Abstract
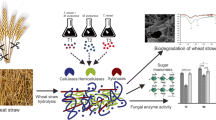
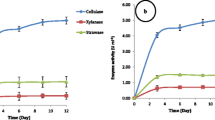
Lignocellulosic biomass from agricultural waste seems promising feedstock for biofuel production; however, its degradation to fermentable sugars is challenging. Interestingly, fungi have shown substantial potential for the breakdown of lignocellulosic biomass and thus could be employed in lignocellulose-based biorefinery. Aiming at this, the current study was focused on screening the novel cellulolytic fungi from the surrounding environment. The preliminary molecular/morphological screening of 107 samples narrowed the experiment to 5 different fungi, designated: Aspergillus tubingensis AKF2, Aspergillus flavus AKF3, Pyricularia oryzae AKF4, Aspergillus nominus AKF5, and Aspergillus oryzae AKF6. The selected fungi were evaluated for their cellulolytic potential utilizing wheat straw, corn cob, and rice husk. The highest enzyme activity (CMCase: 68.2 U ml−1; FPase: 343.3 U ml−1; β-Gase: 86.3 U ml−1; xylanase: 234.6 U ml−1) was observed on the 8th day in the cultures of A. tubingensis AKF2 using wheat straw as compared to corn cob and rice husk. The total reducing sugars released after enzymatic breakdown were also high (437.05 mg g−1) in the wheat straw cultures of A. tubingensis AKF2 comprehending the higher cellulolytic activity of this fungus. To further investigate the compositional breakdown, scanning electron microscopy and FTIR were performed. Significant (LSD > 0.05) results were observed in the wheat straw cultures of A. tubingensis AKF2 with 48%, 36%, 39%, and 56% reductions in the cellulose, hemicellulose, lignin, and total mass contents, respectively. The superseding fungus (A. tubingensis AKF2) identified here could be further optimized for an eco-sustainable biorefinery and may have industrial applications for cellulase production.
Graphical abstract







Similar content being viewed by others
References
Van Zessen E, Weismann M, Bakker R, Elbersen H, Reith J, Den Uil H (2003) Lignocellulosic ethanol, a second opinion. Report 2GAVE-03.11. NetherlandsAgency for Energy & Environment. Available from:, https://library.wur.nl/WebQuery/wurpubs/339809.
Rasmussen H, Tanner D, Sørensen H, Meyer AS (2017) New degradation compounds from lignocellulosic biomass pretreatment: routes for formation of potent oligophenolic enzyme inhibitors. Green Chem 19(2):464–473
Bar-On YM, Phillips R, Milo R (2018) The biomass distribution on Earth. Proc Natl Acad Sci U S A 115(25):6506–6511
Baykara SZ (2018) Hydrogen: a brief overview on its sources, production and environmental impact. Int J Hydrogen Energy 43(23):10605–10614
Hanif I, Raza SMF, Gago-de-Santos P, Abbas Q (2019) Fossil fuels, foreign direct investment, and economic growth have triggered CO2 emissions in emerging Asian economies: some empirical evidence. Energy 171:493–501
Patel SJ, Onkarappa R, Shobha KS (2007) Comparative study of ethanol production from microbial pretreated agricultural residues. J Appl Environ Manag 11(4):137–141
Chen H (2014) Chemical composition and structure of natural lignocellulose, Biotechnology of lignocellulose. Springer, pp 25–71
Baadhe RR, Potumarthi R, Mekala NK (2014) Influence of dilute acid and alkali pretreatment on reducing sugar production from corncobs by crude enzymatic method: a comparative study. Bioresour Technol 162:213–217
Salehi SA, Karimi K, Behzad T, Poornejad NJE (2012) Efficient conversion of rice straw to bioethanol using sodium carbonate pretreatment. Fuels 26(12):7354–7361
Goto M, Kamiya N (2016) Powerful peracetic acid–ionic liquid pretreatment process for the efficient chemical hydrolysis of lignocellulosic biomass. Bioresource Technol 214:487–495
Karimi K, Kheradmandinia S, Taherzadeh MJ (2006) Conversion of rice straw to sugars by dilute-acid hydrolysis. Biomass Bioenergy 30(3):247–253
Irshad MN, Anwar Z, But HI, Afroz A, Ikram N, Rashid UJB (2013) The industrial applicability of purified cellulase complex indigenously produced by Trichoderma viride through solid-state bio-processing of agro-industrial and municipal paper wastes. BioResources 8(1):145–157
Ahmed I, Zia MA, Iqbal HMNJC (2010) Bioprocessing of proximally analyzed wheat straw for enhanced cellulase production through process optimization with Trichoderma viride under SSF. Cellulose 2(W3):100
Ilyas U, Majeed A, Hussain K, Nawaz K, Ahmad S, Nadeem MJWASJ (2011) Solid state fermentation of Vignamungo for cellulase production by Aspergillus niger. World Applied Science Journal 12:1172–1178
Sharma A, Tewari R, Rana SS, Soni R, Soni SK (2016) Cellulases: classification, methods of determination and industrial applications. Appl Biochem Biotechnol 179(8):1346–1380
Kogo T, Yoshida Y, Koganei K, Matsumoto H, Watanabe T, Ogihara J, Kasumi T (2017) Production of rice straw hydrolysis enzymes by the fungi Trichoderma reesei and Humicola insolens using rice straw as a carbon source. Bioresour Technol 233:67–73
Arnthong J, Chuaseeharonnachai C, Boonyuen N, Tachaapaikun C, Chimchana D, Eurwilaichitr L, Champreda V, Chantasingh D (2018) Cooperative decomposition of rice straw by co-cultivation of cellulolytic fungI. J Sci 45:645–652
Fatma S, Saleem A, Tabassum R (2020) Wheat straw hydrolysis by using co-cultures of Trichoderma reesei and Monascus purpureus toward enhanced biodegradation of the lignocellulosic biomass in bioethanol biorefinery. Biomass Convers Biorefin 11(3):743–754
Boer W, Folman LB, Summerbell RC, Boddy L (2005) Living in a fungal world: impact of fungi on soil bacterial niche development. FEMS Microbiol Rev 29(4):795–811
Mandels M, Reese ET (1957) Induction of cellulase in Trichoderma viride as influenced by carbon sources and metals. J Bacteriol 73(2):269
Gritzali M, Brown RD Jr (1979) The cellulase system of Trichoderma: relationships between purified extracellular enzymes from induced or cellulose-grown cells. Adv Chem 181:237–260
Benny GL (2008) Methods used by Dr. RK Benjamin, and other mycologists, to isolate zygomycetes. Aliso J Syst Evol Bot 26(1):37–61
White TJ, Lee S-H, Taylor L, Shawe-Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic, San Diego, pp 315–322
Sazci A, Erenler K, Radford A (1986) Detection of cellulolytic fungi by using Congo red as an indicator: a comparative study with the dinitrosalicyclic acid reagent method. J Appl Bacteriol 61(6):559–562
Teather RM, Wood PJ (1982) Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl Environ Microbiol 43(4):777–780
Spano L, Medeiros J, Mandels MJUA, Natick, (1975) Enzymatic hydrolysis of cellulosic waste to glucose. Resource Recovery and Conservation 1(3):279–294
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2008) Determination of structural carbohydrates and lignin in biomass. Laboratory analytical procedure 1617(1):1–16
Ghose T (1987) Measurement of cellulase activities. Pure Appl Chem 59(2):257–268
Herr D (1979) Secretion of cellulase and β-glucosidase by Trichoderma viride ITCC-1433 in submerged culture on different substrates. Biotechnol Bioeng 21(8):1361–1371
Xu X, Xu Z, Shi S, Lin M (2017) Lignocellulose degradation patterns, structural changes, and enzyme secretion by Inonotus obliquus on straw biomass under submerged fermentation. Bioresour Technol 241:415–423
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31(3):426–428
Godin B, Agneessens R, Gerin PA, Delcarte JJT (2011) Composition of structural carbohydrates in biomass: precision of a liquid chromatography method using a neutral detergent extraction and a charged aerosol detector. Talanta 85(4):2014–2026
Anasontzis GE, Thuy NT, Hang DTM, Huong HT, Thanh DT, Hien DD, Thanh VN, Olsson L (2017) Rice straw hydrolysis using secretomes from novel fungal isolates from Vietnam. Biomass Bioenergy 99:11–20
Dias AA, Freitas GS, Marques GS, Sampaio A, Fraga IS, Rodrigues MA, Evtuguin DV, Bezerra RM (2010) Enzymatic saccharification of biologically pre-treated wheat straw with white-rot fungi. Bioresour Technol 101(15):6045–6050
McIntosh S, Vancov T (2011) Optimisation of dilute alkaline pretreatment for enzymatic saccharification of wheat straw. Biomass Bioenergy 35(7):3094–3103
Aggarwal NK, Goyal V, Saini A, Yadav A, Gupta R (2017) Enzymatic saccharification of pretreated rice straw by cellulases from Aspergillus niger BK01. 3 Biotech 7(3):158
Bakri Y, Masson M, Thonart P (2010) Isolation and identification of two new fungal strains for xylanase production. Appl Biochem Biotechnol 162(6):1626–1634
Hui L, Wan C, Hai-Tao D, Xue-Jiao C, Qi-Fa Z, Yu-Hua Z (2010) Direct microbial conversion of wheat straw into lipid by a cellulolytic fungus of Aspergillus oryzae A-4 in solid-state fermentation. Biores Technol 101(19):7556–7562
Gomathi D, Muthulakshmi C, Kumar DG, Ravikumar G, Kalaiselvi M, Uma C (2012) Submerged fermentation of wheat bran by Aspergillus flavus for production and characterization of carboxy methyl cellulase. Asian Pac J Trop Biomed 2(1):S67–S73
Prajapati BP, Kumar Suryawanshi R, Agrawal S, Ghosh M, Kango N (2018) Characterization of cellulase from Aspergillus tubingensis NKBP-55 for generation of fermentable sugars from agricultural residues. Bioresour Technol 250:733–740
Rais A, Shakeel M, Hafeez FY, Hassan MN (2016) Plant growth promoting rhizobacteria suppress blast disease caused by Pyricularia oryzae and increase grain yield of rice. Biocontrol 61(6):769–780
Kumar AK, Sharma S (2017) Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review. Bioresour Bioprocess 4(1):7
Zhang F, Bunterngsook B, Li J-X, Zhao X-Q, Champreda V, Liu C-G, Bai F-W (2019) Regulation and production of lignocellulolytic enzymes from Trichoderma reesei for biofuels production. In Advances in bioenergy (Vol. 4, pp. 79-119). Elsevier
Seiboth, B., Ivanova, C. and Seidl-Seiboth, V., 2011. Trichoderma reesei: a fungal enzyme producer for cellulosic biofuels. Biofuel production-recent developments and prospects, pp.309-340
Khokhar Z-U, Syed Q, Nadeem M, Irfan M, Wu J, Samra Z, Gul I, Athar A (2014) Enhanced production of cellulase by Trichoderma reesei using wheat straw as a carbon source. World Appl Sci J 30(9):1095–1104
Manavalan T, Manavalan A, Heese K (2015) Characterization of lignocellulolytic enzymes from white-rot fungi. Curr Microbiol 70(4):485–498
Chang AJ, Fan J, Wen X (2012) Screening of fungi capable of highly selective degradation of lignin in rice straw. Int Biodeterior Biodegrad 72:26–30
Singh A, Bajar S, Bishnoi NR, Singh N (2010) Laccase production by Aspergillus heteromorphus using distillery spent wash and lignocellulosic biomass. J Hazard Mater 176(1–3):1079–1082
Gopalakrishnan RM, Manavalan T, Ramesh J, Thangavelu KP, Heese K (2020) Improvement of saccharification and delignification efficiency of Trichoderma reesei Rut-C30 by genetic bioengineering. Microorganisms 8(2):159
Vasina DV, Pavlov AR, Koroleva OV (2016) Extracellular proteins of Trametes hirsuta st. 072 induced by copper ions and a lignocellulose substrate. BMC Microbiol 16(1):106
Jiang Y, Duarte AV, van den Brink J, Wiebenga A, Zou G, Wang C, de Vries RP, Zhou Z, Benoit I (2016) Enhancing saccharification of wheat straw by mixing enzymes from genetically-modified Trichoderma reesei and Aspergillus niger. Biotechnol Lett 38(1):65–70
Tsegaye B, Balomajumder C, Roy P (2018) Biodegradation of wheat straw by Ochrobactrum oryzae BMP03 and Bacillus sp. BMP01 bacteria to enhance biofuel production by increasing total reducing sugars yield. Environ Sci Pollut Res Int 25(30):30585–30596
Dhiman SS, Haw J-R, Kalyani D, Kalia VC, Kang YC, Lee J-K (2015) Simultaneous pretreatment and saccharification: green technology for enhanced sugar yields from biomass using a fungal consortium. Bioresour Technol 179:50–57
Mishra V, Jana AK, Jana MM, Gupta A (2017) Enhancement in multiple lignolytic enzymes production for optimized lignin degradation and selectivity in fungal pretreatment of sweet sorghum bagasse. Bioresour Technol 236:49–59
Castoldi R, Bracht A, de Morais GR, Baesso ML, Correa RCG, Peralta RA, Moreira RDFPM, de Moraes MDLT, de Souza CGM, Peralta RM (2014) Biological pretreatment of Eucalyptus grandis sawdust with white-rot fungi: study of degradation patterns and saccharification kinetics. Chem Eng J 258:240–246
Prasad S, Singh A, Joshi H (2007) Ethanol as an alternative fuel from agricultural, industrial and urban residues. Resour Conserv Recycl 50(1):1–39
Asghar U, Irfan M, Iram M, Huma Z, Nelofer R, Nadeem M, Syed Q (2015) Effect of alkaline pretreatment on delignification of wheat straw. Nat Prod Res 29(2):125–131
Talebnia F, Karakashev D, Angelidaki I (2010) Production of bioethanol from wheat straw: an overview on pretreatment, hydrolysis and fermentation. Bioresour Technol 101(13):4744–4753
Shahryari Z, Fazaelipoor MH, Setoodeh P, Nair RB, Taherzadeh MJ, Ghasemi Y (2018) Utilization of wheat straw for fungal phytase production. Int J Recycl Org Waste Agric 7(4):345–355
Zhang A-P, Liu C-F, Sun R-C, Xie J (2013) Extraction, purification, and characterization of lignin fractions from sugarcane bagasse. BioResources 8(2):1604–1614
Salvachúa D, Prieto A, López-Abelairas M, Lu-Chau T, Martínez ÁT, Martínez MJ (2011) Fungal pretreatment: an alternative in second-generation ethanol from wheat straw. Biores Technol 102(16):7500–7506
Kannaiyan R, Mahinpey N, Kostenko V, Martinuzzi RJ (2017) Enhanced delignification of wheat straw by the combined effect of hydrothermal and fungal treatments. Chem Eng Commun 204(7):803–812
Shah TA, Ullah R (2019) Pretreatment of wheat straw with ligninolytic fungi for increased biogas productivity. Int J Environ Sci Technol 16(11):7497–7508
Liu Q, Gao R, Li J, Lin L, Zhao J, Sun W, Tian C (2017) Development of a genome-editing CRISPR/Cas9 system in thermophilic fungal Myceliophthora species and its application to hyper-cellulase production strain engineering. Biotechnol Biofuels 10(1):1
Xu G, Li J, Liu Q, Sun W, Jiang M, Tian C (2018) Transcriptional analysis of Myceliophthora thermophila on soluble starch and role of regulator AmyR on polysaccharide degradation. Bioresour Technol 265:558–562
Funding
This study was supported by the “Akhuwat Foundation,” a non-profit micro-finance organization working in Pakistan for the welfare of humanity. Akhuwat started working in 2001 to provide a small interest-free loan to poor families enabling them to become self-reliant. To fulfill the dream of a poverty-free society, Akhuwat started to provide “free education” to deserving students from all over Pakistan. Akhuwat-FIRST is one of the leading research institutes in Pakistan supported by “Akhuwat Foundation.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Fungi collected from the environment and prescreening through CMC-agar staining and ITS sequencing resulted in 5 different fungal isolates with cellulase activity.
• The selected fungi were evaluated for the enzymatic hydrolysis of wheat straw, rice straw, and corn cob through lignocellulolytic enzyme assays.
• Compositional and structural modifications of biodegraded wheat straw proofread the higher cellulolytic activity of fungi.
• The potential fungus “Aspergillus tubingensis AKF2” identified in this study have a great capacity for lignocellulose biodegradation and could be promising for biofuel extraction.
Supplementary Information
Below is the link to the electronic supplementary material.
13399_2021_1932_MOESM1_ESM.jpg
Supplementary file1 Morphological characterization of the 20 different fungi (AKF1-AKF20) taken after 5 days of culture on PDA Petri plates. (JPG 161 KB)
13399_2021_1932_MOESM2_ESM.jpg
Supplementary file2 A: Molecular confirmation of 5 fungi (AKF2, AKF3, AKF4, AKF5, AKF6) for the ITS band (500–700 bp). B: CMC-agar plates with clearing halo (diameter) of hydrolysis. (JPG 51 KB)
Rights and permissions
About this article
Cite this article
Awais, M., Fatma, S., Naveed, A. et al. Enhanced biodegradation of organic waste treated by environmental fungal isolates with higher cellulolytic potential. Biomass Conv. Bioref. (2021). https://doi.org/10.1007/s13399-021-01932-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-021-01932-w