with disease severity among malaria infected children: a cross-sectional study at Pawe General Hospital, Northwest Ethiopia. Interdiscip Perspect Infect Dis. 2017;2017.
Dhangadamajhi G, Panigrahi S, Roy S, Tripathy S. Effect of Plasmodium falciparum infection on blood parameters and their association with clinical severity in adults of Odisha. India Acta Trop. 2019;190:1–8.
Zhang L, Orban M, Lorenz M, Barocke V, Braun D, Urtz N, et al. A novel role of sphingosine 1-phosphate receptor S1pr1 in mouse thrombopoiesis. J Exp Med. 2012;209(12):2165–81.
El Tahir A, Malhotra P, Chauhan VS. Uptake of proteins and degradation of human serum albumin by Plasmodium falciparum–infected human erythrocytes. Malar J. 2003;2(1):1–8.
Visser BJ, Wieten RW, Nagel IM, Grobusch MP. Serum lipids and lipoproteins in malaria-a systematic review and meta-analysis. Malar J. 2013;12(1):1–16.
Yeo TW, Weinberg JB, Lampah DA, Kenangalem E, Bush P, Chen Y, et al. Glycocalyx breakdown is associated with severe disease and fatal outcome in Plasmodium falciparum malaria. Clin Infect Dis. 2019;69(10):1712–20.
Cannon RE, Peart JC, Hawkins BT, Campos CR, Miller DS. Targeting blood-brain barrier sphingolipid signaling reduces basal P-glycoprotein activity and improves drug delivery to the brain. Proc Natl Acad Sci. 2012;109(39):15930–5.
Oggungwan K, Glaharn S, Ampawong S, Krudsood S, Viriyavejakul P. FTY720 restores endothelial cell permeability induced by malaria sera. Sci Rep. 2018;8(1):1–6.
Yanagida K, Liu CH, Faraco G, Galvani S, Smith HK, Burg N, et al. Size-selective opening of the blood–brain barrier by targeting endothelial sphingosine 1–phosphate receptor 1. Proc Natl Acad Sci. 2017;114(17):4531–6.
Hempel C, Sporring J, Kurtzhals JAL. Experimental cerebral malaria is associated with profound loss of both glycan and protein components of the endothelial glycocalyx. FASEB J. 2019;33(2):2058–71.
Oo ML, Chang S-H, Thangada S, Wu M-T, Rezaul K, Blaho V, et al. Engagement of S1P 1-degradative mechanisms leads to vascular leak in mice. J Clin Invest. 2011;121(6):2290–300.
Li P, Kondo T, Numaguchi Y, Kobayashi K, Aoki M, Inoue N, et al. Role of bradykinin, nitric oxide, and angiotensin II type 2 receptor in imidapril-induced angiogenesis. Hypertension. 2008;51(2):252–8.
Jensen AR, Adams Y, Hviid L. Cerebral Plasmodium falciparum malaria: The role of PfEMP1 in its pathogenesis and immunity, and PfEMP1-based vaccines to prevent it. Immunol Rev. 2020;293(1):230–52.
Gillrie MR, Avril M, Brazier AJ, Davis SP, Stins MF, Smith JD, et al. Diverse functional outcomes of P lasmodium falciparum ligation of EPCR: potential implications for malarial pathogenesis. Cell Microbiol. 2015;17(12):1883–99.
Moussiliou A, Alao MJ, Denoeud-Ndam L, Tahar R, Ezimegnon S, Sagbo G, et al. High plasma levels of soluble endothelial protein C receptor are associated with increased mortality among children with cerebral malaria in Benin. J Infect Dis. 2015;211(9):1484–8.
Biancardi VC, Son SJ, Ahmadi S, Filosa JA, Stern JE. Circulating angiotensin II gains access to the hypothalamus and brain stem during hypertension via breakdown of the blood–brain barrier. Hypertension. 2014;63(3):572–9.
Beare NA, Glover SJ, Lewallen S, Taylor TE, Harding SP, Molyneux ME. Prevalence of raised intracranial pressure in cerebral malaria detected by optic nerve sheath ultrasound. Am J Trop Med Hyg. 2012;87(6):985–8.
Patil HV, others. Clinical profile and outcome of complicated Plasmodium falciparum malaria. Int J Med Public Health. 2012;2(1).
Abdulazeez A, Ya’u M, Kurfi B, et al. Association of hypertension and activity of angiotensin converting enzyme in malaria patients attending Sheik Muhammad Jidda General Hospital, Kano State, Nigeria. Niger J Basic Clin Sci. 2017;14(2):121.
Hoffmeister B, Valdez ADA. Hypertension is associated with an increased risk for severe imported falciparum malaria: a tertiary care hospital based observational study from Berlin, Germany. Malar J. 2019;18(1):410.
Punsawad C, Viriyavejakul P. Reduction in serum sphingosine 1-phosphate concentration in malaria. PLoS ONE. 2017;12(6):e0180631.
Khan SJ, Abbass Y, Marwat MA. Thrombocytopenia as an indicator of malaria in adult population. Malar Res Treat. 2012;2012.
Dondorp A. Clinical significance of sequestration in adults with severe malaria. Transfus Clin Biol. 2008;15(1–2):56–7.
Liles WC, Kain KC. Endothelial activation and dysfunction in the pathogenesis of microvascular obstruction in severe malaria—a viable target for therapeutic adjunctive intervention. J Infect Dis. 2014;210(1):163–4.
Hanson J, Lam SW, Mahanta KC, Pattnaik R, Alam S, Mohanty S, et al. Relative contributions of macrovascular and microvascular dysfunction to disease severity in falciparum malaria. J Infect Dis. 2012;206(4):571–9.
Graça L, Abreu IG, Santos AS, Graça L, Dias PF, Santos ML. Descriptive acute respiratory distress syndrome (ARDS) in adults with imported severe Plasmodium falciparum malaria: a 10 year-study in a Portuguese tertiary care hospital. PLoS ONE. 2020;15(7):e0235437.
Taylor WR, Hanson J, Turner GD, White NJ, Dondorp AM. Respiratory manifestations of malaria. Chest. 2012;142(2):492–505.
Viriyavejakul P, Punsawad C. Overexpression of sphingosine kinase-1 and sphingosine-1-phosphate receptor-3 in severe plasmodium falciparum malaria with pulmonary edema. BioMed Res Int. 2020;2020.
Zhao J, Tan Y, Wang L, Su X, Shi Y. Serum sphingosine-1-phosphate levels and Sphingosine-1-Phosphate gene polymorphisms in acute respiratory distress syndrome: a multicenter prospective study. J Transl Med. 2020;18:1–11.
Biollaz J, Brunner HR, Gavras I, Waeber B, Gavras H. Antihypertensive therapy with MK 4211: angiotensin II-renin relationships to evaluate efficacy of converting enzyme blockade. J Cardiovasc Pharmacol. 1982;4(6):966–72.
Klein N, Gembardt F, Supé S, Kaestle SM, Nickles H, Erfinanda L, et al. Angiotensin-(1–7) protects from experimental acute lung injury. Crit Care Med. 2013;41(11):e334–43.
Acknowledgement
The present review is an upshot of the project proposal submitted to ICMR, Govt. of India for funding by both the authors. The funding agency has no role in designing, drafting, and decision to submit the review article for publication. We thankfully acknowledge the authors whose contributions have been cited and would like to apologize to those whose published articles could not be cited due to space constraints.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dhangadamajhi, G., Singh, S. Malaria link of hypertension: a hidden syndicate of angiotensin II, bradykinin and sphingosine 1-phosphate. Human Cell 34, 734–744 (2021). https://doi.org/10.1007/s13577-021-00513-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13577-021-00513-3