Abstract
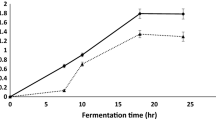
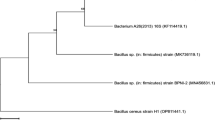
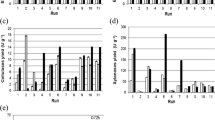
The present work aims to exploit the zero-cost inducer bread waste (BW) for the production of cold-adapted α-amylase (CA-AM21) by the hyperproducer marine psychrotolerant Glutamicibacter soli strain AM6 EMCCN 3074. Incubation temperature (20 °C), incubation time, BW, (NH4)2SO4, and modified seawater-based minimal medium were inferred to be significant factors influencing CAM-21 production in OVAT and Plackett-Burman investigations. BW (3.8%w/v), (NH4)2SO4 (0.098%w/v), and incubation time (2.329 days) prompted CA-AM21 (56.85 U/mL) with 7.58-fold enhancement, Box-Behnken design, and ridge steepest ascent path inferences. A two-step strategy was used to purify CA-AM21: fractional (NH4)2SO4 precipitation (60-80%) and a starch affinity chromatography. Specific activity, yield, and fold purification of purified CA-AM21 were 884.5 U/mg, 12.45%, and 285.3, respectively. CA-AM21 exhibited an approximate molecular mass of 14 kDa, deduced from SDS-PAGE analyses. The pH and temperature optimums for CA-AM21 were 7.0 and 40 °C, respectively. At 5–25 °C, full activity was maintained after 2 h. At pH 6.0–8.0, almost full activity (90–100%) was retained after 15 h. CA-AM21 demonstrated full stability after 30 min at 1.0–5.0 M NaCl, with 200% increased activity at 1.0–4.0 M NaCl. Full stability of CA-AM21 in the presence of non-ionic detergents, such as Tween 20, Tween 80, and Triton X-100, with 100% activity sustained after 30 min was retained. On soluble starch, purified CA-AM21 exhibited km and Vmax of 0.039 mg.mL−1 and 115.71 μmol glucose. min−1. mg−1, respectively. The purified CA-AM21 demonstrated remarkable significant washing efficiency with commercial laundry detergents thus implying its applicability for detergent formulations.






Similar content being viewed by others
Data availability
All data are available and included in the article.
Code availability
Not applicable.
References
Kuddus M (2014) Cold-active microbial enzymes. Biochem Physiol Open Access 04. https://doi.org/10.4172/2168-9652.1000e132
Nandanwar S, Borkar S, Lee J, Kim HJ (2020) Taking advantage of promiscuity of cold-active enzymes Appl Sci 10:8128. https://doi.org/10.3390/app10228128
van der Maarel MJEC, van der Veen B, Uitdehaag JCM, Leemhuis H, Dijkhuizen L (2002) Properties and applications of starch-converting enzymes of the alpha-amylase family. J Biotechnol 94:137–155. https://doi.org/10.1016/s0168-1656(01)00407-2
Maharana A, Singh S (2018) Cold active amylases producing psychrotolerants isolated from Nella Lake, Antarctica Biosci Biotechnol Res Asia 15. 10.13005/bbra/2603
Kuddus M, Saima R, Ahmad I (2012) Cold-active extracellular α-amylase production from novel bacteria Microbacterium foliorum GA2 and Bacillus cereus GA6 isolated from Gangotri glacier, Western Himalaya. J Genet Eng Biotechnol 10:151–159. https://doi.org/10.1016/j.jgeb.2012.03.002
Morita RY (1975) Psychrophilic bacteria. Bacteriol Rev 39:144–167. https://doi.org/10.1128/br.39.2.144-167.1975
Kim S, Park H, Choi J (2017) Cloning and characterization of cold-adapted α-amylase from Antarctic Arthrobacter agilis. Appl. Biochem Biotechnol 181:1048–1059. https://doi.org/10.1007/s12010-016-2267-5
Roohi KM (2014) Bio-statistical approach for optimization of cold-active α-amylase production by novel psychrotolerant M. foliorum GA2 in solid state fermentation. Biocatal Agri Biotechnol 3:175–181. https://doi.org/10.1016/j.bcab.2013.09.007
Roohi R, Kuddus M, Saima S (2013) Cold-active detergent-stable extracellular α-amylase from Bacillus cereus GA6: biochemical characteristics and its perspectives in laundry detergent formulation. J Biochem Technol 4:636–644
Hamid DB (2015) Cold-active α-amylase from psychrophilic and psychrotolerant yeast. J Glob Biosci 4:2670–2677
Fenice M, Selbmann L, Zucconi L, Onofri S (1997) Production of extracellular enzymes by Antarctic fungal strains. Polar Biol 17:275–280. https://doi.org/10.1007/s003000050132
Melikoglo M (2008) Production of sustainable alternatives to petrochemicals and fuels using waste bread as a raw material. The University of Manchester
Leung C, Cheung A, Zhang A, Lam K, Lin C (2012) Utilisation of waste bread for fermentative succinic acid production. Biochem Eng J 65:10–15. https://doi.org/10.1016/j.bej.2012.03.010
Han W, Hu Y, Li S, Huang J, Nie Q, Zhao H, Tang J (2017) Simultaneous dark fermentative hydrogen and ethanol production from waste bread in a mixed packed tank reactor. J Clean Prod 141:608–611. https://doi.org/10.1016/j.jclepro.2016.09.143
Cerda A, El-Bakry M, Gea T, Sánchez A (2016) Long term enhanced solid-state fermentation: inoculation strategies for amylase production from soy and bread wastes by Thermomyces sp. in a sequential batch operation. J Environ Chem Eng:4. https://doi.org/10.1016/j.jece.2016.04.022
Gómez-Villegas P, Vigara J, Romero L, Gotor C, Raposo S, Gonçalves B, Léon R (2021) Biochemical characterization of the amylase activity from the new haloarchaeal strain Haloarcula sp. HS isolated in the Odiel marshlands. Biology 10: 337.
Männistö MK, Puhakka JA (2002) Psychrotolerant and microaerophilic bacteria in boreal groundwater. FEMS microbiology ecology 41(1):9–16
Eden PA, Schmidt TM, Blakemore RP, Pace NR (1991) Phylogenetic analysis of Aquaspirillum magnetotacticum using polymerase chain reaction amplified 16S rRNA-specific DNA. Int J Syst Bacteriol 41:324–325
Embaby AM, Heshmat Y, Hussein A, Marey HS (2014) A sequential statistical approach towards an optimized production of a broad spectrum bacteriocin substance from a soil bacterium Bacillus sp. YASI strain. Sci World J 2014:396304
Asgher M, Asad MJ, Rahman SU, Legge R. L (2007) A thermostable α-amylase from a moderately thermophilic Bacillus subtilis strain for starch processing. J Food Eng 79: 950–955. https://doi.org/10.1016/j.jfoodeng.2005.12.053.
Nguyen T (2018) Preparation of artificial sea water (ASW) for culturing marine bacteria. 10.13140/RG.2.2.20641.71528.
Okolo BN, Ezeogu LI, Mba CN (1995) Production of raw starch digesting amylase by Aspergillus niger grown on native starch sources. J. Sci. Food Agric 69:109–115
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428. https://doi.org/10.1021/ac60147a030
Plackett RL, Burman JP (1946) The design of optimum multifactorial experiments. Biometrika 33:305–325
Box GEP, Behnken DW (1960) Some new three level designs for the study of quantitative variables. Technometrics 2:455–475
Rao MD, Ratnam BVV, VenkataRamesh D, Ayyanna C (2005) Rapid method for the affinity purification of thermostable α-amylase from Bacillus licheniformis. World J Microbiol Biotechnol 21:371–375. https://doi.org/10.1007/s11274-004-3908-3
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Liu G, Wu S, Jin W, Sun C (2016) Amy63, a novel type of marine bacterial multifunctional enzyme possessing amylase, agarase and carrageenase activities. Sci Rep 6(1):1-. https://doi.org/10.1038/srep18726.
Shukla RJ, Singh SP (2015) Characteristics and thermodynamics of α-amylase from thermophilic actinobacterium, Laceyella sacchari TSI-2. Process Biochem 50:2128–2136. https://doi.org/10.1016/j.procbio.2015.10.013
Yemm EW, Willis A (1954) The estimation of carbohydrates in plant extracts by anthrone. Biochem J 57(3):508–514
Myers RH (1976) Response surface methodology. Edwards Brothers, Ann Arbor, MI
Draper NR (1963) “Ridge analysis” of response surfaces. Technometrics 5:469–479
Matrawy AA, Khalil AI, Marey HS, Embaby AM (2020) Biovalorization of the raw agro-industrial waste rice husk through directed production of xylanase by Thermomyces lanuginosus strain A3-1 DSM 105773: a statistical sequential model. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-00824-9
Embaby AM, Hussein MN, Hussein A (2018) Monascus orange and red pigments production by Monascus purpureus ATCC16436 through co-solid state fermentation of corn cob and glycerol: an eco-friendly environmental low cost approach. PLoS One 13:e0207755
Anan A, Ghanem KM, Embaby AM, Hussein A, El-Naggar MY (2018) Statistically optimized ceftriaxone sodium biotransformation through Achromobacter xylosoxidans strain Cef6: an unusual insight for bioremediation. J Basic Microbiol 58:120–130. https://doi.org/10.1002/jobm.201700497
Embaby AM, Melika RR, Hussein A, El-Kamel AH, S Marey H (2018) Biosynthesis of chitosan-Oligosaccharides (COS) by non-aflatoxigenic Aspergillus sp. strain EGY1 DSM 101520: a robust biotechnological approach. Process Biochem. 64: 16–30. https://doi.org/10.1016/j.procbio.2017.09.030
Embaby AM, Melika RR, Hussein A, El-Kamel AH, Marey HS (2016) A novel non-cumbersome approach towards biosynthesis of pectic-oligosaccharides by non-aflatoxigenic Aspergillus sp. section flavi strain EGY1 DSM 101520 through citrus pectin fermentation. PLoS One 11:e0167981. https://doi.org/10.1371/journal.pone.0167981
Kim H, Im Y, Choi J, Han SJ (2016) Optimization of physical factor for amylase production by Arthrobacter sp. by response surface methodology. Korean J Chem Eng 54:140–144
Smith MR, Zahnley JC (2005) Production of amylase by Arthrobacter psychrolactophilus. J Ind Microbiol Biotechnol 32:277–283. https://doi.org/10.1007/s10295-005-0240-3
Fan H, Liu Y, Liu Z (2009) [Optimization of fermentation conditions for cold-adapted amylase production by Micrococcus antarcticus and its enzymatic properties]. Huan jing ke xue= Huanjing kexue 30: 2473–2478.
Cotârlet M, Negoiţă TG, Bahrim GE, Stougaard P (2011) Partial characterization of cold active amylases and proteases of Streptomyces sp. from Antarctica. Braz J Microbiol 42:868–877. https://doi.org/10.1590/S1517-83822011000300005
Ji-lian WANG, Yu REN, Ming-yuan LI (2020) Medium optimization of cold-active amylase by Pseudomonas sp. using response surface methodology [J]. J Biol 37(4):101
Krishna C (2005) Solid-state fermentation systems-an overview. Crit Rev Biotechnol 25:1–30. https://doi.org/10.1080/07388550590925383
Ottoni JR, Rodrigues e Silva T, Maia de Oliveira V, Zambrano Passarini MR
Characterization of amylase produced by cold-adapted bacteria from Antarctic samples. Biocatal Agric Biotechnol 23: 101452
Zhang J, Zeng R (2007) Psychrotrophic amylolytic bacteria from deep sea sediment of Prydz Bay, Antarctic: diversity and characterization of amylases. World J Microbiol Biotechnol 23:1551–1557. https://doi.org/10.1007/s11274-007-9400-0
Arabacı N, Arıkan B (2018) Isolation and characterization of a cold-active, alkaline, detergent stable α-amylase from a novel bacterium Bacillus subtilis N8. Prep. Biochem Biotechnol 48:419–426. https://doi.org/10.1080/10826068.2018.1452256
Liu J, Zhang Z, Liu Z, Zhu H, Dang H, Lu J, Cui Z (2011) Production of cold-adapted amylase by marine bacterium Wangia sp. C52: optimization, modeling, and partial characterization. Mar Biotechnol (NY) 13:837–844. https://doi.org/10.1007/s10126-010-9360-5
Pandey A, Nigam P, Soccol CR, Soccol VT, Singh D, Mohan R (2000) Advances in microbial amylases. Biotechnol Appl Biochem 31:135–152. https://doi.org/10.1042/ba19990073
Gupta R, Gigras P, Mohapatra H, Goswami VK, Chauhan B (2003) Microbial α-amylases: a biotechnological perspective. Process Biochem 38:1599–1616. https://doi.org/10.1016/S0032-9592(03)00053-0
Qin Y, Huang Z, Liu Z (2014) A novel cold-active and salt-tolerant α-amylase from marine bacterium Zunongwangia profunda: molecular cloning, heterologous expression and biochemical characterization. Extremophiles 18:271–281. https://doi.org/10.1007/s00792-013-0614-9
Rathour R, Gupta J, Tyagi B, Thakur I (2020) Production and characterization of psychrophilic α-amylase from a psychrophilic bacterium, Shewanella sp. ISTPL2. Amylase. https://doi.org/10.1515/amylase-2020-0001
William Konigsberg, Reduction of disulfide bonds in proteins with dithiothreitol (1972) Methods in enzymology 25: 185-188
Siddiqui KS, Poljak A, Guilhaus M, Feller G, D'Amico S, Gerday C, Cavicchioli R (2005) Role of disulfide bridges in the activity and stability of a cold-active α–amylase. J Bacteriol 187:6206–6212
Jensen MT, Gottschalk TE, Svensson B (2003) Differences in conformational stability of barley alpha-amylase isozymes 1 and 2. Role of charged groups and isozyme 2 specific salt-bridges. J Cereal Sci 38:289–300
Pons J, Planas A, Querol E (1995) Contribution of a disulfide bridge to the stability of 1,3-1,4-beta-D-glucan 4-glucanohydrolase from Bacillus licheniformis. Protein Eng 8:939–945. https://doi.org/10.1093/protein/8.9.939
Otzen D (2011) Protein–surfactant interactions: a tale of many states. Biochim Biophys Acta - Proteins Proteomics 1814:562–591. https://doi.org/10.1016/j.bbapap.2011.03.003
Wang X, Kan G, Shi C, Xie Q, Ju Y, Wang R, Qiao Y, Ren X (2019) Purification and characterization of a novel wild-type α-amylase from Antarctic sea ice bacterium Pseudoalteromonas sp. M175. Protein Expression and Purification 164:105444
Xie F, Quan S, Liu D, Ma H, Li F, Zhou F, Chen G (2014) Purification and characterization of a novel α-amylase from a newly isolated Bacillus methylotrophicus strain P11-2. Process Biochem 49: 47–53. https://doi.org/10.1016/j.procbio.2013.09.025
Fincan SA, Enez B, Özdemir S, Bekler FM (2014) Purification and characterization of thermostable α-amylase from thermophilic Anoxybacillus flavithermus. Carbohydrate polymers 102:144–150
Author information
Authors and Affiliations
Contributions
AK: supervision, conceptualization, and revising of the manuscript. AE: supervision, methodology, conceptualization, data curation, and writing — reviewing and editing the manuscript. AM: visualization, investigation, validation, data curation, and writing — original draft preparation.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information

Fig. S1
Eadie-Hofstee plot of purified CA-AM21 using soluble starch as a substrate. The plot was drawn by Hyper32 software (PNG 37 kb)
Rights and permissions
About this article
Cite this article
Matrawy, A.A., Khalil, A.I. & Embaby, A.M. Bioconversion of bread waste by marine psychrotolerant Glutamicibacter soli strain AM6 to a value-added product: cold-adapted, salt-tolerant, and detergent-stable α-amylase (CA-AM21). Biomass Conv. Bioref. 13, 12125–12142 (2023). https://doi.org/10.1007/s13399-022-02325-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02325-3